Adsorption performance and mechanism of MnO2/Ti3C2TX composite towards U(VI) in water
-
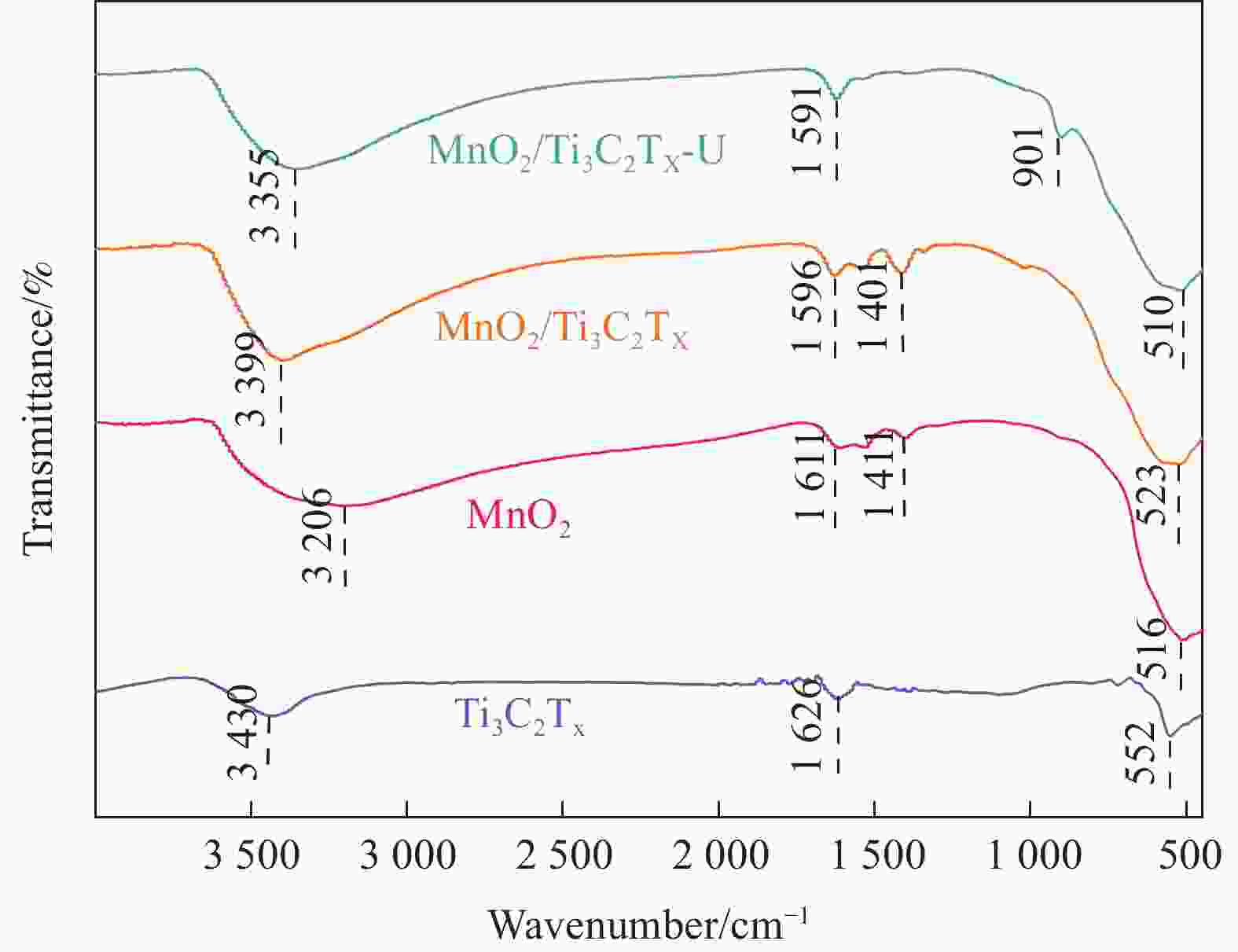
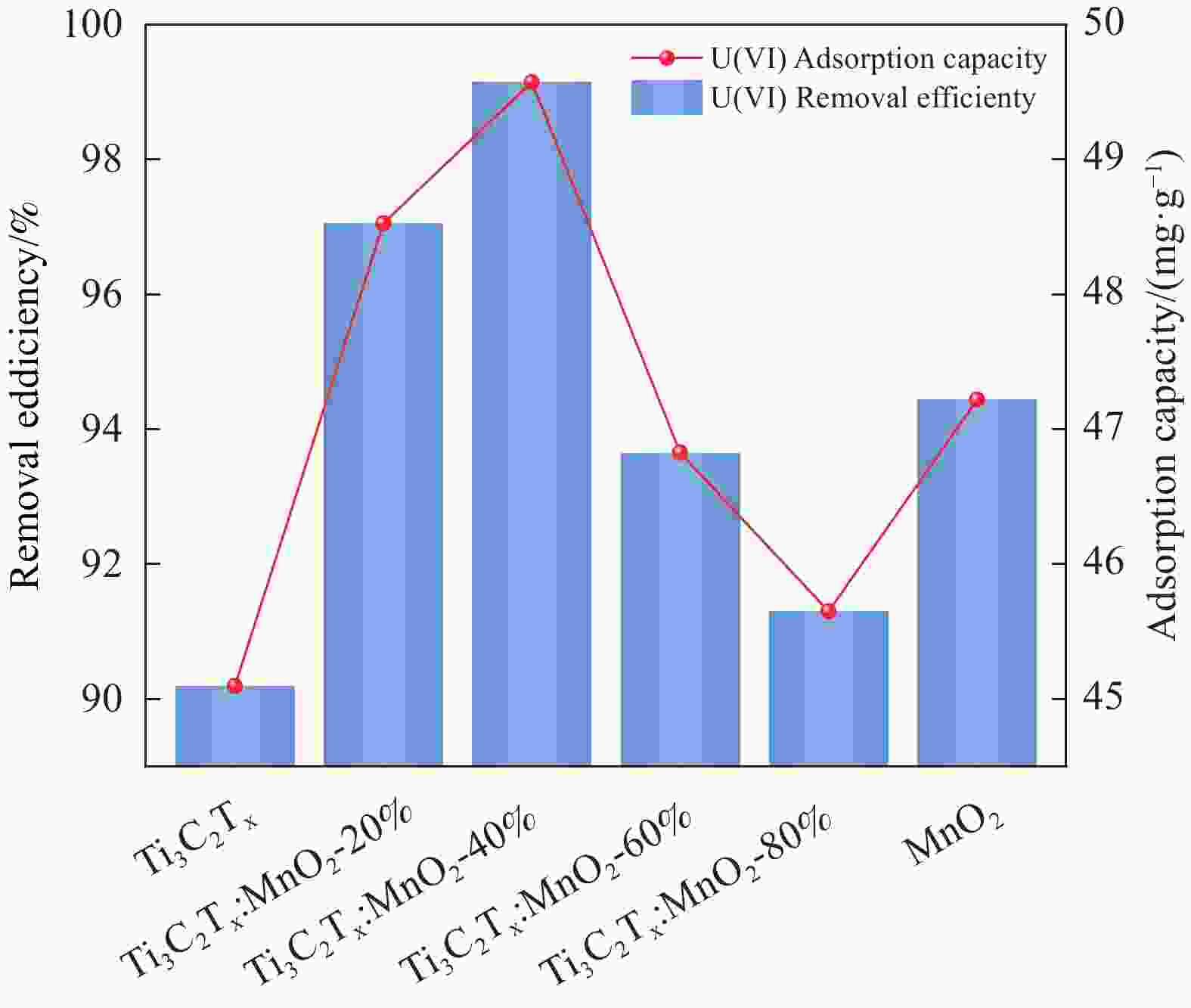
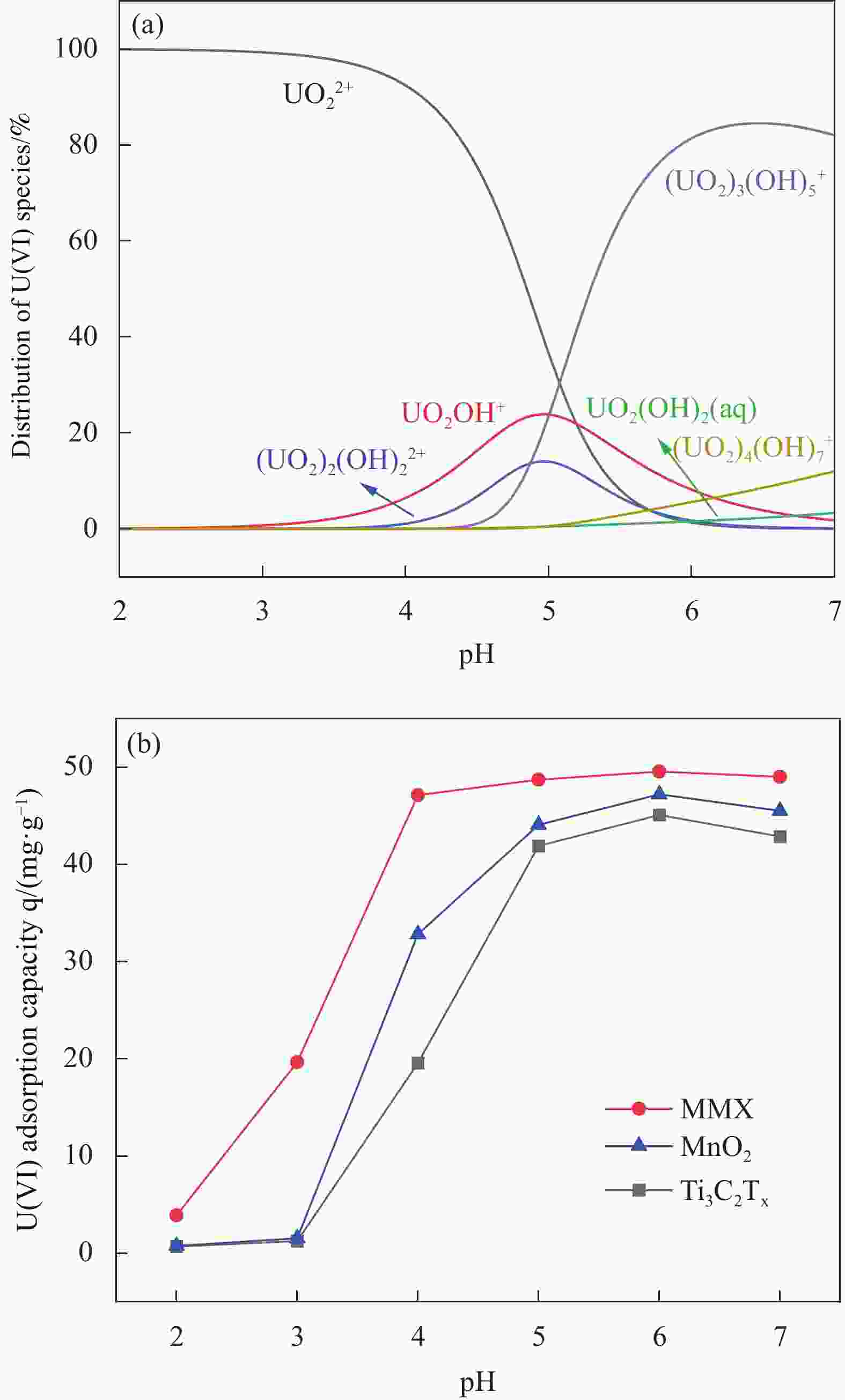
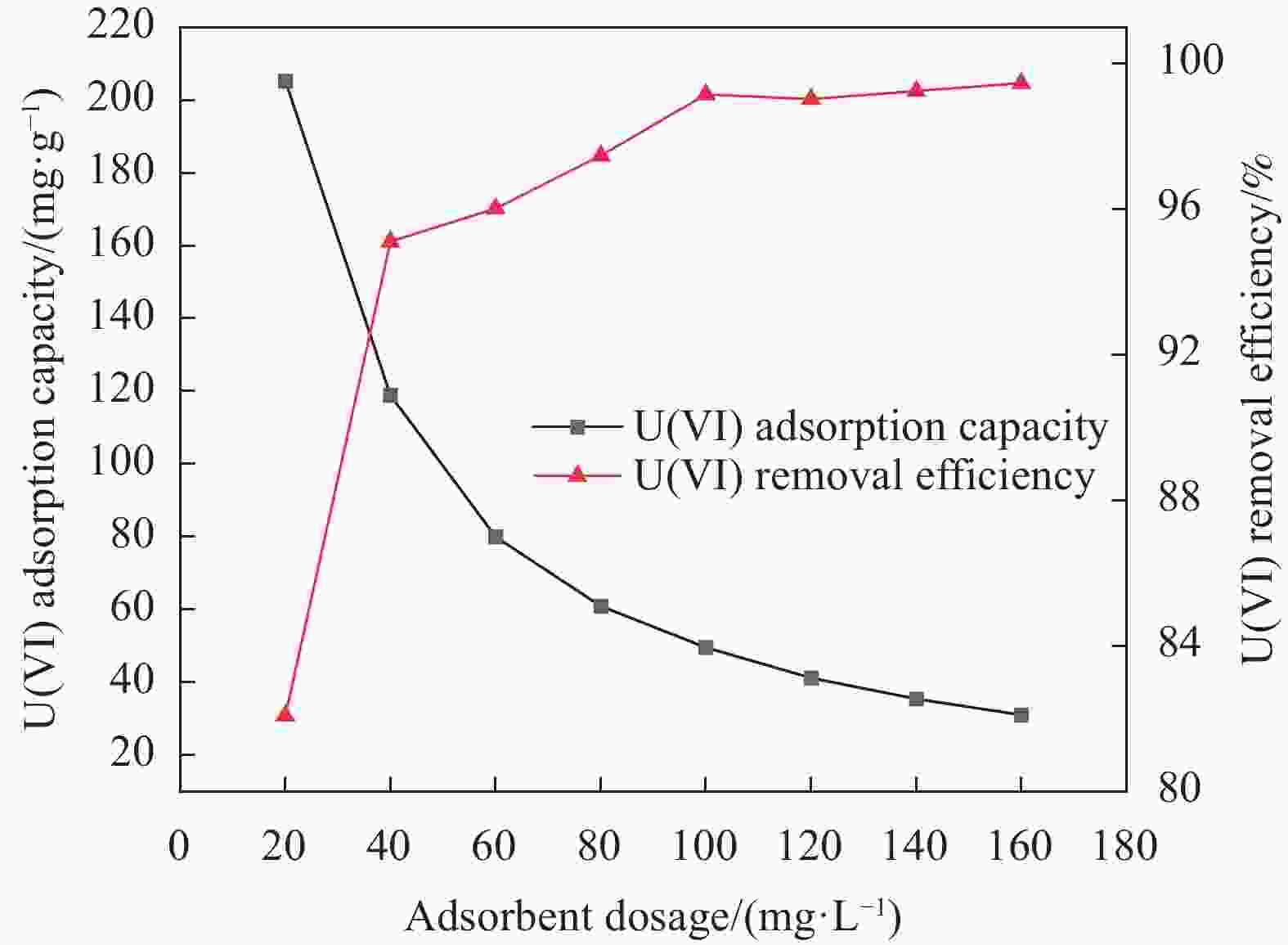
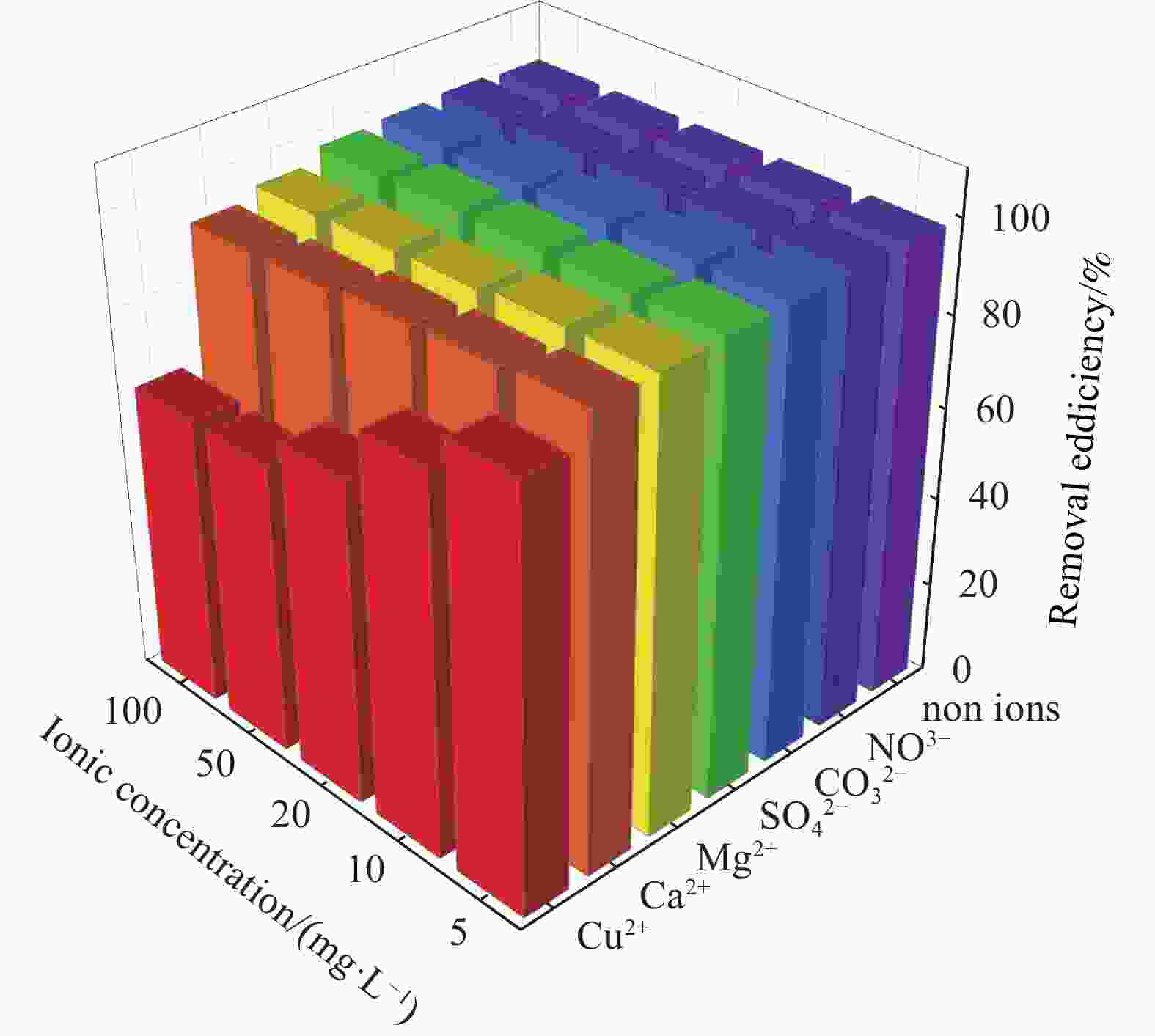
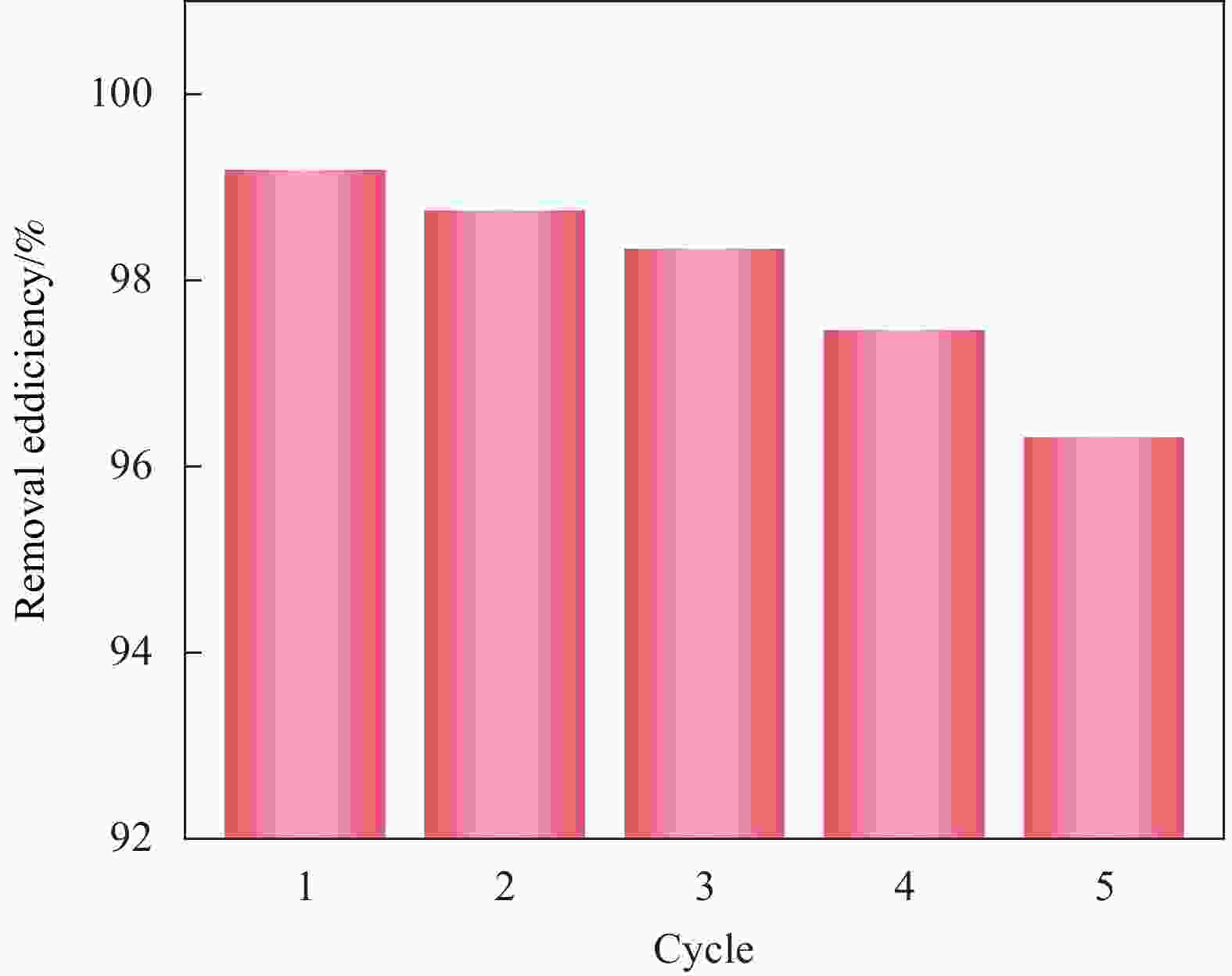
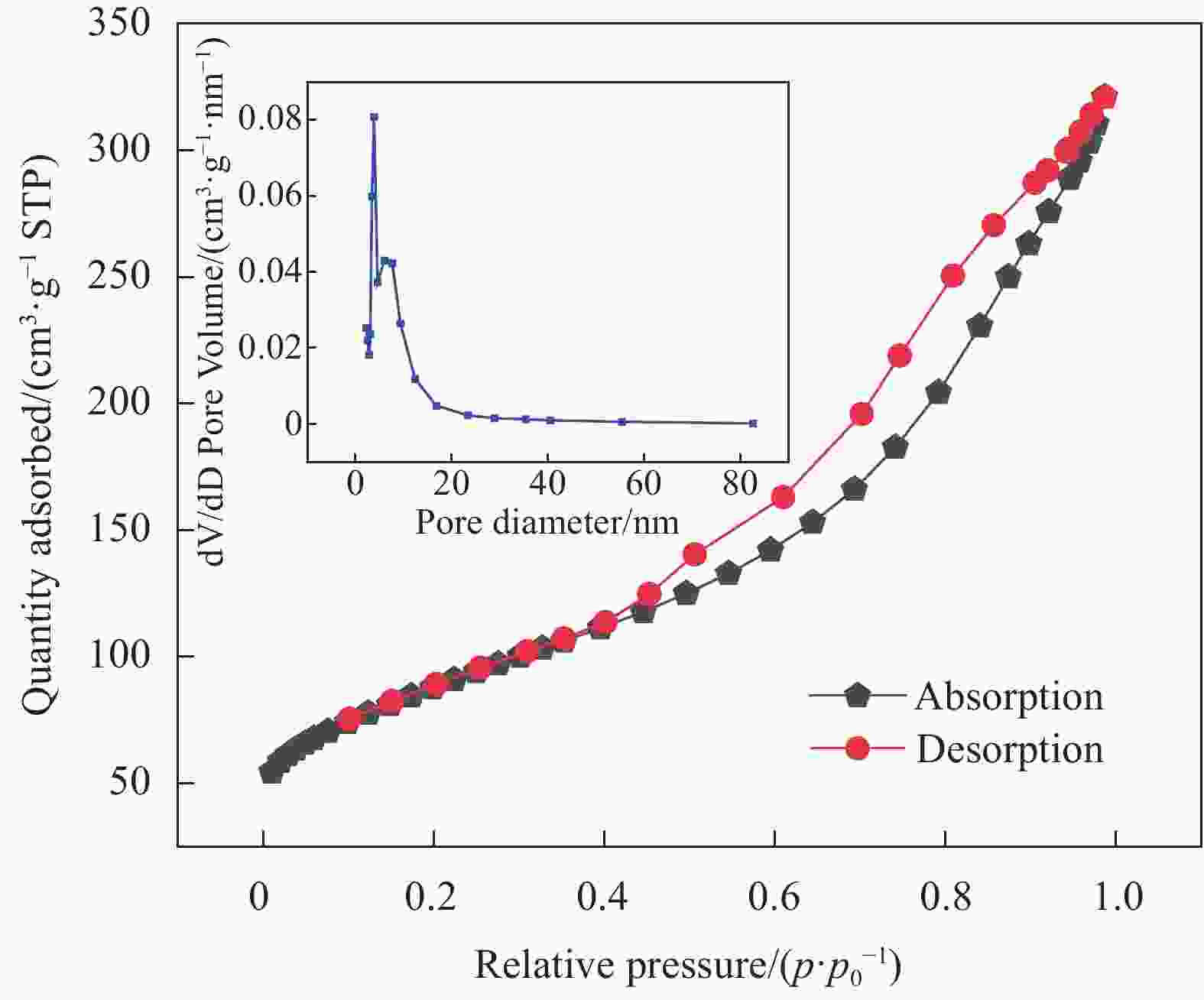
摘要: 针对Ti3C2TX纳米片层易堆叠和吸附位点少的不足,采用水热法制备了MnO2/Ti3C2TX复合剂。通过单因素试验探讨铀的初始浓度、投加量、pH值、时间和共存离子对其吸附U(VI)的影响,利用现代表征手段分析MnO2/Ti3C2TX的表面性质及吸附U(VI)的作用机制。试验结果表明:在U(VI)初始浓度5 mg·L−1、MnO2/Ti3C2TX投加量为0.1 g·L−1、温度303 K、pH值为6时,30 s内U(VI)浓度降至0.41 mg·L−1,30 min后吸附达到平衡,其对U(VI)的吸附率达99.15%,吸附容量为49.58 mg·g−1。经过5次循环后,MnO2/Ti3C2TX对U(VI)的吸附效率仍保持在96.3%,具有良好的可再生利用性。整个吸附过程为自发吸热过程,符合拟二级动力学模型和Freundlich等温线模型。BET分析表明MnO2/Ti3C2TX的比表面积达318.3 m2·g−1,较Ti3C2TX高55.9倍。FTIR和XPS分析表明MnO2/Ti3C2TX对U(VI)吸附主要是表面含氧基团与铀的配位络合。Abstract: To address the shortcomings of Ti3C2TX nanosheets, which tend to stack and have limited adsorption sites, a MnO2/Ti3C2TX composite was prepared using the hydrothermal method. The influence of uranium initial concentration, dosage, pH, time, and interfering ions on U(VI) adsorption was investigated through single-factor adsorption experiments. Modern characterization techniques were employed to analyze the surface properties of MnO2/Ti3C2TX and the mechanism of U(VI) adsorption. Experimental results revealed that with an initial U(VI) concentration of 5 mg·L−1, MnO2/Ti3C2TX dosage of 0.1 g·L−1, and a temperature of 303 K, pH of 6, the U(VI) concentration dropped to 0.41mg·L−1 within 30 seconds. Adsorption equilibrium was reached after 30 minutes, with an adsorption rate of 99.15% and an adsorption capacity of 49.58 mg·g−1. After five cycles, the adsorption efficiency of MnO2/Ti3C2TX remained at 96.3%, demonstrating its potential for regeneration and reuse.The entire adsorption process was endothermic and spontaneous, fitting the pseudo-second-order kinetic model and the Freundlich isotherm model. BET analysis showed that the specific surface area of MnO2/Ti3C2TX reached 318.3 m2·g−1, which is 55.9 times higher than that of Ti3C2TX. FTIR and XPS analyses indicated that the primary mechanism of U(VI) adsorption on MnO2/Ti3C2TX is the coordination complexation between surface oxygen-containing groups and uranium.
-
Key words:
- Ti3C2TX /
- MXene /
- MnO2 /
- adsorption /
- U(VI)
-
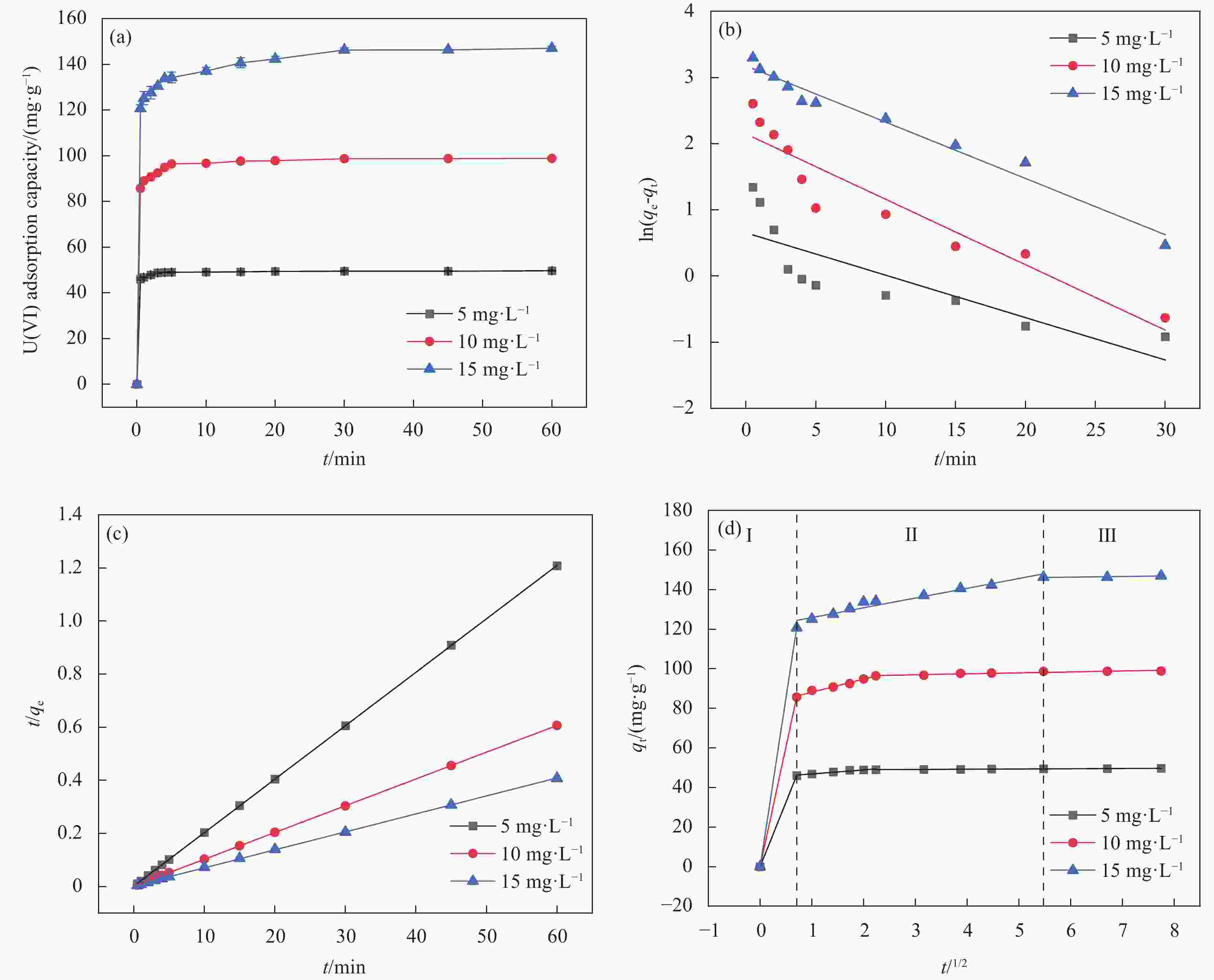
图 10 (a)吸附时间对MnO2/Ti3C2TX吸附不同浓度 U(VI)的影响; (b)拟一级动力学模型拟合曲线;(c)拟二级动力学模型拟合曲线;(d)颗粒内扩散模型拟合曲线
Figure 10. (a) Effect of adsorption time on MnO2/Ti3C2TX adsorption of different concentrations of U (VI); (b) the fitting curve of pseudo-first-order kinetic model; (c) the fitting curve of quasi-second-order kinetic model; (d) the fitting curve of intra-particle diffusion model
qt−Adsorption capacity at t time; qe−Equilibrium adsorption capacity; t−Adsorption time
表 1 MnO2/ Ti3C2Tx与其他吸附剂比表面积对比
Table 1. Comparison of BET surface area between MnO2/ Ti3C2Tx and other adsorbents
表 2 MnO2/Ti3C2TX吸附U(VI)的动力学模型参数
Table 2. Kinetic model parameters of uranium adsorption by MnO2/Ti3C2TX
C0/(mg·L−1) qe.exp/(mg·g−1) Pseudo- first Kinetics model Pseudo- second Kinetics model K1 qe.cal R2 K2 qe.cal R2 5 49.792 0.085 1.923 0.687 0.020 49.751 1 10 99.112 0.099 8.575 0.893 0.010 99.010 1 15 146.825 0.064 24.016 0.972 0.007 147.059 0.999 Notes: qe is the adsorption capacity at equilibrium time; K1 and K2 are reaction rate constants of pseudo-first-order and pseudo-second-order equations, respectively; R2 is the correlation coefficient. 表 3 MnO2/Ti3C2TX吸附等温线模型的相关参数
Table 3. Relevant parameters of MnO2/Ti3C2TX adsorption isotherm model
T/K Langmuir Freundlich qmax/(mg·g−1) b R2 KF n R2 293 372.801 0.853 0.933 162.314 3.058 0.994 303 380.414 1.157 0.941 176.716 3.139 0.994 313 434.837 0.717 0.936 196.977 3.145 0.992 Notes: qmax is Adsorption capacity per unit mass of the adsorbent; b is Langmuir coefficient related to the affinity of binding site; KF , n-Constants that are related to the adsorption capacity and the adsorption intensity, respectively. 表 4 MnO2/Ti3C2TX吸附U(VI)性能与其他吸附剂对比
Table 4. Comparison of MnO2/Ti3C2TX adsorption performance for U (VI) with other adsorbents
Adsorbent pH Temperature/K Equilibrium
time/minMaximum adsorption
capacity /(mg·g−1)References MnO2@BBC 5 298 240 97.40 Chen etal[33](2022) HPC/POSS-OH modified MXene 5 298 90 307.67 Zhao etal[34](2022) Chloroacetic acid modified Ti3C2TX 5 308 150 165.43 Xie etal[35](2022) γ-Fe2O3/CSS 6 298 120 214.1 Fan etal[36](2022) Ti3C2@FeS-PDA/PEI 6 298 240 88.5 Liu etal[37](2023) MnO2/ Ti3C2TX 6 303 30 380.41 This work Notes: MnO2@BBC-MnO2 modified bamboo-derived biochar composite;HPC/POSS-OH- hydroxypropyl cellulose (HPC) and polyhedral oligomeric silsesquioxane (POSS-OH);CSS-corn stalk starch; PDA/PEI-polydopamine and polyethyleneimine 表 5 MnO2/Ti3C2TX吸附U(VI)的热力学参数
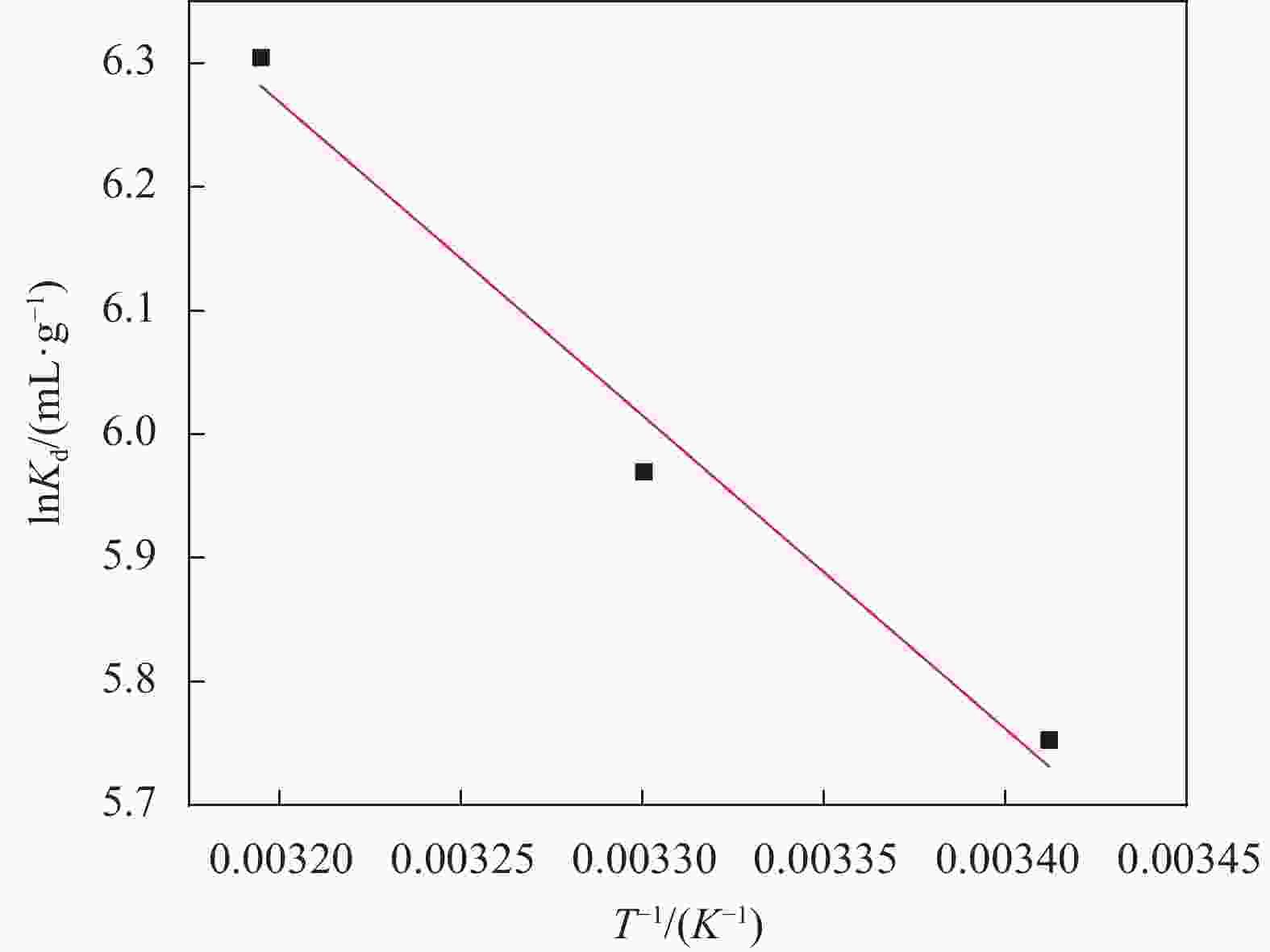
Table 5. Thermodynamic parameters of uranium adsorption by MnO2/Ti3C2TX
∆G0/(kJ·mol−1) ∆H0/(kJ·mol−1) ∆S0/(kJ·mol−1) 293 K 303 K 313 K 21.073 119.551 −14.014 −15.038 −16.406 Notes: ΔG0 −Standard free energy change; ΔH0 −Standard enthalpy change; ΔS0 −Standard entropy change. -
[1] WANG S, WANG L, LI Z, et al. Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron[J/OL][J]. Journal of Hazardous Materials, 2021, 408: 124949. doi: 10.1016/j.jhazmat.2020.124949 [2] CHAI W S, CHEUN J Y, KUMAR P S, et al. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application[J/OL][J]. Journal Of Cleaner Production, 2021, 296: 126589. doi: 10.1016/j.jclepro.2021.126589 [3] SHETH Y, DHARASKAR S, CHAUDHARY V, et al. Prospects of titanium carbide-based MXene in heavy metal ion and radionuclide adsorption for wastewater remediation: A review[J/OL][J]. Chemosphere, 2022, 293: 133563. doi: 10.1016/j.chemosphere.2022.133563 [4] DU Y, YU B, WEI L, et al. Efficient removal of Pb(II) by Ti3C2TX powder modified with a silane coupling agent[J/OL][J]. Journal of Materials Science, 2019, 54(20): 13283-13297. doi: 10.1007/s10853-019-03814-z [5] WANG L, TAO W, YUAN L, et al. Rational control of the interlayer space inside two-dimensional titanium carbides for highly efficient uranium removal and imprisonment[J/OL][J]. Chemical Communications, 2017, 53(89): 12084-12087. doi: 10.1039/C7CC06740B [6] CHEN L, WAKEEL M, UL HAQ T, et al. Recent progress in environmental remediation, colloidal behavior and biological effects of MXene: a review[J/OL][J]. Environmental Science-Nano, 2022, 9(9): 3168-3205. doi: 10.1039/D2EN00340F [7] MENG C, GAO X, ZOU S, et al. Unraveling the adsorption behaviors of uranium and thorium on the hydroxylated titanium carbide MXene[J/OL][J]. Computational Materials Science, 2022, 210: 111460. doi: 10.1016/j.commatsci.2022.111460 [8] LI M, KUANG S, DONG J, et al. Performance and mechanisms of Cr(VI) removal by nano-MnO2 with different lattices[J/OL][J]. Journal Of Molecular Structure, 2023, 1275: 134624. doi: 10.1016/j.molstruc.2022.134624 [9] DING Y, XIAN Q, WANG E, et al. Mesoporous MnO2 /SBA-15 as a synergetic adsorbent for enhanced uranium adsorption[J/OL][J]. New Journal of Chemistry, 2020, 44(32): 13707-13715. doi: 10.1039/D0NJ02966A [10] KIM S, GHOLAMIRAD F, YU M, et al. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene[J/OL][J]. Chemical Engineering Journal, 2021, 406: 126789. doi: 10.1016/j.cej.2020.126789 [11] BI L, MA J, NIU Z, et al. Synthesis of β-cyclodextrin derivatives and their selective separation behaviors for U(VI) in solution[J/OL][J]. Journal of Radioanalytical and Nuclear Chemistry, 2020, 326(1): 719-736. doi: 10.1007/s10967-020-07343-x [12] MA J, ZHAO Q, ZHOU L, et al. Mutual effects of U(VI) and Eu(III) immobilization on interpenetrating 3-dimensional MnO2/graphene oxide composites[J/OL][J]. Science Of The Total Environment, 2019, 695: 133696. doi: 10.1016/j.scitotenv.2019.133696 [13] ZHAO X, VASHISTH A, PREHN E, et al. Antioxidants Unlock Shelf-Stable Ti3C2TX (MXene) Nanosheet Dispersions[J/OL][J]. MATTER, 2019, 1(2): 513-526. doi: 10.1016/j.matt.2019.05.020 [14] JIANG H, WANG Z, YANG Q, et al. A novel MnO2/Ti3C2TX MXene nanocomposite as high performance electrode materials for flexible supercapacitors[J/OL][J]. Electrochimica Acta, 2018, 290: 695-703. doi: 10.1016/j.electacta.2018.08.096 [15] ZHOU Y, CHEN T, ZHANG J, et al. Amorphous MnO2 as Cathode Material for Sodium-ion Batteries[J/OL][J]. Chinese Journal Of Chemistry, 2017, 35(8): 1294-1298. doi: 10.1002/cjoc.201600915 [16] WANG H, CUI H, SONG X, et al. Facile synthesis of heterojunction of MXenes/TiO2 nanoparticles towards enhanced hexavalent chromium removal[J/OL][J]. Journal of Colloid and Interface Science, 2020, 561: 46-57. doi: 10.1016/j.jcis.2019.11.120 [17] ZENG X, WANG Y, HE X, et al. Enhanced removal of Cr(VI) by reductive sorption with surface-modified Ti3C2TX MXene nanocomposites[J/OL][J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106203. doi: 10.1016/j.jece.2021.106203 [18] MA J, ZHAO Q, WEI D, et al. Simple construction of core- shell MnO2@ TiO2 with highly enhanced U( VI) adsorption performance and evaluated adsorption mechanism[J/OL][J]. Inorganic Chemistry Frontiers, 2019, 6(4): 1011-1021. doi: 10.1039/C8QI01379A [19] GU P, ZHANG S, MA R, et al. Layered double hydroxides nanosheets in-situ anchored on ultrathin MXenes for enhanced U(VI) and Eu(III) trapping: Excavating from selectivity to mechanism[J/OL][J]. Separation and Purification Technology, 2022, 288: 120641. doi: 10.1016/j.seppur.2022.120641 [20] KHAN A R, HUSNAIN S M, SHAHZAD F, et al. Two-dimensional transition metal carbide (Ti3C2TX) as an efficient adsorbent to remove cesium (Cs+)[J/OL][J]. Dalton Transactions, 2019, 48(31): 11803-11812. doi: 10.1039/C9DT01965K [21] MAHMOOD M, RASHEED A, AYMAN I, et al. Synthesis of Ultrathin MnO2 Nanowire-Intercalated 2D-MXenes for High-Performance Hybrid Supercapacitors[J/OL][J]. Energy & Fuels, 2021, 35(4): 3469-3478. [22] KHAN A R, AWAN S K, HUSNAIN S M, et al. 3D flower like δ-MnO2/MXene Nano-hybrids for the removal of hexavalent Cr from wastewater[J/OL][J]. Ceramics International, 2021, 47(18): 25951-25958. doi: 10.1016/j.ceramint.2021.05.326 [23] ZHANG Q, PENG Y, DENG F, et al. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants[J/OL][J]. Separation And Purification Technology, 2020, 246: 116890. doi: 10.1016/j.seppur.2020.116890 [24] FANG R, FENG Q, HUANG H, et al. Effect of K+ ions on efficient room-temperature degradation of formaldehyde over MnO2 catalysts[J/OL][J]. Catalysis Today, 2019, 327: 154-160. doi: 10.1016/j.cattod.2018.05.019 [25] LIU Y, DENG H, LU Z, et al. The study of MnO2 with different crystalline structures for U(VI) elimination from aqueous solution[J/OL][J]. Journal of Molecular Liquids, 2021, 335: 116296. doi: 10.1016/j.molliq.2021.116296 [26] XIA C, LUO Y, BIN X, et al. Rational design of flower-like MnO2/Ti3C2TX composite electrode for high performance supercapacitors[J/OL][J]. Nanotechnology, 2023, 34(25): 255602. doi: 10.1088/1361-6528/acc744 [27] GUO Y, LIU X, XIE S, et al. 3D ZnO modified biochar-based hydrogels for removing U(VI) in aqueous solution[J/OL][J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2022, 642: 128606. doi: 10.1016/j.colsurfa.2022.128606 [28] ZHANG C, LI X, CHEN Z, et al. Synthesis of ordered mesoporous carbonaceous materials and their highly efficient capture of uranium from solutions[J/OL][J]. Science China-Chemistry, 2018, 61(3): 281-293. doi: 10.1007/s11426-017-9132-7 [29] LI K, XIONG T, LIAO J, et al. Design of MXene/graphene oxide nanocomposites with micro-wrinkle structure for efficient separating of uranium(VI) from wastewater[J/OL][J]. Chemical Engineering Journal, 2022, 433: 134449. doi: 10.1016/j.cej.2021.134449 [30] WANG H, WU F, WANG Z, et al. Ultra-fast and ultra-efficient removal of Cr (VI) by the aqueous solutions of monolayer MXene (Ti3C2TX). [J/OL]. Chemosphere, 2022, 308(Pt 3): 136573. [31] WANG D, XU Y, XIAO D, et al. Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption[J/OL][J]. Journal of Hazardous Materials, 2019, 371: 83-93. doi: 10.1016/j.jhazmat.2019.02.091 [32] LI S, WANG L, PENG J, et al. Efficient thorium(IV) removal by two-dimensional Ti2CTX MXene from aqueous solution[J/OL][J]. Chemical Engineering Journal, 2019, 366: 192-199. doi: 10.1016/j.cej.2019.02.056 [33] CHEN X, WANG Y, LV J, et al. Simple one-pot synthesis of manganese dioxide modified bamboo-derived biochar composites for uranium(VI) removal[J/OL][J]. New Journal of Chemistry, 2022, 46(30): 14427-14438. doi: 10.1039/D2NJ02292C [34] ZHAO W, CHI H, ZHANG X, et al. Cellulose/silsesquioxane grafted Ti3C2TX MXene for synergistically enhanced adsorption of uranium[J/OL][J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2022, 650: 129610. [35] XIE L, YAN J, LIU Z, et al. Synthesis of a Two-Dimensional MXene Modified by Chloroacetic Acid and Its Adsorption of Uranium[J/OL][J]. Chemistryselect, 2022, 7(1): e202103583. doi: 10.1002/slct.202103583 [36] 范甲, 胡世琴, 魏柏, 等. 磁性γ-Fe_2O_3/玉米秸秆淀粉的制备及其对废水中U(VI)吸附性能[J/OL][J]. 复合材料学报, 2022, 39(10): 4898-4907.Fan Jia, Hu ShiQin, Wei Bai, et al. Preparation of magnetic γ-Fe2O3/corn stalk starch and its adsorption performance for U(VI) in wastewater [J/OL][J]. Acta Materiae Compositae Sinica, 2022, 39(10): 4898-4907. (in chinese [37] LIU F, WANG S, HU B. Electrostatic self-assembly of nanoscale FeS onto MXenes with enhanced reductive immobilization capability for U(VI) and Cr(VI)[J/OL][J]. Chemical Engineering Journal, 2023, 456: 141100. doi: 10.1016/j.cej.2022.141100 [38] CHEN X, XIE S, WANG G, et al. The performance and mechanism of U(VI) removal from aqueous solutions by a metal-organic framework (DUT-69)[J/OL][J]. Journal Of Radioanalytical And Nuclear CHEMISTRY, 2021, 328(1): 181-194. doi: 10.1007/s10967-021-07645-8 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 81
- HTML全文浏览量: 62
- 被引次数: 0




 下载:
下载: