Improved Extraction Performance of SnO2 ETL for Perovskite Solar Cells by a Combined Hydrolysis Oxidation and Sol-Gel Method
-
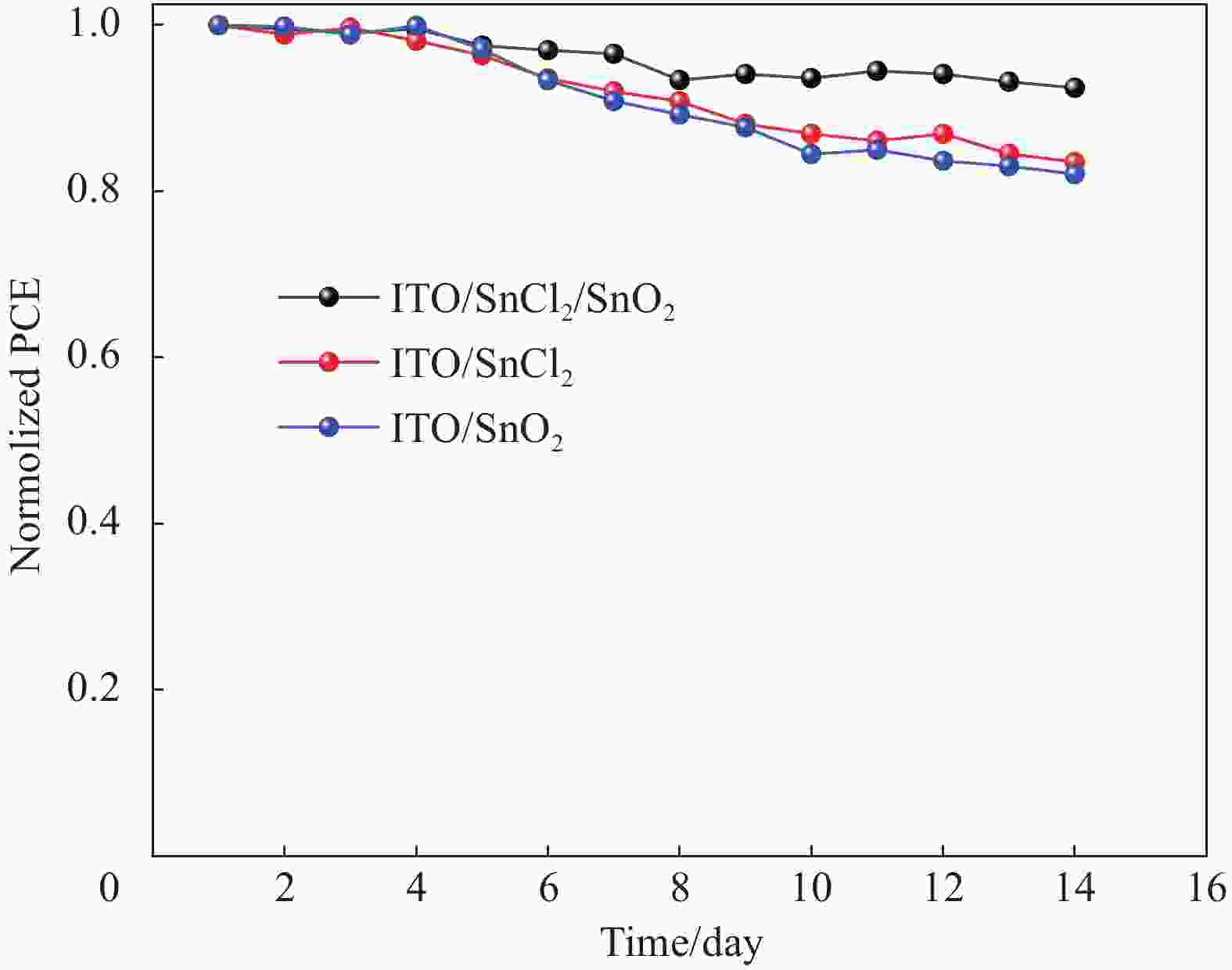
摘要: 二氧化锡(SnO2)由于其高电子迁移率、良的传导性和低温制备特性,在钙钛矿太阳能电池(PSCs)中得到了广泛的应用。目前,制备SnO2最常用的两种方法是SnCl2水解氧化法和SnO2溶胶-凝胶法。然而,SnCl2水解氧化虽然可以产生结晶良好的SnO2,但其可控性较差,使得器件性能的重复性较低。另一方面,溶胶-凝胶法制备的基于SnO2电子输运层的器件具有良好的重复性,但结晶度较差,导致电子输运性能下降。在本研究中,采用水解氧化和溶胶-凝胶相结合的方法制备了SnO2电子传输层。研究结果表明,采用SnCl2水解氧化法制备高质量的SnO2结晶层可以作为预生长模板,提高溶胶-凝胶法制备SnO2的结晶质量。此外,用溶胶-凝胶法制备的SnO2结晶层覆盖水解氧化SnO2层可以提高器件制备的重复性。由此制备的电子传递层方法可以有效地提高薄膜晶体的生长质量和电荷的提取能力,最终有助于提高器件的效率及稳定性并减少迟滞。
-
关键词:
- 二氧化锡 /
- 复合电子传输层 /
- 水解氧化 /
- 溶胶-凝胶法 /
- 有机无-机杂化钙钛矿太阳电池
Abstract: Tin dioxide (SnO2) is widely used in perovskite solar cells (PSCs) due to its high electron mobility, suitable conduction band and low-temperature preparation characteristics. Currently, the two most commonly used methods for preparing SnO2 are SnCl2 hydrolysis oxidation or SnO2 sol-gel preparation. However, although SnCl2 hydrolysis oxidation can produce well-crystallized SnO2, its controllability is poor, resulting in low device performance repeatability. On the other hand, the devices based on SnO2 electronic transport layer prepared by the sol-gel method have good repeatability, but usually have poor crystallinity, leading to a decrease in electron transport performance. In this study, a combination of hydrolysis oxidation and sol-gel methods was used to prepare SnO2 electronic transport layers. The results of the study demonstrate that using SnCl2 hydrolysis oxidation to prepare high-quality SnO2 crystalline layers can serve as a pre-growth template to improve the crystalline quality of sol-gel generated SnO2. Additionally, covering the hydrolysis oxidation-based SnO2 layer with sol-gel prepared SnO2 crystalline layer can improves the repeatability of device preparation. The electron transport layers prepared by this method can effectively enhance the quality of thin film crystal growth and charge extraction capability, ultimately contributing to improving the efficiency, stability, and reducing hysteresis of the devices.-
Key words:
- tin dioxide /
- composite electron transport layer /
- hydrolysis oxidation /
- sol-gel method /
- hybrid PSCs
-
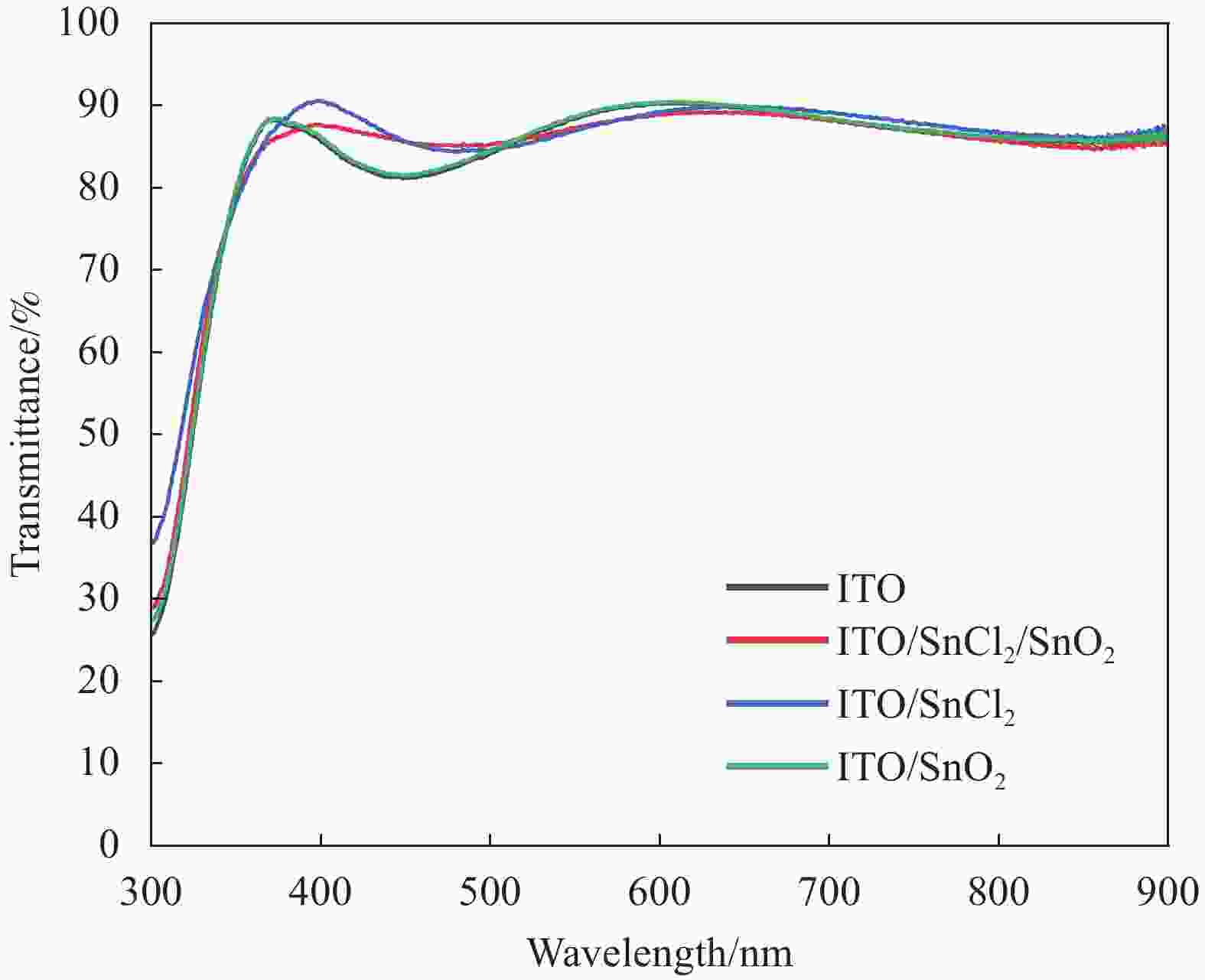
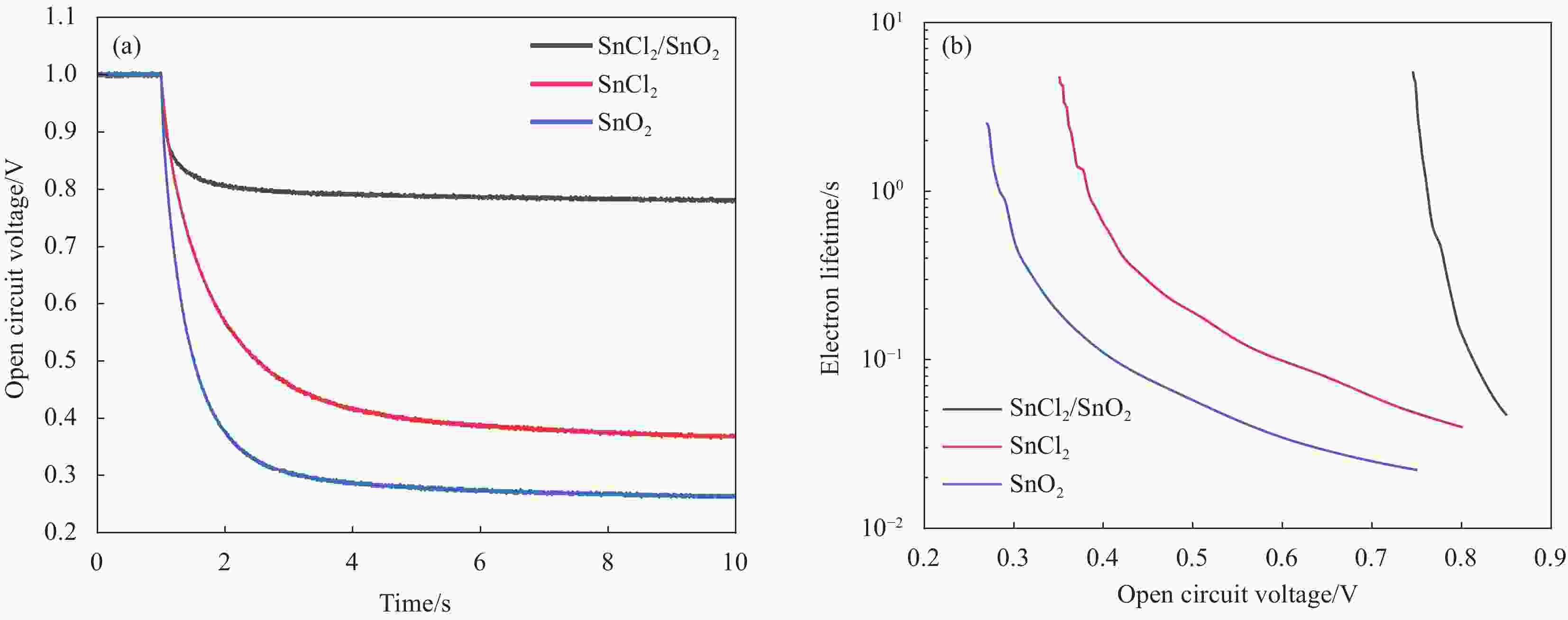
图 6 (FAPbI3)0.83(MAPbBr3)0.17在ITO/SnCl2/SnO2、ITO/SnCl2和ITO/SnO2三种衬底上制备的器件;(a)归一化开电压衰减曲线和(b)基于开电压衰减计算的电子寿命
Figure 6. (FAPbI3)0.83(MAPbBr3)0.17 devices prepared on three ITO/SnCl2/SnO2, ITO/SnCl2, and ITO/SnO2 substrates; (a) Normalized open voltage decay curves and (b) electron lifetime calculated by open voltage decay
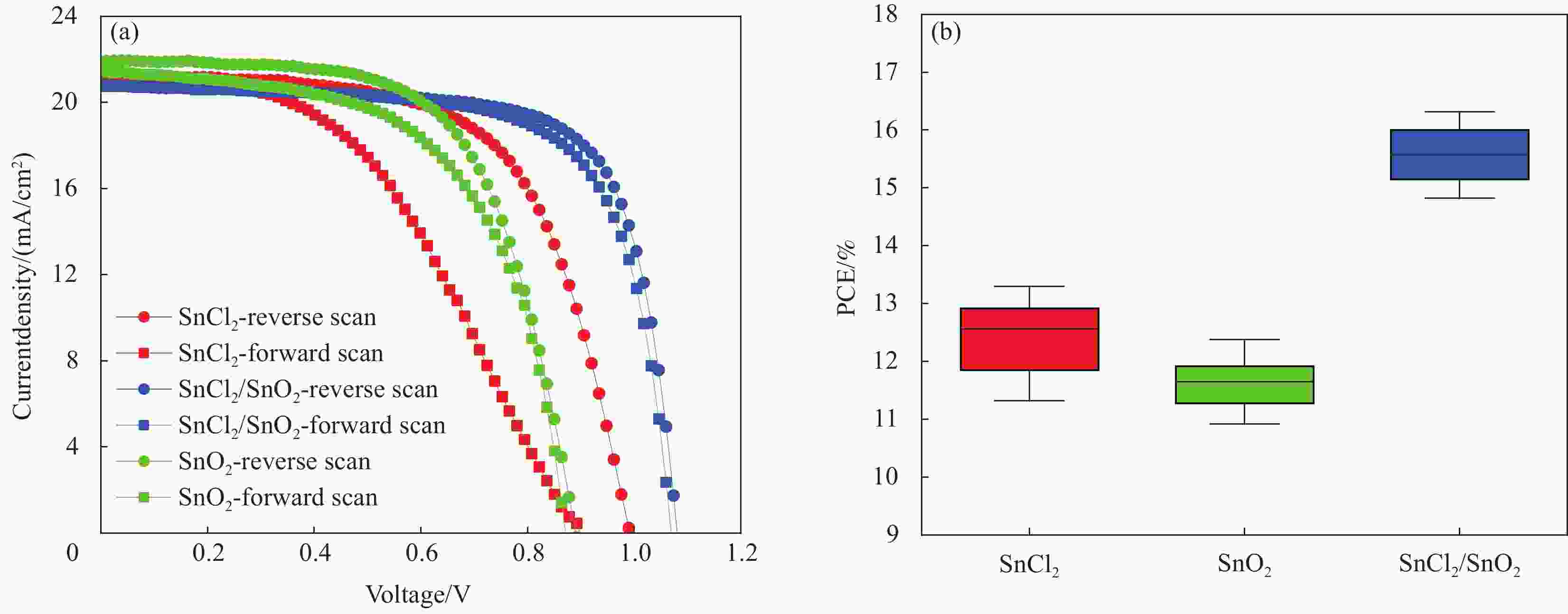
图 7 (a)三种方法制备的ETL制备的(FAPbI3)0.83(MAPbBr3)0.17太阳能电池的J-V曲线; (b)基于三种方法制备的ETL, 20个器件(FAPbI3)0.83(MAPbBr3)0.17器件的效率箱形图。
Figure 7. (a) J-V curves of (FAPbI3)0.83(MAPbBr3)0.17 solar cells fabricated with ETLs prepared by using the three methods; (b) Box plot of efficiencies of 20 devices for (FAPbI3)0.83(MAPbBr3)0.17 devices based on different ETLs.
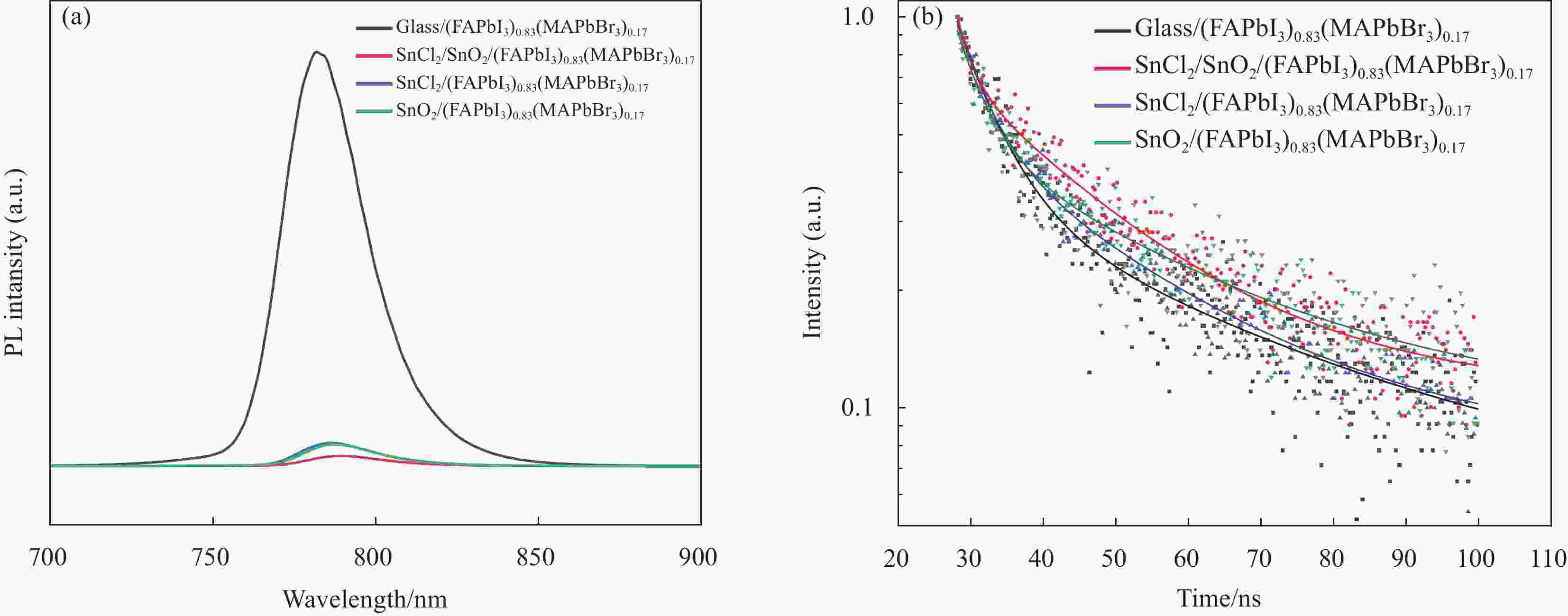
表 1 不同衬底(FAPbI3)0.83(MAPbBr3)0.17薄膜的瞬态荧光光谱拟合参数
Table 1. TRPL fitting parameters of (FAPbI3)0.83(MAPbBr3)0.17 films prepared on different substrates
Samples Glass SnCl2/SnO2 SnCl2 SnO2 τ1 Value /ns 5.52 2.09 4.32 4.04 τ1 Rel./% 68.48 35.87 55.79 58.24 τ2 Value /ns 38.16 20.63 25.56 29.91 τ2 Rel./% 31.52 64.13 44.21 41.76 τave Value /ns 30.36 19.64 21.83 25.81 Notes: τ1 Value and τ2 Value are fast decay life and slow decay life; τ1 Rel. and τ2 Rel. are the proportion of fast decay lifespan and the proportion of slow decay lifespan; τave Value is fluorescence lifetime of perovskite charge carriers 表 2 不同制备方法制备的(FAPbI3)0.83(MAPbBr3)0.17器件作为电子传输层的光电参数
Table 2. Optoelectronic parameters of (FAPbI3)0.83(MAPbBr3)0.17 devices prepared using different preparation methods as electron transport layers
ETLs Voc/V Jsc /(mA·cm−2) FF/% PCE/% SnCl2-revese 0.99 21.31 62.90 13.30 SnCl2-forward 0.90 21.25 45.95 8.78 SnO2-reverse 0.89 21.92 63.43 12.38 SnO2-forward 0.87 21.44 59.74 11.16 SnCl2/SnO2-reverse 1.08 20.81 72.56 16.32 SnCl2/SnO2-forward 1.07 20.75 70.51 15.64 Notes: Jsc is short-circuit current; FF is fill factor; PCE is the photoelectric conversion efficiency of perovskite solar cells. -
[1] MARTIN A. Green , ANITA Ho-Baillie , Henry J Snaith , The Emergence of Perovskite Solar Cells[J]. Nature Photonics, 2014, 8 (7): 506-514. [2] ABDULAZIZ S. R. Bati, ZHONG Yu Lin, PAUL L. Burn , et al. Next-Generation Applications for Integrated Perovskite Solar Cells[J]. Communications Materials, 2023, 4 (1): 2. [3] SHI Jiangjian, XU Xin, LI Dongmei, et al. Interfaces in Perovskite Solar Cells[J]. Small, 2015, 11(21): 2472-2486. doi: 10.1002/smll.201403534 [4] SEO Jangwon, NOH Jun Hong, SEOK Sang Il, Rational Strategies for Efficient Perovskite Solar Cells[J]. Accounts of chemical research, 2016, 49 (3): 562-572. [5] CHENG Ming, ZUO Chuantian, WU Yongzhen, et al. Charge-Transport Layer Engineering in Perovskite Solar Cells[J]. Sci Bull, 2020, 65(15): 1237-1241. doi: 10.1016/j.scib.2020.04.021 [6] MOHAMAD Noh Mohamad Firdaus, HOONG Teh Chin, RUSLI Daik , et al. Teridi Mohd Asri Mat, The Architecture of the Electron Transport Layer for a Perovskite Solar Cell[J]. JOURNAL OF MATERIALS CHEMISTRY C, 2018, 6 (4): 682-712. [7] SHINI Foo , MARIYAPPAN Thambidurai, PONNUSAMY Senthil Kumar , et al. Recent Review on Electron Transport Layers in Perovskite Solar Cells[J]. International Journal of Energy Research, 2022, 46 (15): 21441-21451. [8] LI Fumin, SHEN Zhitao, WENG Yujuan, et al. Novel Electron Transport Layer Material for Perovskite Solar Cells with over 22% Efficiency and Long-Term Stability[J]. ADVANCED FUNCTIONAL MATERIALS, 2020, 30(45): 2004933. doi: 10.1002/adfm.202004933 [9] JAE Paik Min , WOOK Yoo Jin , JAEWANG Park , et al. SnO2–TiO2 Hybrid Electron Transport Layer for Efficient and Flexible Perovskite Solar Cells[J]. ACS Energy Letters, 2022, 7 (5): 1864-1870. [10] WANG Yongling, WAN Jiawei, DING Jie, et al. A Rutile TiO2 Electron Transport Layer for the Enhancement of Charge Collection for Efficient Perovskite Solar Cells[J]. Angewandte Chemie International Edition, 2019, 58(28): 9414-9418. doi: 10.1002/anie.201902984 [11] JIANG Qi, ZHANG Xingwang, YOU Jingbi, SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells[J]. Small, 2018, 14 (31): 1801154. [12] HUANG Xiangping, DU Jianhui, GUO Xing, et al. Polyelectrolyte-Doped SnO2 as a Tunable Electron Transport Layer for High-Efficiency and Stable Perovskite Solar Cells[J]. Solar RRL, 2020, 4 (1): 1900336. [13] MOHAMAD Noh Mohamad Firdaus , AFFIQAH Arzaee Nurul , JAVAD Safaei , et al. Eliminating Oxygen Vacancies in SnO2 Films Via Aerosol-Assisted Chemical Vapour Deposition for Perovskite Solar Cells and Photoelectrochemical Cells[J]. Journal of Alloys and Compounds, 2019, 773: 997-1008. [14] KUANG Yinghuan, VALERIO Zardetto , RODERIKE van Gils , et al Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells[J]. ACS APPLIED MATERIALS & INTERFACES, 2018, 10 (36): 30367-30378. [15] JIANG Xixi, XIONG Yuli, ZHANG Zhihui, et al. Efficient Hole-Conductor-Free Printable Mesoscopic Perovskite Solar Cells Based on Sno2 Compact Layer[J]. Electrochimica Acta, 2018, 263: 134-139. [16] DONG Qingshun, SHI Yantao, ZHANG Chunyang, et al. Energetically Favored Formation of SnO2 Nanocrystals as Electron Transfer Layer in Perovskite Solar Cells with High Efficiency Exceeding 19%[J]. Nano Energy, 2017, 40: 336-344. doi: 10.1016/j.nanoen.2017.08.041 [17] DONG Qingshun, SHI Yantao, WANG Kai, et al. Insight into Perovskite Solar Cells Based on Sno2 Compact Electron-Selective Layer[J]. The Journal of Physical Chemistry C, 2015, 119(19): 10212-10217. doi: 10.1021/acs.jpcc.5b00541 [18] MARYAM Haghighi , NAHID Ghazyani , SABA Mahmoodpour , et al. Low-Temperature Processing Methods for Tin Oxide as Electron Transporting Layer in Scalable Perovskite Solar Cells[J]. Solar RRL, 2023: 2201080. [19] LI Jing, BU Tongle, LIU Yifan, et al. Enhanced Crystallinity of Low-Temperature Solution-Processed SnO2 for Highly Reproducible Planar Perovskite Solar Cells[J]. ChemSusChem, 2018, 11(17): 2898-2903. doi: 10.1002/cssc.201801433 [20] KE Weijun, ZHAO Dewei, ALEXANDER J. Cimaroli , et al. Effects of Annealing Temperature of Tin Oxide Electron Selective Layers on the Performance of Perovskite Solar Cells[J]. Journal of Materials Chemistry A, 2015, 3 (47): 24163-24168. [21] KE Weijun, FANG Guojia, LIU Qin, et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells[J]. Journal of the American Chemical Society, 2015, 137(21): 6730-6733. doi: 10.1021/jacs.5b01994 [22] LIU Aqiang, LIU Kang, ZHOU Huimin, et al. Solution Evaporation Processed High Quality Perovskite Films[J]. Science Bulletin, 2018, 63 (23): 1591-1596. [23] LARRY Partain , Solar Cell Device Physics. In Solar Cells and Their Applications, John Wiley & Sons, Inc: 2010; pp 67-109. [24] WANG Minhuan, ZHAO Yepin, JIANG Xiaoqing, et al. Rational Selection of the Polymeric Structure for Interface Engineering of Perovskite Solar Cells[J]. Joule, 2022, 6(5): 1032-1048. doi: 10.1016/j.joule.2022.04.002 [25] TABU Mbumba Manala , MAURICE Malouangou Davy , MATONDO Tsiba Jadel , et al. Compositional Engineering Solutions for Decreasing Trap State Density and Improving Thermal Stability in Perovskite Solar Cells[J]. JOURNAL OF MATERIALS CHEMISTRY C, 2021, 9 (40): 14047-14064. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 63
- HTML全文浏览量: 60
- 被引次数: 0





 下载:
下载: