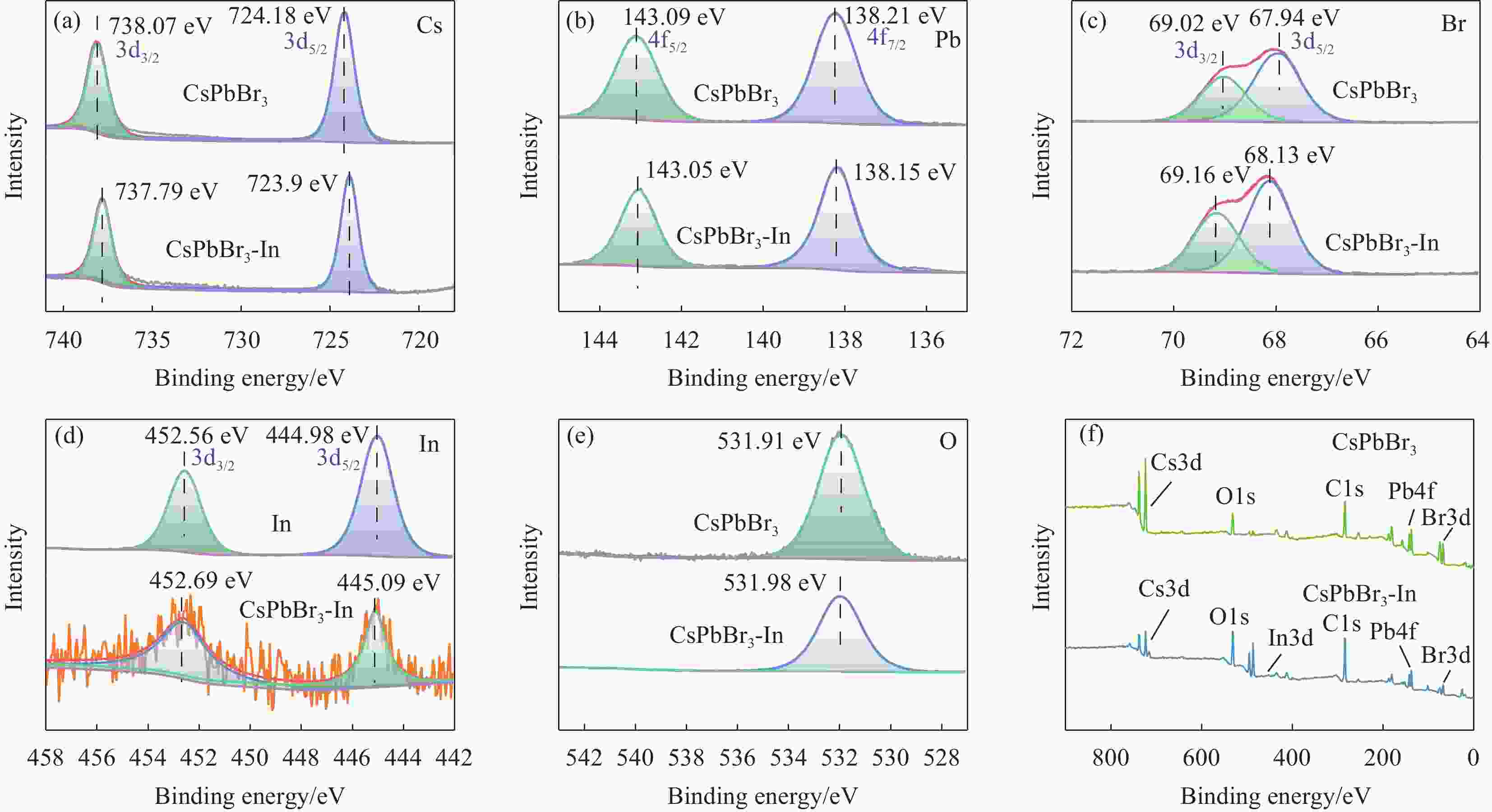
CsPbBr3 quantum dots passivated by acetylacetone indium and their room-temperature methanol gas sensitivity
-
摘要: 甲醇气体对人体具有毒性,会损害人的神经系统和血液循环系统,开发能够检测甲醇的器件有十分重要的意义。传感器法检测甲醇具有成本低、灵敏度高且可实时监测的优点,但目前主流的甲醇气体传感器主要以金属氧化物为主,存在工作温度高的缺点。本文通过简单的溶液合成法合成了钙钛矿量子点CsPbBr3,并采用乙酰丙酮铟配体(In(Acac)3)来对其表面缺陷进行钝化,获得了室温对甲醇具有较好气敏性的材料。在室温下对体积分数为80×10–6甲醇气体相应灵敏度为0.25,响应恢复时间为11.0 s/17.0 s,在紫外光照射下传感器的气体响应性能进一步提高。传感器具有良好的可再现性和稳定性,传感器在体积分数为80×10–6的甲醇浓度下进行多次测试灵敏度均保持在0.25左右,且在15天内均维持在较高水平。同时,在高湿度、无光照等恶劣条件下依然对甲醇有较好的响应。考虑到金属卤化钙钛矿结构可以很容易通过改变元素来调节性质,该研究方法和实验过程可应用到对其他气体的检测。Abstract: Methanol gas is toxic gas that can harm the human nerve system and blood circulation system. Developing devices capable of detecting methanol gas is of great significance. The use of sensors to detect methanol gas has the advantages of low cost, high sensitivity, and real-time monitoring. However, the mainstream methanol gas sensors mainly rely on metal oxides, which have the drawback of high operating temperatures. Therefore, we synthesized perovskite quantum dots CsPbBr3 through a simple solution synthesis method, and passivated their surface defects using an acetylacetone indium ligand (In(Acac)3), obtaining a material with good gas sensitivity to methanol gas at room temperature. The sensitivity to a volume fraction of 80×10–6 methanol gas at room temperature is 0.25, and the response/recovery time is 11.0 s/17.0 s, gas sensitivity is further improved under ultraviolet light irradiation. It also has good reproducibility and stability, the sensitivity of the sensor has been maintained at around 0.25 after multiple tests at a volume fraction of 80×10–6 methanol gas, and the sensor sensitivity has remained at a high level for 15 days. At the same time, it still has a good response to methanol gas under harsh conditions such as high humidity and no light. Considering that the structure of metal halide perovskite can easily adjust its properties by changing elements, this research method and experimental process can be applied to the detection of other gases.
-
Key words:
- perovskite /
- gas sensor /
- methanol /
- metal organic compounds /
- coordination modification
-
图 2 (a) CsPbBr3-In晶体结构和传感器组成图;(b) CsPbBr3-In量子点、CsPbBr3量子点和标准CsPbBr3卡片XRD图谱;((c), (d)) CsPbBr3-In薄膜的SEM、EDS图像;((e), (f)) CsPbBr3-In量子点的TEM、HRTEM图像
Figure 2. (a) CsPbBr3-In crystal structure and sensor composition diagram; (b) CsPbBr3-In quantum dots, CsPbBr3 quantum dots, and standard CsPbBr3 card XRD patterns; ((c), (d)) SEM and EDS images of CsPbBr3-In thin films; ((e), (f)) TEM and HRTEM images of CsPbBr3-In quantum dots
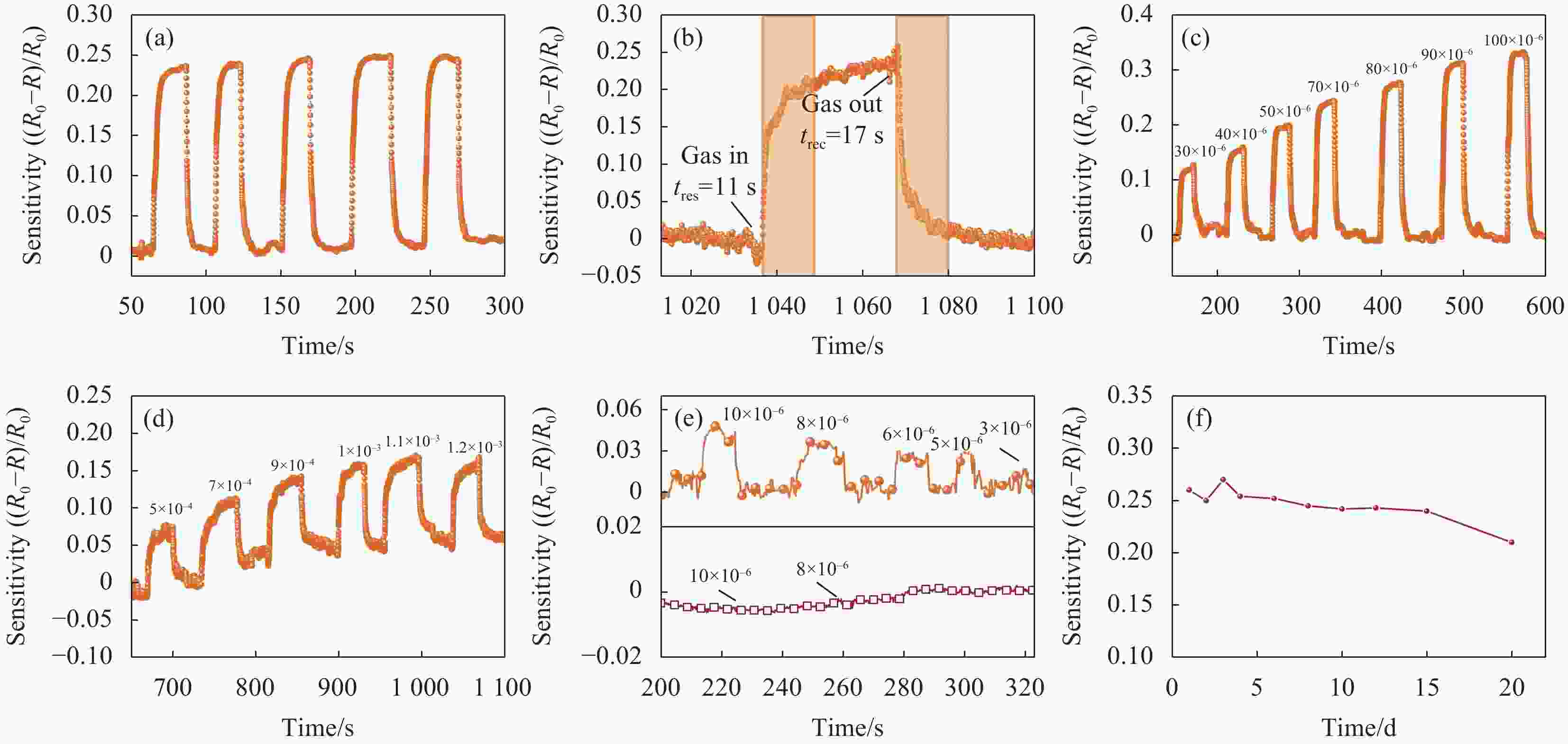
图 5 CsPbBr3-In在室温下的传感性能:(a) 甲醇体积分数为80×10–6下传感器多次响应曲线;(b) 甲醇体积分数为80×10–6下甲醇的响应恢复时间;(c) CsPbBr3-In传感器对不同浓度甲醇的灵敏度;(d) CsPbBr3传感器对不同浓度甲醇的灵敏度;(e) CsPbBr3-In、CsPbBr3传感器对低浓度甲醇气体的响应曲线;(f) CsPbBr3-In传感器在空气中的稳定性
Figure 5. Sensing performance of CsPbBr3-In at room temperature: (a) Multiple response curve of the sensor at a volume fraction of 80×10–6 methanol gas; (b) Response recovery time of methanol at a volume fraction of 80×10–6; (c) Sensitivity of CsPbBr3-In sensor to different concentrations of methanol; (d) Sensitivity of CsPbBr3 sensor to different concentrations of methanol; (e) Response curve of CsPbBr3-In, CsPbBr3 sensor to low concentrations of methanol gas; (f) Stability of CsPbBr3-In sensor in air
tres—Response time; trec—Recovery time; R0—Starting resistance; R—Real time resistance
图 6 CsPbBr3-In传感器在不同光照条件下的响应(气体浓度:体积分数80×10–6):(a) 传感器在黑暗条件下对甲醇气体的响应;(b) 传感器在自然光照射下对甲醇气体的响应;(c) 传感器在紫外光照射下对甲醇气体的响应;(d) 传感器在自然光+紫外灯对甲醇气体的响应
Figure 6. Response of CsPbBr3-In sensor under different light conditions (Gas concentration: 80×10–6): (a) Response of sensor to methanol gas under dark conditions; (b) Response of sensor to methanol gas under natural light irradiation; (c) Response of sensor to methanol gas under ultraviolet light irradiation; (d) Response of sensor under natural light+ultraviolet lamp
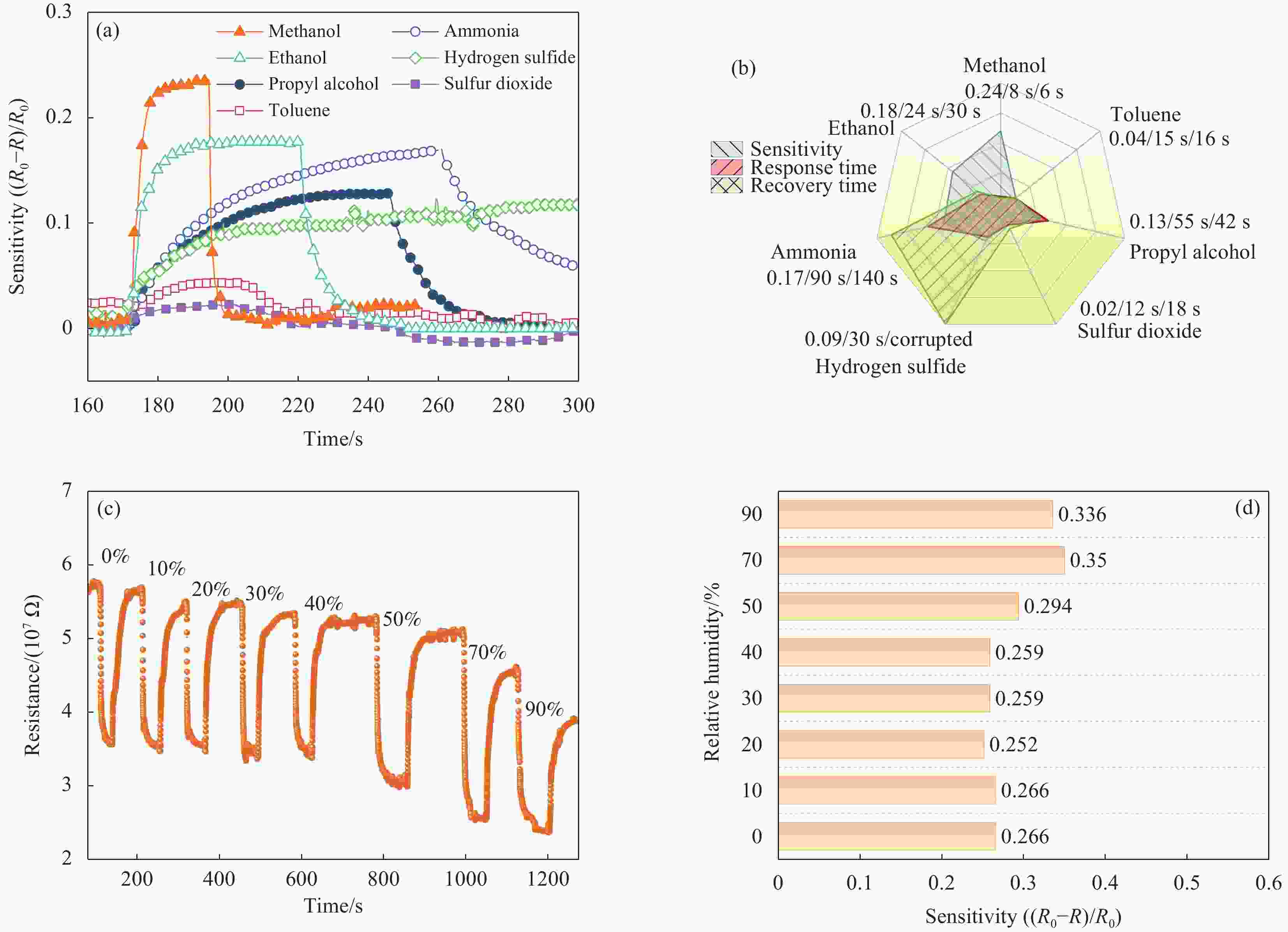
图 7 传感器对对不同气体和不同湿度环境下的响应情况:((a), (b)) 传感器对体积分数为80×10–6不同气体的灵敏度、响应恢复时间测试;((c), (d)) 传感器在不同湿度条件下对体积分数为80×10–6甲醇气体的响应
Figure 7. Sensor response to different gases and humidity environments: ((a), (b)) Sensor sensitivity and response/recovery time test for different gases with a volume fraction of 80×10–6; ((c), (d)) Response of the sensor to a volume fraction of 80×10–6 methanol gas under different humidity conditions
表 1 用于甲醇检测的各种纳米结构金属氧化物的比较
Table 1. Comparison of various nanostructured metal oxides for methanol detection
Material Temperature/℃ Concentration Sensitivity (R0/R) tres/trec/s Ref. CsPbBr3-In RT 80×10–6 1.33 11/17 This work SnO2 200 100×10–6 58 4/8 [33] CoFe2O4 90 100×10–6 1.42 430/252 [34] Zn2SnO4 450 50×10–6 2.25 39/138 [35] MoS2/TiO2 240 200×10–6 1.8 80/100 [36] In/NiO 300 200×10–6 10.9 273/26 [37] Note: RT—Room temperature. -
[1] LEVY P, HEXDALL A, GORDON P, et al. Methanol contamination of Romanian home-distilled alcohol[J]. Journal of Toxicology: Clinical Toxicology, 2003, 41(1): 23-28. doi: 10.1081/CLT-120018267 [2] 陈金合, 刘小晖. 甲醇汽油毒性研究[J]. 安全与健康, 2016(1): 43-45.CHEN Jinhe, LIU Xiaohui. Study on the toxicity of methanol gasoline[J]. Safety and Health, 2016(1): 43-45(in Chinese). [3] 冉茂霞, 李莹, 林佳如, 等. 急性甲醇中毒临床特征分析[J]. 中国医药, 2019, 14(9): 1361-1365. doi: 10.3760/j.issn.1673-4777.2019.09.020RAN Maoxia, LI Ying, LIN Jiaru, et al. Clinical features of acute methanol poisoning[J]. China Medicine, 2019, 14(9): 1361-1365(in Chinese). doi: 10.3760/j.issn.1673-4777.2019.09.020 [4] 陈捷敏. 甲醇中毒视网膜功能结构和蛋白质组学研究[D]. 上海: 复旦大学, 2012.CHEN Jiemin. Retina functional and morphologic alteration and proteomic analysis after methanol intoxication[D]. Shanghai: Fudan University, 2012(in Chinese). [5] KRUSE J A. Methanol poisoning[J]. Intensive Care Medicine, 1992, 18(7): 391-397. doi: 10.1007/BF01694340 [6] 王泽君, 王梓昂, 郭峰. 纯露中甲醇经口经皮吸入路径下含量合理阈值[J]. 山东化工, 2022, 51(7): 133-135. doi: 10.19319/j.cnki.issn.1008-021x.2022.07.055WANG Zejun, WANG Ziang, GUO Feng. Deriving the threshold of methanol in water floral[J]. Shandong Chemical Industry, 2022, 51(7): 133-135(in Chinese). doi: 10.19319/j.cnki.issn.1008-021x.2022.07.055 [7] JONES A W, MÅRDH G, ÄNGGÅRD E. Determination of endogenous ethanol in blood and breath by gas chromatography-mass spectrometry[J]. Pharmacology Biochemistry and Behavior, 1983, 18: 267-272. doi: 10.1016/0091-3057(83)90184-3 [8] LAAKSO O, HAAPALA M, JAAKKOLA P, et al. FT-IR breath test in the diagnosis and control of treatment of methanol intoxications[J]. Journal of Analytical Toxicology, 2001, 25: 26-30. doi: 10.1093/jat/25.1.26 [9] GÜNTNER A T, PINEAU N J, MOCHALSKI P, et al. Sniffing entrapped humans with sensor arrays[J]. Jounal of Analytical Chemistry, 2018, 90(8): 4940-4945. doi: 10.1021/acs.analchem.8b00237 [10] GHOSH R, GARDNER J W, GUHA P K. Air pollution monitoring using near room temperature resistive gas sensors: A review[J]. IEEE Transactions on Electron Devices, 2019, 66(8): 3254-3264. [11] MARKIEWICZ N, CASALS O, FABREGA C, et al. Micro light plates for low-power photoactivated (gas) sensors[J]. Applied Physics Letters, 2019, 114(5): 053508. doi: 10.1063/1.5078497 [12] AL-ASHOURI A, KÖHNEN E, LI B, et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction[J]. Science, 2020, 370(6522): 1300-1309. [13] LU H Z, LIU Y H, AHLAWAT P, et al. Vapor-assisted deposition of highly efficient, stable black-phase FAPbI3 perovskite solar cells[J]. Science, 2020, 370(6512): eabb8985. [14] XUAN W F, CHEN Y Y, HU D A, et al. Smart mask based on lead-free perovskite humidity sensor for labor intensity grading by breath monitoring[J]. Sensors and Actuators B: Chemical, 2023, 397: 134622. [15] YE W, CAO Q C, CHENG X F, et al. A lead-free Cs2PdBr6 perovskite-based humidity sensor for artificial fruit waxing detection[J]. Journal of Materials Chemistry A, 2020, 8(34): 17675-17682. doi: 10.1039/D0TA05193D [16] SHAN H S, XUAN W F, LI Z, et al. Room-temperature hydrogen sulfide sensor based on tributyltin oxide functionalized perovskite CsPbBr3 quantum dots[J]. ACS Applied Nano Materials, 2022, 5(5): 6801-6809. doi: 10.1021/acsanm.2c00791 [17] SIEGLER T D, DUNLAP-SHOHL W A, MENG Y H, et al. Water-accelerated photooxidation of CH3NH3PbI3 perovskite[J]. Journal of the American Chemical Society, 2022, 144(12): 5552-5561. [18] BI E B, CHEN H, XIE F X, et al. Diffusion engineering of ions and charge carriers for stable efficient perovskite solar cells[J]. Nature Communications, 2017, 8: 15330. doi: 10.1038/ncomms15330 [19] WANG C C, GAO Y L. Stability of perovskites at the surface analytic level[J]. Journal of Physical Chemistry Letters, 2018, 9(16): 4657-4666. doi: 10.1021/acs.jpclett.8b00381 [20] GARCÍA DE ARQUER F P, ARMIN A, MEREDITH P, et al. Solution-processed semiconductors for next-generation photodetectors[J]. Nature Reviews Materials, 2017, 2(3): 16100. doi: 10.1038/natrevmats.2016.100 [21] CHEN X, HU H W, XIA Z M, et al. CsPbBr3 perovskite nanocrystals as highly selective and sensitive spectrochemical probes for gaseous HCl detection[J]. Journal of Materials Chemistry C, 2017, 5(2): 309-313. doi: 10.1039/C6TC04136A [22] 李玥, 谢启飞, 王新中, 等. 提纯溶剂对室温合成CsPbBr3量子点性能的影响[J]. 光子学报, 2019, 48(10): 85-92.LI Yue, XIE Qifei, WANG Xinzhong, et al. Effect of purified solvents on the performance of quantum dots synthesized at room temperature[J]. Acta Photonica Sinica, 2019, 48(10): 85-92(in Chinese). [23] ZHU L, XU W J, XUAN W F, et al. Perovskite CsPbBr3 quantum dots capped with zinc acetylacetonate: Gas sensing of ethanol in humidity with aid of machine-learning[J]. Materials Science in Semiconductor Processing, 2023, 167: 107790. [24] 夏冬林, 付陈承. Ce3+掺杂CsPbBr3纳米晶的制备与性能[J]. 人工晶体学报, 2021, 50(12): 2246-2254.XIA Donglin, FU Chencheng. Preparation and properties of Ce3+ doped CsPbBr3 nanocrystals[J]. Journal of Synthetic Crystals, 2021, 50(12): 2246-2254(in Chinese). [25] HOAT P D, YUN Y, PARK B, et al. Synthesis of Cs2TeI6 thin film and its NO2 gas-sensing properties under blue-light illumination[J]. Scripta Materialia, 2022, 207: 114305. doi: 10.1016/j.scriptamat.2021.114305 [26] DIMESSO L, DAS C, STӦHR M, et al. Properties of cesium tin iodide (Cs-Sn-I) systems after annealing under different atmospheres[J]. Materials Chemistry & Physics, 2017, 197: 27-35. [27] HUANG W X, MANSER J S, SADHU S, et al. Direct observation of reversible transformation of CH3NH3PbI3 and NH4PbI3 induced by polar gaseous molecules[J]. Journal of Physical Chemistry Letters, 2016, 7(24): 5068-5073. [28] QIN M C, CAO J, ZHANG T K, et al. Fused-ring electron acceptor ITIC-Th: A novel stabilizer for halide perovskite precursor solution[J]. Advanced Energy Materials, 2018, 8(18): 1703399. doi: 10.1002/aenm.201703399 [29] SONG J Z, FANG T, LI J H, et al. Organic-inorganic hybrid passivation enables perovskite QLEDs with an EQE of 16.48[J]. Advanced Materials, 2018, 30(50): 1805409. doi: 10.1002/adma.201805409 [30] SONG J Z, LI J H, XU L M, et al. Room-temperature triple-ligand surface engineering synergistically boosts ink stability, recombination dynamics, and charge injection toward EQE-11.6% perovskite QLEDs[J]. Advanced Materials, 2018, 30(30): 1800764. doi: 10.1002/adma.201800764 [31] GONG B, SHI T L, ZHU W, et al. UV irradiation-assisted ethanol detection operated by the gas sensor based on ZnO nanowires/optical fiber hybrid structure[J]. Sensors and Actuators B: Chemical, 2017, 245: 821-827. [32] DA SILVAL F, M'PEKO J C, CATTO A C, et al. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature[J]. Sensors and Actuators B: Chemical, 2017, 240: 573-579. [33] SONG L M, LUKIANOV A,BUTENKO D, et al. Facile synthesis of hierarchical tin oxide nanoflowers with ultra-high methanol gas sensing at low working temperature[J]. Nanoscale Research Letters, 2019, 14(1): 84. [34] HALVAEE P, DEHGHANI S, HOGHOGHIFARD S. Low temperature methanol sensors based on cobalt ferrite nanoparticles, nanorods, and porous nanoparticles[J]. IEEE Sensors Journal, 2020, 20(8): 4056-4062. [35] HANH N H, NGOC T M, VAN DUY L, et al. A comparative study on the VOCs gas sensing properties of Zn2SnO4 nanoparticles, hollow cubes, and hollow octahedra towards exhaled breath analysis[J]. Sensors and Actuators B: Chemical, 2021, 343: 130147. [36] SINGH S, SHARMA S. Temperature dependent selective detection of ethanol and methanol using MoS2/TiO2 composite[J]. Sensors and Actuators B: Chemical, 2022, 350: 130798. doi: 10.1016/j.snb.2021.130798 [37] FENG C H, KOU X Y, CHEN B, et al. One-pot synthesis of in doped NiO nanofibers and their gas sensing properties[J]. Sensors and Actuators B: Chemical, 2017, 253: 584-591. doi: 10.1016/j.snb.2017.06.115 -






 下载:
下载: