Structural and property modulation of cyclohexanedimethanol-based polycarbonates by bio-based tetrahydrofuran dimethanol with cyclic ether structures
-
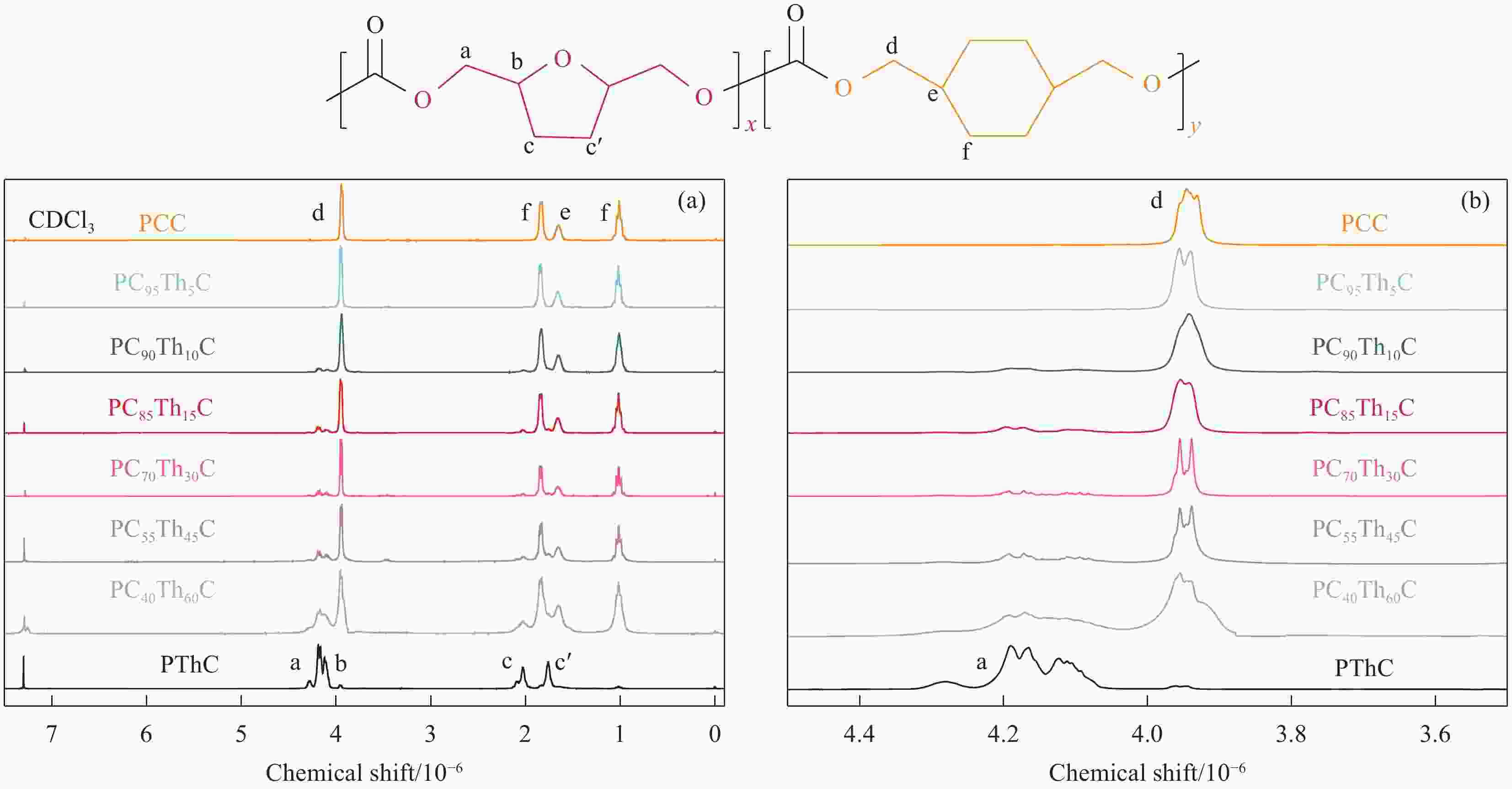
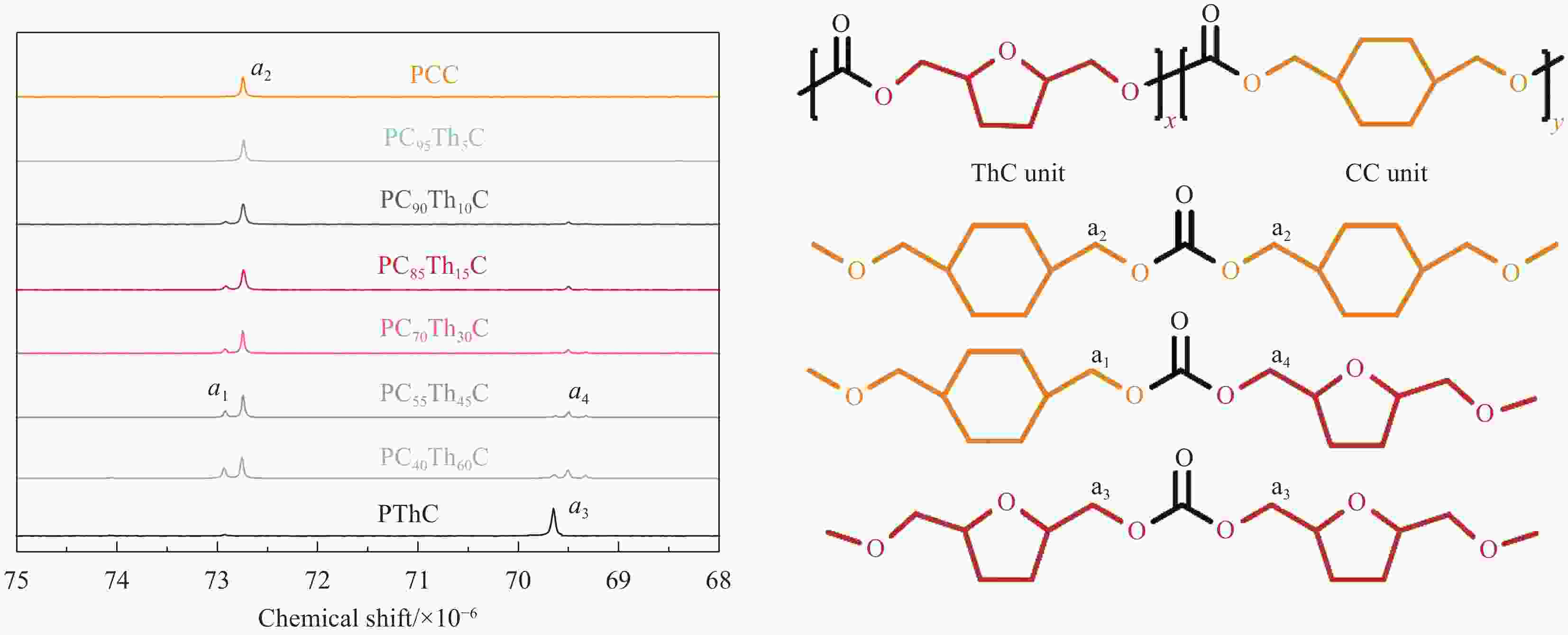
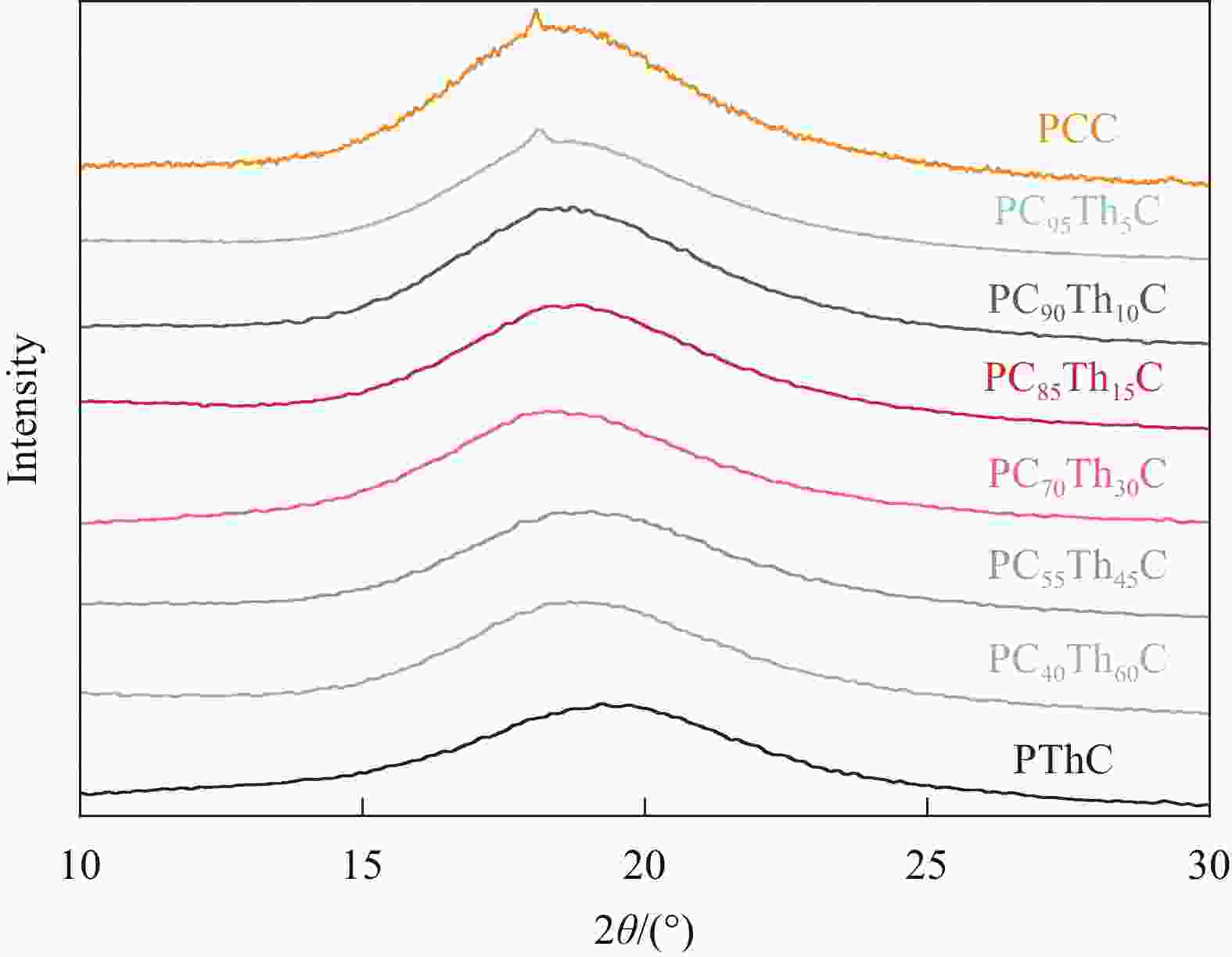
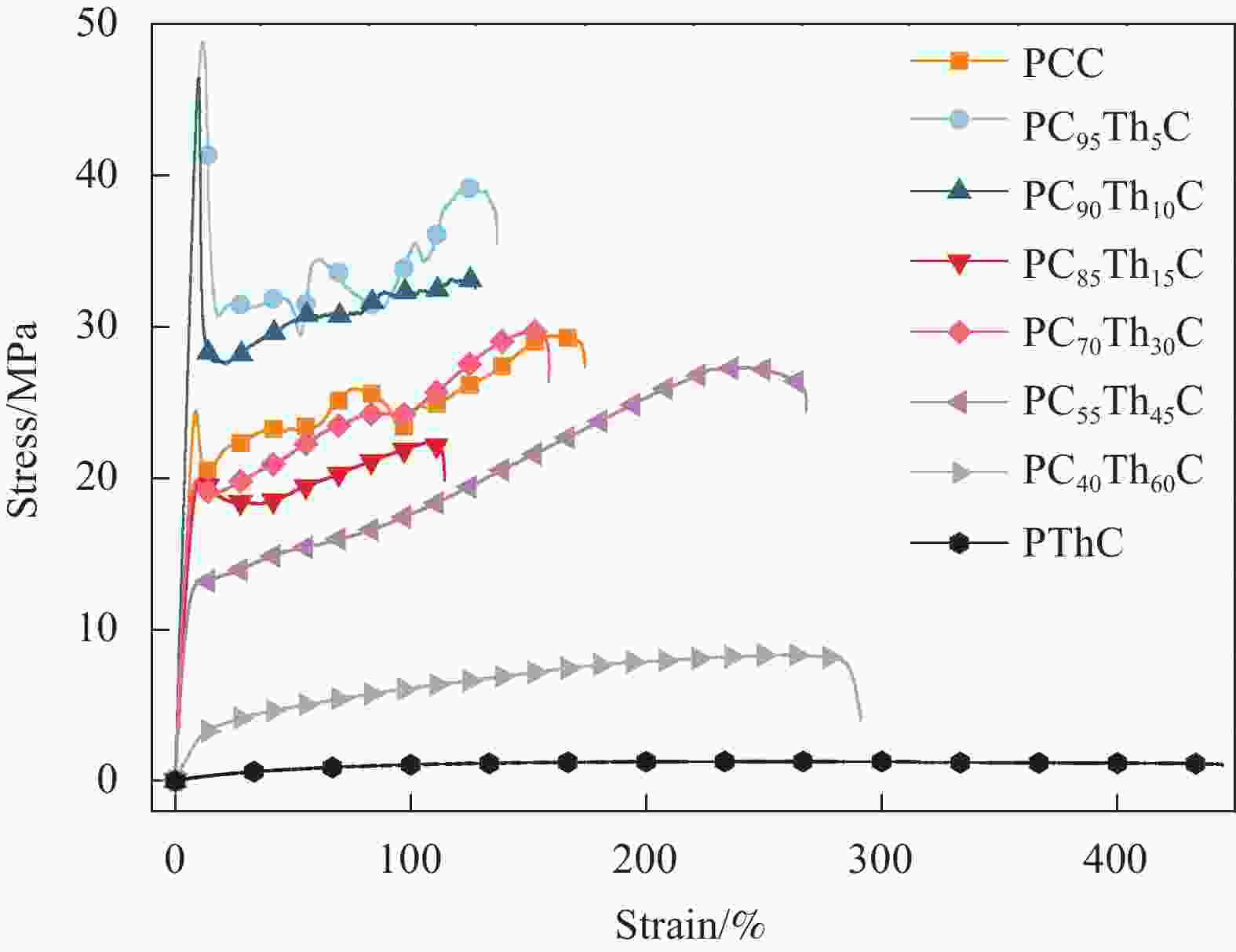
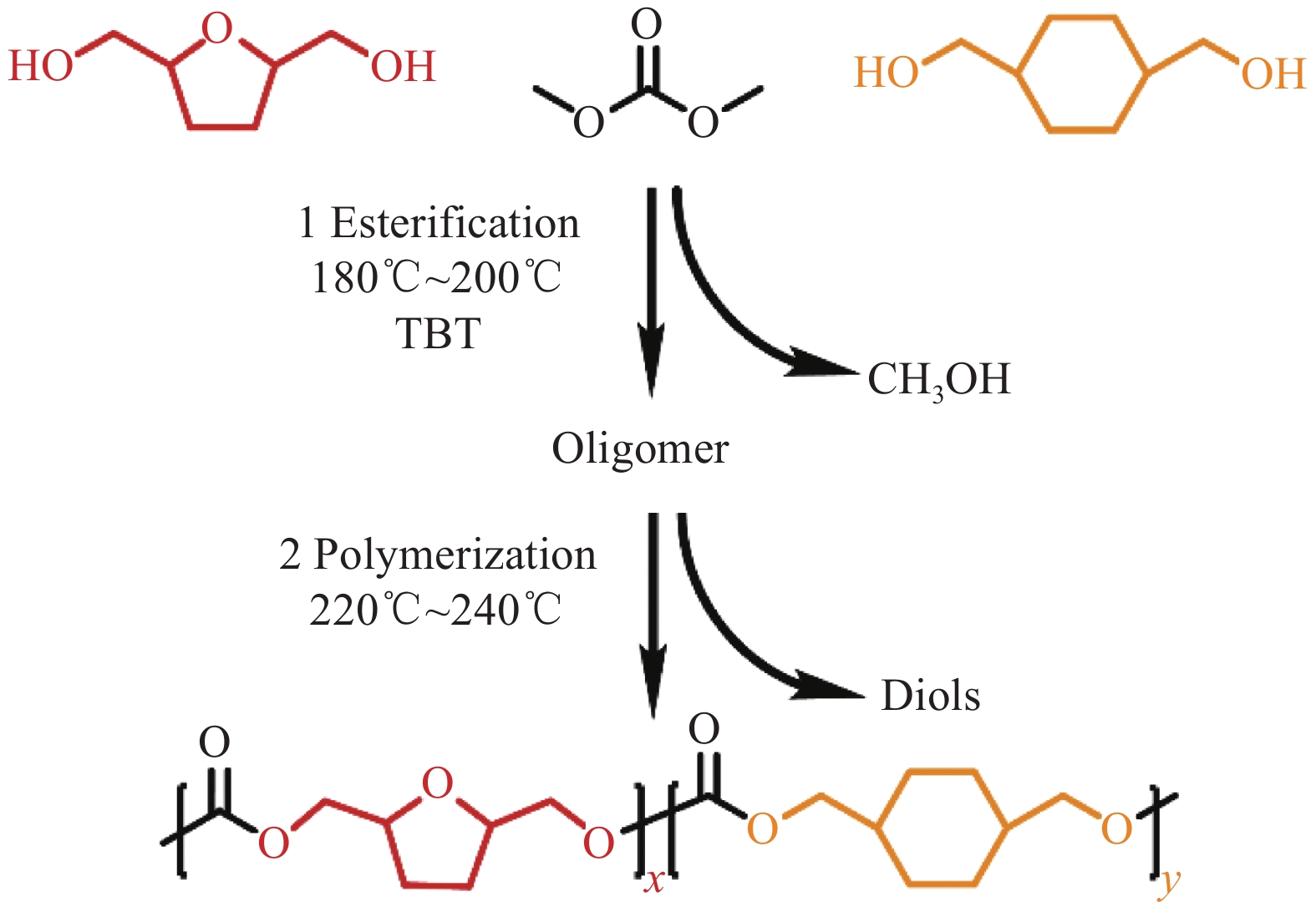
摘要: 1,4-环己烷二甲醇基聚碳酸酯具有优异的热力学性能,但因其降解性能的影响,商业应用受到极大的限制。向其分子链中引入脂肪族单元可以有效提高降解能力,但却以牺牲其热力学性能为代价。因此,为了维持热力学与降解性能间的平衡,本文以具有环醚刚性的生物基2,5-四氢呋喃二甲醇(THFDM)为改性单元,采用熔融酯交换缩聚法成功制备了高分子量聚(碳酸1,4-环己烷二甲醇酯-co-碳酸2,5-四氢呋喃二甲醇酯)(PCThC)共聚物。通过NMR探究共聚物的组成和微观结构,证实了共聚物组分间的无规分布;DSC和WAXD结果表明,THFDM单元的引入破坏了PCThC分子链的规整排列,使其结晶能力降低,共聚物从半结晶型过渡到无定型。THFDM中的刚性环结构有效阻止了共聚物Tg的快速下降,保持其较好的热稳定性;THFDM分子中的环结构赋予了聚合物更高的刚度,提高了聚合物的力学性能;此外,THFDM中的醚键改善了PCThC共聚物的亲水性,加快了水解速率,不论是在酸性或碱性缓冲溶液中,共聚物均表现出一定的降解能力。Abstract: 1,4-Cyclohexanedimethanol-based polycarbonates have desired thermodynamic property yet poor degradation capability. Introducing aliphatic units into the molecular chains could effectively improve the degradability at the severe expense of thermodynamic properties. For this reason, biobased 2,5-tetrahydrofurandimethanol (THFDM) with cyclic ether rigidity was selected as the modification unit and Poly(1,4-cyclohexyldimethylene-co-2,5-tetrahydrofurandimethanol carbonate) (PCThC) copolymers with higher molecular weights were synthesized via melt ester exchange polycondensation method. The composition and microstructure were investigated by NMR, which confirms the random distribution among the copolymer components. DSC and WAXD results revealed that the introduction of THFDM units break the regular arrangement of the molecular chains in PCThCs, reduce the crystallisation ability, which promote the transition from the semi-crystalline to amorphous. Furthermore, the rigid ring structure in THFDM prevents the rapid declination of Tg and keeps the good thermal stability. The ring structure in the THFDM endow higher stiffness and better mechanical performance for the polymer. The ether bonds in THFDM enhance the hydrophilicity of the PCThC copolymers, resulting in a gradual acceleration of the hydrolysis rate. The copolymers exhibit pleasant degradation ability either under acidic or alkaline environment.
-
表 1 PCC、PThC和PCThC共聚物的分子量和特性粘度
Table 1. Molecular weights and characteristic viscosities of PCC, PThC, and PCThC copolymers
Sample Mn/(g·mol−1) Mw/(g·mol−1) Ð [η]/(dL·g−1) PCC 33500 50000 1.49 0.94 PC95Th5C 42700 65400 1.53 1.10 PC90Th10C 50500 76100 1.51 1.49 PC85Th15C 47600 72400 1.52 1.30 PC70Th30C 41500 68500 1.65 1.19 PC55Th45C 41300 60200 1.46 1.03 PC40Th60C 40500 64300 1.59 1.09 PThC 7300 10700 1.46 0.64 Notes: Mn and Mw are the number average molecular weight and weight average molecular weight of the polymer; Ð is the molecular weight distribution; [η] is the characteristic viscosity. 表 2 PCC、PThC和PCThC共聚物的组成
Table 2. Composition of PCC, PThC, and PCThC copolymers
Sample ThC unitin feedsa ThC unit in polmersb LnCC LnThC R PCC 0 0 — — — PC95Th5C 0.05 0.03 27.70 1.05 0.99 PC90Th10C 0.10 0.09 8.36 1.07 1.05 PC85Th15C 0.15 0.13 5.91 1.14 1.05 PC70Th30C 0.30 0.22 5.16 1.22 1.01 PC55Th45C 0.45 0.33 3.97 1.28 1.03 PC40Th60C 0.60 0.52 2.71 1.42 1.07 PThC 1 1 — — — Notes: aMolar fraction of THFDM in the diol (CHDM + THFDM) feed. bMolar fraction of ThC units in PCThC copolymers calculated by 1H-NMR spectroscopy. LnCC and LnThC are the average sequence lengths of CC and ThC units. R is the randomness. 表 3 PCC、PThC和PCThC共聚物的热性能参数
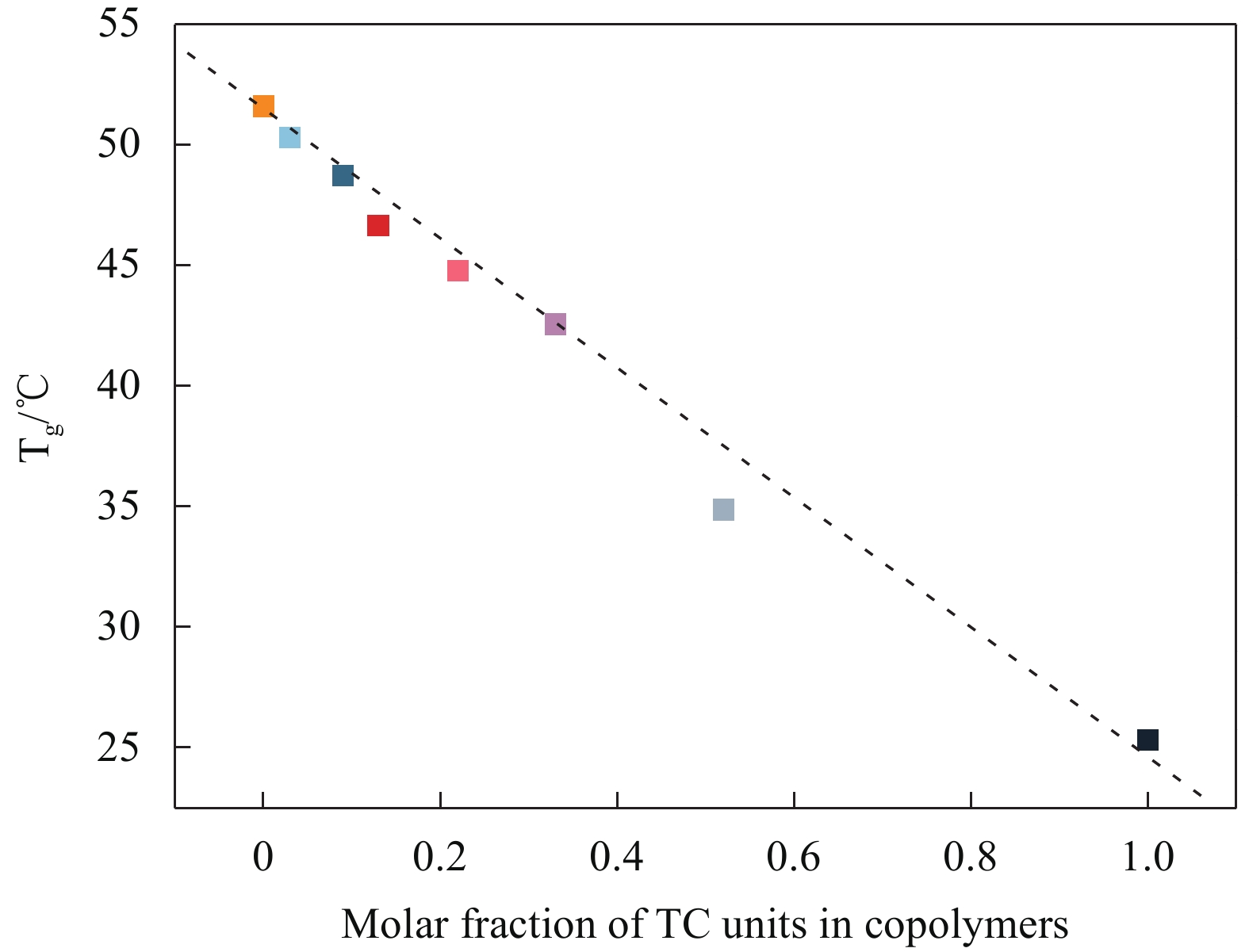
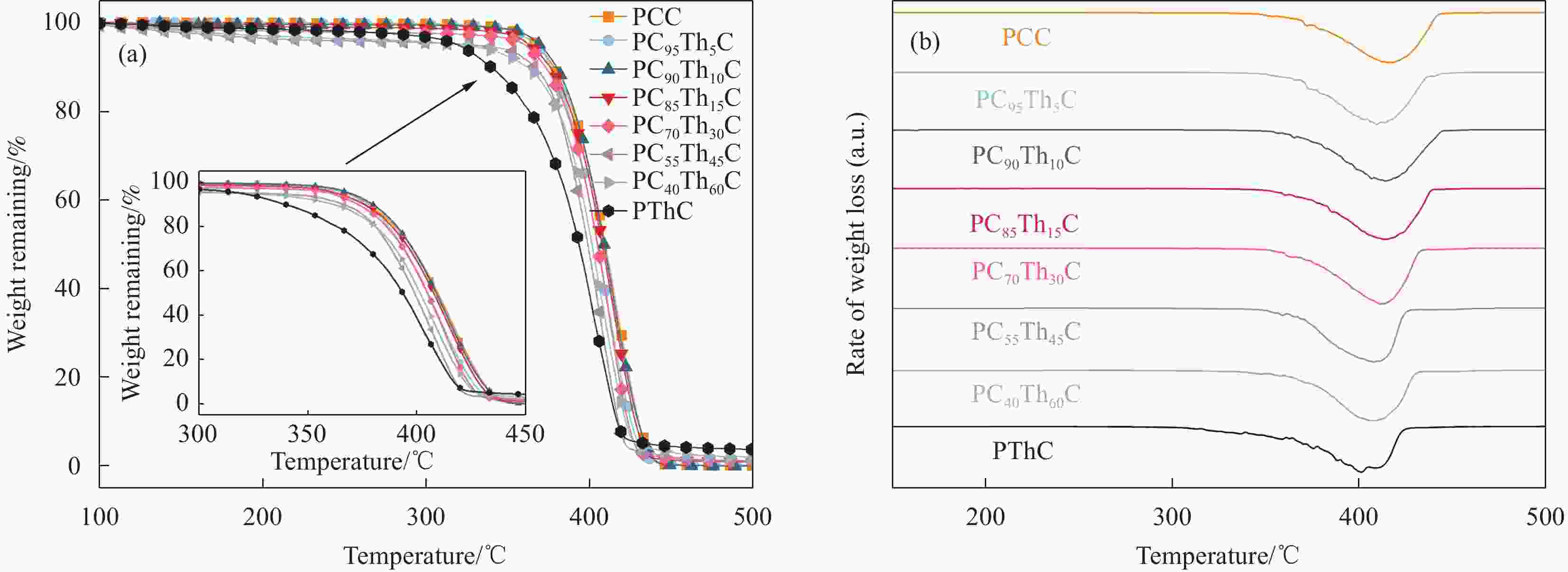
Table 3. Thermal property parameters of PCC, PThC, and PCThC copolymers
Sample Tg/℃ Tm/℃ ΔHm/(J·g−1) Td,5%/℃ Td,max/℃ Xc/% PCC 51.6 137.7 21.6 368 418 27.7 PC95Th5C 50.3 136.6 18.1 367 410 17.7 PC90Th10C 48.7 — — 369 414 — PC85Th15C 46.7 — — 363 414 — PC70Th30C 44.8 — — 361 412 — PC55Th45C 42.6 — — 328 408 — PC40Th60C 34.9 — — 321 409 — PThC 25.3 — — 320 401 — Notes: Tg and Tm are the glass transition temperature and melting temperature; ΔHm is the enthalpy of melting; Td,5% and Td,max are the decomposition temperature at 5% weight loss and the maximum decomposition rate; Xc is the degree of crystallinity. 表 4 PCC、PThC和PCThC共聚物的力学性能参数
Table 4. Mechanical property parameters of PCC, PThC, and PCThC copolymers
Sample E/MPa σb/MPa εb/% PCC 351±15 29.5±1.8 174±24 PC95Th5C 511±30 49.6±3.4 137±13 PC90Th10C 515±27 46.2±2.2 127±16 PC85Th15C 570±32 22.4±1.1 114±7 PC70Th30C 501±23 29.7±2.5 159±21 PC55Th45C 514±17 28.5±1.2 284±16 PC40Th60C 93±25 8.1±0.2 261±34 PThC 8±2 1±0.1 445±43 Notes: E is the Young’s modulus; σb is the tensile strength; εb is the elongation at break. -
[1] 王志凯. 高品质改性双酚A型聚碳酸酯的研究[D]. 长沙: 湖南师范大学, 2014.WANG Zhikai. Research on high-quality modified bisphenol A polycarbonate[D]. Changsha: Hunan Normal University, 2014(in Chinese). [2] 苏甜, 马念, 胡涛, 等. 双酚A型聚碳酸酯的结晶增速改性研究进展[J]. 高分子通报, 2016, (10): 29-40. doi: 10.14028/j.cnki.1003-3726.2016.010.003SU Tian, MA Nian, HU Tao, et al. Modification of bisphenol-a polycarbonate for crystallization-rate enhancement: A state-of-the-art review[J]. Polymer Bulletin, 2016, (10): 29-40(in Chinese). doi: 10.14028/j.cnki.1003-3726.2016.010.003 [3] LIU Ye, LU Xiaobing. Chemical recycling to monomers: Industrial Bisphenol-A-Polycarbonates to novel aliphatic polycarbonate materials[J]. Journal of Polymer Science, 2022, 60(24): 3256-3268. doi: 10.1002/pol.20220118 [4] YU Y, PANG C C, JIANG X S, et al. Copolycarbonates based on a bicyclic diol derived from citric acid and flexible 1, 4-cyclohexanedimethanol: From synthesis to properties[J]. ACS Macro Letters, 2019, 8(4): 454-459. doi: 10.1021/acsmacrolett.9b00184 [5] YAN S D, WU G Z. Hydrolytic degradation of isosorbide-based polycarbonates: Effects of terminal groups, additives, and residue catalysts[J]. Polymer Degradation and Stability, 2021, 192: 109703-109712. doi: 10.1016/j.polymdegradstab.2021.109703 [6] CHATTI S, SCHWARZ G, KRICHELDORF H R. Cyclic and noncyclic polycarbonates of isosorbide (1, 4:3, 6-dianhydro-d-glucitol)[J]. Macromolecules, 2006, 39(26): 9064-9070. doi: 10.1021/ma0606051 [7] PARK S A, CHOI J, JU S, et al. Copolycarbonates of bio-based rigid isosorbide and flexible 1, 4-cyclohexanedimethanol: Merits over bisphenol-A based polycarbonates[J]. Polymer, 2017, 116: 153-159. doi: 10.1016/j.polymer.2017.03.077 [8] 李鑫, 张小舟, 姜佳伟, 等. 异山梨醇型聚碳酸酯共聚物的合成及性能[J]. 塑料, 2021, 50(4): 140-144, 155.LI Xin, ZHANG Xiaozhou, JIANG Jiawei, et al. Copolycarbonates synthesis and performance of isosorbide[J]. Plastics, 2021, 50(4): 140-144, 155(in Chinese). [9] 刘静月, 侯洪波, 李贤勇, 等. 1, 4-环己烷二甲醇型聚碳酸酯二元醇的合成与表征[J]. 四川轻化工大学学报(自然科学版), 2021, 34(4): 18-24.LIU Jingyue, HOU Hongbo, LI Xianyong, et al. Synthesis and characterization of 1, 4-cyclohexane dimethyl alcohol modified polycarbonate diol[J]. Journal of Sichuan University of Science & Engineering (Natural Science Edition), 2021, 34(4): 18-24(in Chinese). [10] CADU A, SEKINE K, MORMUL J, et al. Homogeneous catalysed hydrogenation of HMF[J]. Green Chemistry, 2018, 20(14): 3386-3393. doi: 10.1039/C8GC01025K [11] JIANG F Y, QIU Z B. Crystallization kinetics, mechanical properties, and hydrolytic degradation of novel eco-friendly poly(butylene diglycolate) containing ether linkages[J]. Journal of Applied Polymer Science, 2016, 133(46): 9074-9085. [12] GIGLI M, LOTTI N, GAZZANO M, et al. Novel eco-friendly random copolyesters of poly(butylene succinate) containing ether-linkages[J]. Reactive and Functional Polymers, 2012, 72(5): 303-310. doi: 10.1016/j.reactfunctpolym.2012.02.013 [13] JIN C H, TIAN W H, TU Z, et al. Kilogram-scale production of sustainable PCF copolyesters based on novel cyclic diol THFDM derived from 5-hydroxymethylfurfural: Trade-off between the THFDM structure and various properties of copolyesters[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(39): 13287-13302. [14] JIN C H, WANG B, LIU L P, et al. Biodegradable poly(butylene succinate) copolyesters modified by bioresoured 2, 5-tetrahydrofurandimethanol[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(34): 11203-11214. [15] JIN C H, MA F H, LI C, et al. Kilogram-scale preparation of sustainable PETG modified with a biobased cyclic diol derived from 5-hydroxymethylfurfural: From synthesis to properties[J]. European Polymer Journal, 2021, 161: 110832-110842. doi: 10.1016/j.eurpolymj.2021.110832 [16] COLONNA M, BERTI C, BINASSI E, et al. Poly(1, 4-cyclohexylenedimethylene-1, 4-cyclohexanedicarboxylate): Analysis of parameters affecting polymerization and cis–trans isomerization[J]. Polymer International, 2011, 60(11): 1607-1613. doi: 10.1002/pi.3128 [17] BERTI C, CELLI A, MARCHESE P, et al. Novel copolyesters based on poly(alkylene dicarboxylate)s: 2. Thermal behavior and biodegradation of fully aliphatic random copolymers containing 1, 4-cyclohexylene rings[J]. European Polymer Journal, 2009, 45(8): 2402-2412. doi: 10.1016/j.eurpolymj.2009.04.034 [18] JIN C H, LIU L P, TU Z, et al. Melt polycondensation of 2, 5-tetrahydrofurandimethanol with various dicarboxylic acids towards a variety of biobased polyesters[J]. Polymer Chemistry, 2022, 13(40): 5718-5729. doi: 10.1039/D2PY00975G [19] DIAO L C, SU K M, LI Z H, et al. Furan-based co-polyesters with enhanced thermal properties: poly(1, 4-butylene-co-1, 4-cyclohexanedimethylene-2, 5-furandicarboxylic acid)[J]. RSC Advances, 2016, 6(33): 27632-27639. doi: 10.1039/C5RA27617A [20] HU H, ZHANG R Y, YING W B, et al. Sustainable and rapidly degradable poly(butylene carbonate-co-cyclohexanedicarboxylate): Influence of composition on its crystallization, mechanical and barrier properties[J]. Polymer Chemistry, 2019, 10(14): 1812-1822. doi: 10.1039/C9PY00083F [21] CAI X D, YANG X G, ZHANG H, et al. Modification of biodegradable poly(butylene carbonate) with 1, 4-cyclohexanedimethylene to enhance the thermal and mechanical properties[J]. Polymer Degradation and Stability, 2017, 143: 35-41. doi: 10.1016/j.polymdegradstab.2017.06.018 [22] MATOS M, SOUSA A F, SILVA N H C S, et al. Furanoate-based nanocomposites: A case study using poly(butylene 2, 5-furanoate) and poly(butylene 2, 5-furanoate)-co-(butylene diglycolate) and bacterial cellulose[J]. Polymers, 2018, 10(8): 810-825. doi: 10.3390/polym10080810 [23] LI C T, ZHANG M, WENG Y X, et al. Influence of ether linkage on the enzymatic degradation of PBS copolymers: Comparative study on poly (butylene succinate-co-diethylene glycol succinate) and poly (butylene succinate-co-butylene diglycolic acid)[J]. International Journal of Biological Macromolecules, 2018, 61(6): 1-10. [24] FOX T G. Influence of diluent and of copolymer composition on the glass temperature of a poly-mer system[J]. Bull. Am. Phys. Soc., 1956, 1: 123. [25] YU D Y, ZHONG J C, PU Z J, et al. Synthesis and properties of biobased polycarbonate based on isosorbitol[J]. Journal of Polymer Research, 2023, 30(6): 204. doi: 10.1007/s10965-023-03562-4 [26] HONG S, MIN K D, NAM B U, et al. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization[J]. Green Chemistry, 2016, 18(19): 5142-5150. doi: 10.1039/C6GC01060A [27] WEI Z Y, LIU L A, QU C, et al. Microstructure analysis and thermal properties of l-lactide/ɛ-caprolactone copolymers obtained with magnesium octoate[J]. Polymer, 2009, 50(6): 1423-1429. doi: 10.1016/j.polymer.2009.01.015 [28] WANG J G, LIU X Q, ZHU J, et al. Copolyesters based on 2, 5-furandicarboxylic acid (FDCA): Effect of 2, 2, 4, 4-tetramethyl-1, 3-cyclobutanediol units on their properties[J]. Polymers, 2017, 9(12): 305-319. [29] GIGLI M, LOTTI N, VERCELLINO M, et al. Novel ether-linkages containing aliphatic copolyesters of poly(butylene 1, 4-cyclohexanedicarboxylate) as promising candidates for biomedical applications[J]. Materials Science and Engineering: C, 2014, 34: 86-97. doi: 10.1016/j.msec.2013.08.013 [30] ARTHAM T, DOBLE M. Biodegradation of aliphatic and aromatic polycarbonates[J]. Macromolecular Bioscience, 2008, 8(1): 14-24. doi: 10.1002/mabi.200700106 -






 下载:
下载: