Research progress of electrolytes for aluminum ion batteries
-
摘要: 由于社会的快速发展,人们对二次离子电池的要求日益提高。铝离子电池具有成本低、安全性高、循环性能好等优点,是未来替代锂离子电池的理想储能体系。电解质作为电池系统重要组成之一,起到传输离子、连通电路的作用,对电池性能具有直接影响。因此,设计和制备具有良好综合性能的电解质一直是铝离子电池领域的研究热点。本文对目前铝离子电池的液态电解质、无机固态电解质和聚合物电解质的研究现状进行了总结,从成本、电化学窗口、化学稳定性和离子电导率等方面对它们的性能进行了分析,并对未来铝离子电池电解质的发展方向进行了展望。Abstract: Due to the rapid development of society, demands for secondary ion batteries are gradually increasing. Aluminum-ion battery has a lot of advantages, such as low cost, high safety and good cycle performance. Thus, it is an ideal energy storage system to replace lithium-ion battery in the future. As an important component of battery system, electrolyte plays a key role in transferring ions and connecting circuits, and has a direct impact on battery performance. Therefore, designing and preparing of electrolytes with good overall performance has become a research hotspot in aluminum-ion batteries. This article summarizes the current research status of liquid electrolytes, inorganic solid electrolytes and polymer electrolytes for aluminum-ion batteries, analyzes their performance in terms of cost, electrochemical window, chemical stability and ionic conductivity. The future development direction of aluminum-ion battery electrolytes is prospected.
-
图 1 使用V2O5纳米线阴极和铝阳极:(a) Al(OTF)3溶于体积比为1∶1的PC/THF溶剂的电解质和; (b)摩尔比为1.1∶1的 AlCl3/[EMIM]Cl电解质在0.2 mV·s−1的扫描速率下的循环伏安图[19]
Figure 1. Typical cyclic voltammograms of Al-ion battery using V2O5 nano-wire cathode and aluminium anode in (a) 1∶1 v/v of Al(OTF)3 in PC/THF;(b) 1.1∶1 molar ratio of AlCl3 in ([EMIM]Cl) at a sweep rate of 0.2 mV·s−1[19]
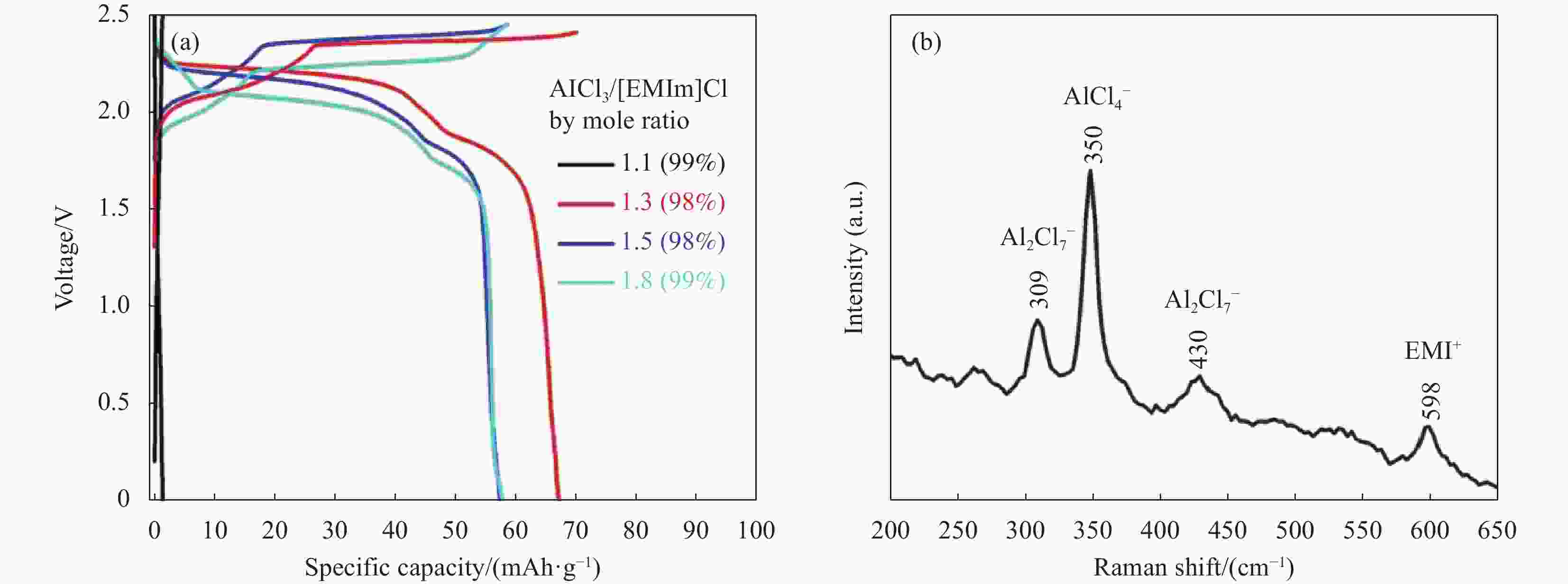
图 2 (a) 世伟洛克型电池中的不同摩尔比(1.1、1.3、1.5 和 1.8)的AlCl3/[EMIM]Cl 离子液体电解质,在电流密度为66 mA·g−1 时 Al/PG 电池的恒电流充放电曲线; (b) 离子液体电解质AlCl3/[EMIM]Cl摩尔比为1.3的拉曼光谱[20]
Figure 2. (a) Galvanostatic charge and discharge curves of Al/PG cells at a current density of 66 mA·g−1 in various mole ratios (1.1, 1.3, 1.5 and 1.8) of AlCl3/[EMIM]Cl ionic liquid electrolytes in a Swagelok-type cell. (b) Raman spectrum of the ionic liquid electrolyte with a mole ratio of AlCl3/[EMIM]Cl=1.3[20]
图 3 (a) 苯的含量与离子电导率和离子液体浓度的关系; (b) 含有不同比例苯的离子液的半电池CV曲线; (c)对应于 (b) 图的阳极和阴极电流密度峰值; (d) 阳极电流密度峰值与半电池CV扫描速率的关系[21]
Figure 3. (a) Concentration and ionic conductivity according to the addition ratio of benzene in ionic liquid; (b) Half-cell CV according to the addition ratio of benzene; (c) Anodic and cathodic current density peaks corresponding to (b). (d) Relationship between anodic current density peak and scan rate of half-cell CV[21]
图 5 未处理和处理后铝阳极表面铝沉积/溶解的示意图以及铝箔在0.5 mol/L Al(OTF)3/[BMIM]OTF和AlCl3/[BMIM]Cl=1.1:1中浸泡24小时前后的SEM图像[25]
Figure 5. Schematic diagram of Al deposition/dissolution on surface of untreated and treated Al anode; SEM images of Al foils before and after immersion in 0.5 mol/L Al(OTF)3/[BMIM]OTF and AlCl3/[BMIM]Cl=1.1 for 24 h[25]
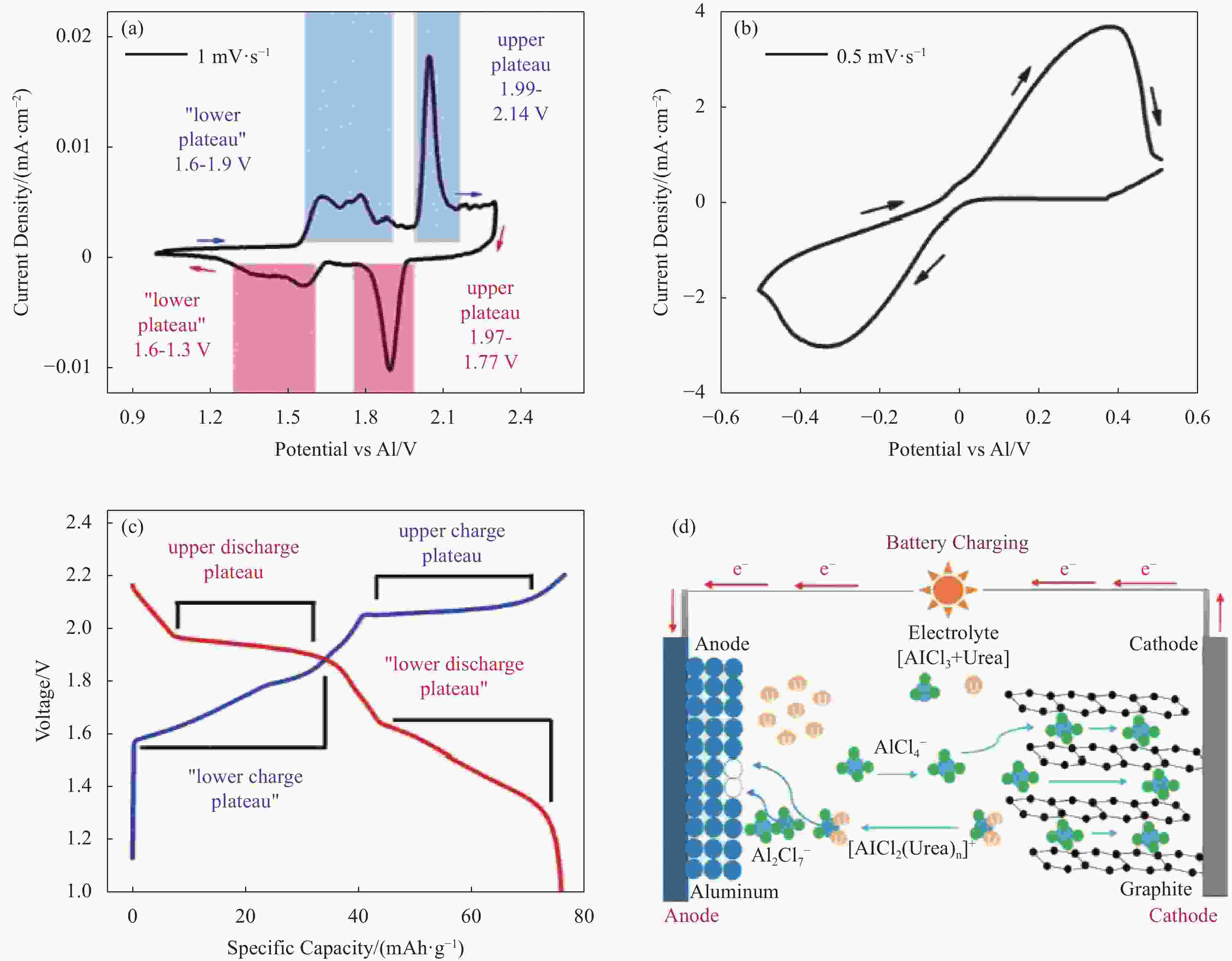
图 7 在摩尔比AlCl3/尿素=1.3电解质中,石墨和铝电极的CV测试: (a) 石墨嵌入/脱嵌(扫速为1 mV·s−1),对应的电池主要充放电曲线; (b) 铝沉积和溶解(扫速为0.5 mV·s−1)使用三电极体系; (c) 使用AlCl3/尿素= 1.3电解质在100 mA·g−1(循环20圈)的恒流充放电曲线; (d) 电池充电示意图(Al沉积和阴离子嵌入石墨)[33]
Figure 7. CV of graphite and aluminum electrodes in AlCl3/urea=1.3 electrolyte (by mole): (a) Graphite intercalation/deintercalation (1 mV·s−1), with corresponding major battery charge/discharge curve features indicated; (b) Aluminum deposition and stripping (0.5 mV·s−1) using three aluminum electrode set up; (c) Galvanostatic charge/discharge curve using AlCl3/urea =1.3 electrolyte at 100 mA·g−1 (cycle 20); (d) Schematic of battery charging (Al deposition and anion intercalation in graphite)[33]
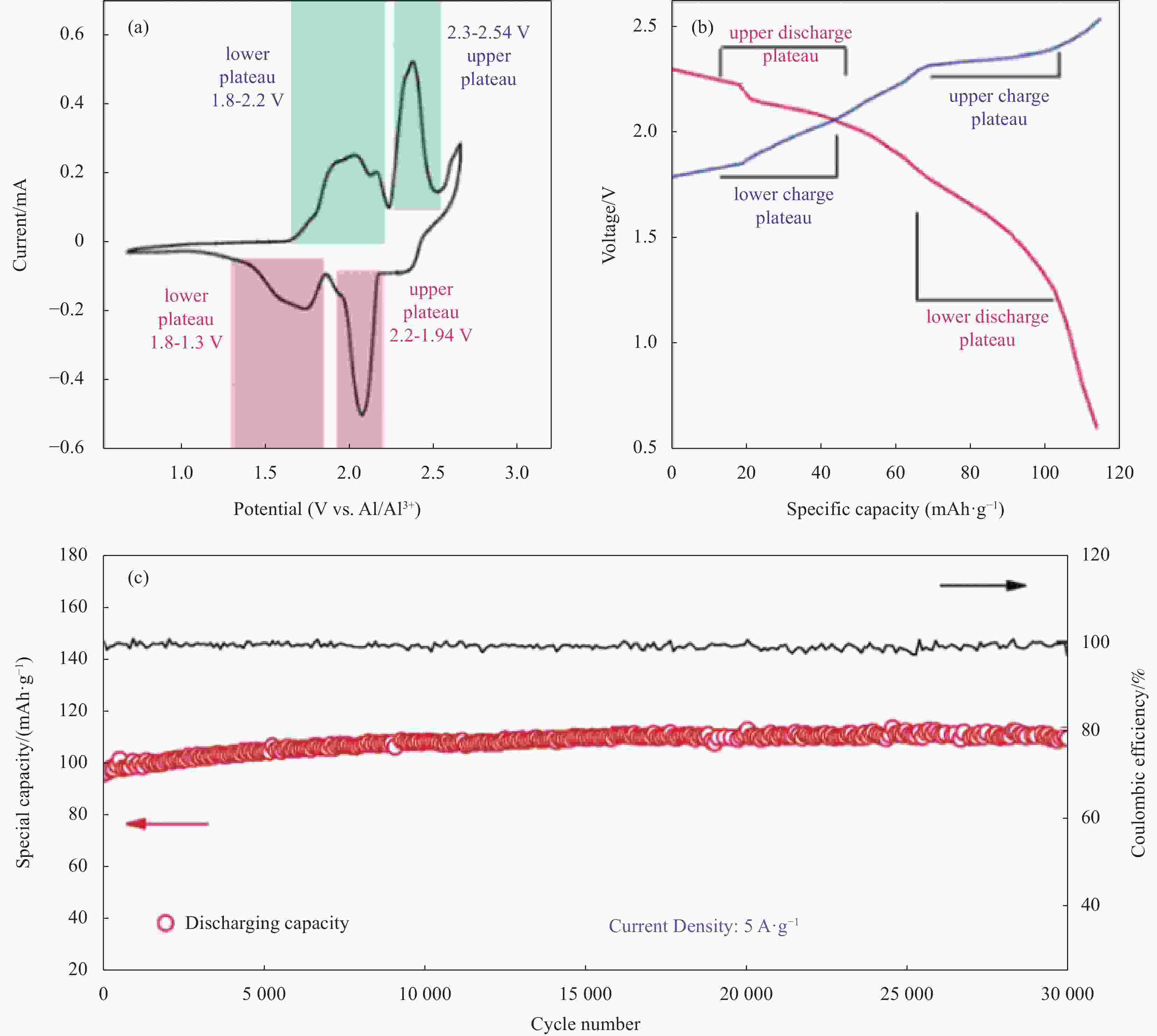
图 8 基于AlCl3/Et3NHCl摩尔比为1.5的Al-G电池的电化学性能:(a) 扫速为1 mV·s−1时的循环伏安曲线; (b) 电流密度为5 A·g−1时具有代表性的恒流充放电曲线; (c) 恒流循环30000次(电流密度为5 A·g−1,上/下截止电压为2.54 V/0.7 V) [34]
Figure 8. Electrochemical performance of the Al-G battery based on the AlCl3/Et3NHCl electrolyte at mole ratio of 1.5: (a) Cyclic voltammogram (CV) curve at 1 mV·s−1; (b) The representative galvanostatic charge/discharge curve at 5 A·g−1; (c) 30000 cycles of galvanostatic cycling (current density at 5 A·g−1 and 2.54 V/0.7 V upper/lower cut-off voltage[34]
图 19 Se/CMK-3复合阴极在铝硒电池中的恒流充放电测试。(a) - (c)分别为100,200和500 mA·g−1的充放电曲线。(d) 阴极在不同电流速率下的循环性能[69]
Figure 19. Galvanostatic charge/discharge measurements of Se/CMK-3 composite cathodes in Al-Se batteries. (a) – (c) Charge/discharge profiles at 100, 200 and 500 mA·g−1, respectively. (d) Cycling performances of the cathodes at different current rates[69]
表 1 25℃下不同AlCl3与4–乙基吡啶摩尔比配制的电解质的离子电导率、粘度和密度
Table 1. Ionic conductivity, viscosity, and density values of 4-ethylpyridine–AlCl3 IL electrolytes with various AlCl3 to 4-ethylpyridine molar ratios measured at 25℃
AlCl3/4-ethylpyridine
molar ratioConductivity/
(mS·cm−1)Viscosity/
(mPa·s)Density/
(g·cm−3)1.1 0.71 17.80 1.209 1.2 0.78 19.62 1.214 1.3 0.89 22.36 1.216 1.4 0.91 23.57 1.217 表 2 不同温度下铝离子固态电解质离子电导率
Table 2. Ion-conductivity of Al-ion solid electrolyte at different temperatures
Temperature/℃ 24 40 50 60 70 80 90 100 Al(CF3SO3)3 (AF) 5.5×10−6
S·cm−16.6×10−6
S·cm−11.19×10−5
S·cm−12.79×10−5
S·cm−11.37×10−4
S·cm−17.65×10−4
S·cm−11.43×10−3
S·cm−11.89×10−3
S·cm−1AlCl3 (ACL) 2.34×10−6
S·cm−12.38×10−6
S·cm−13.01×10−6
S·cm−14.42×10−6
S·cm−16.70×10−6
S·cm−11.76×10−5
S·cm−13.23×10−5
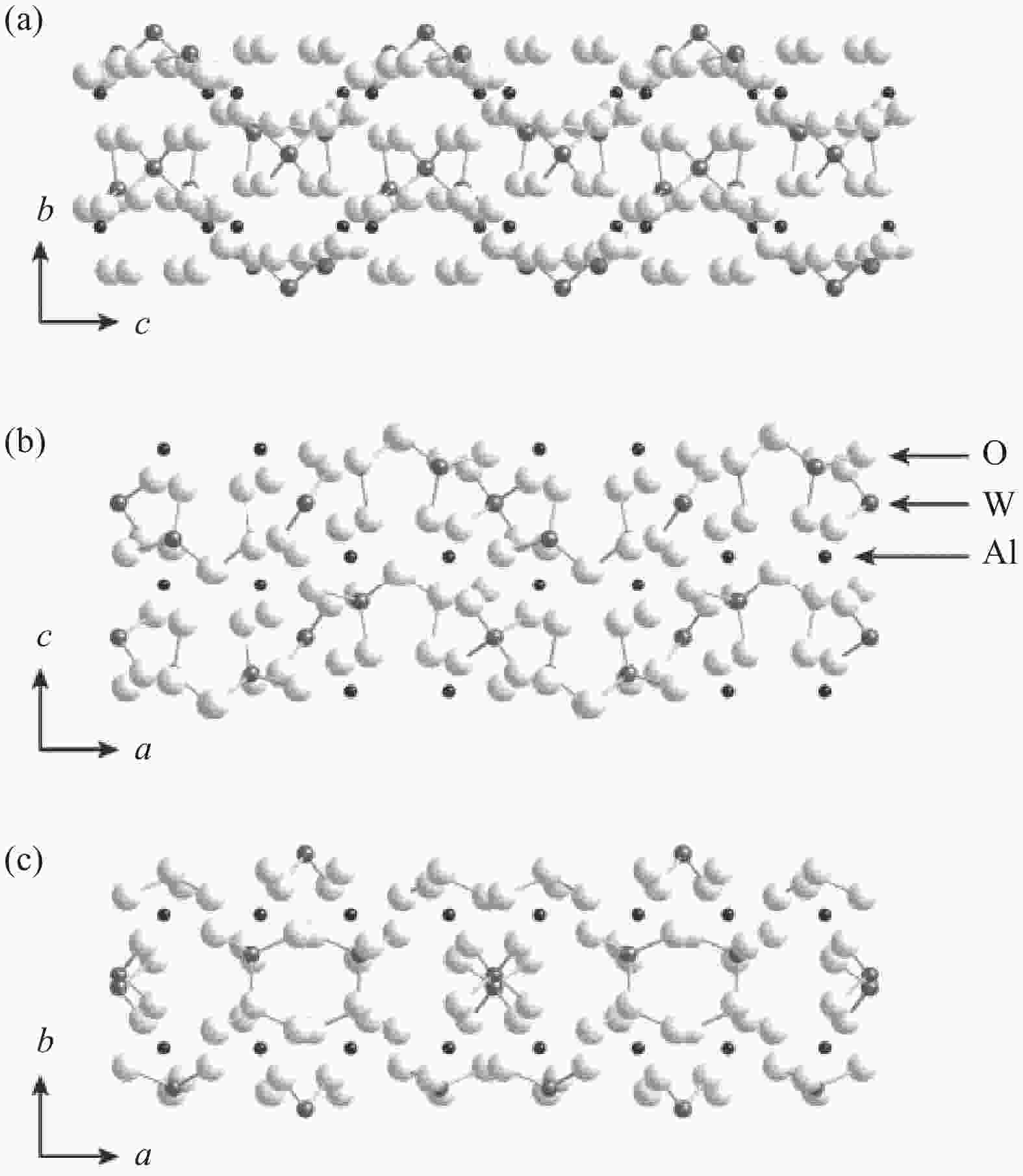
S·cm−14.95×10−5
S·cm−1Al(NO3)3(ANO) 3.98×10−7
S·cm−16.16×10−7
S·cm−19.8×10−7
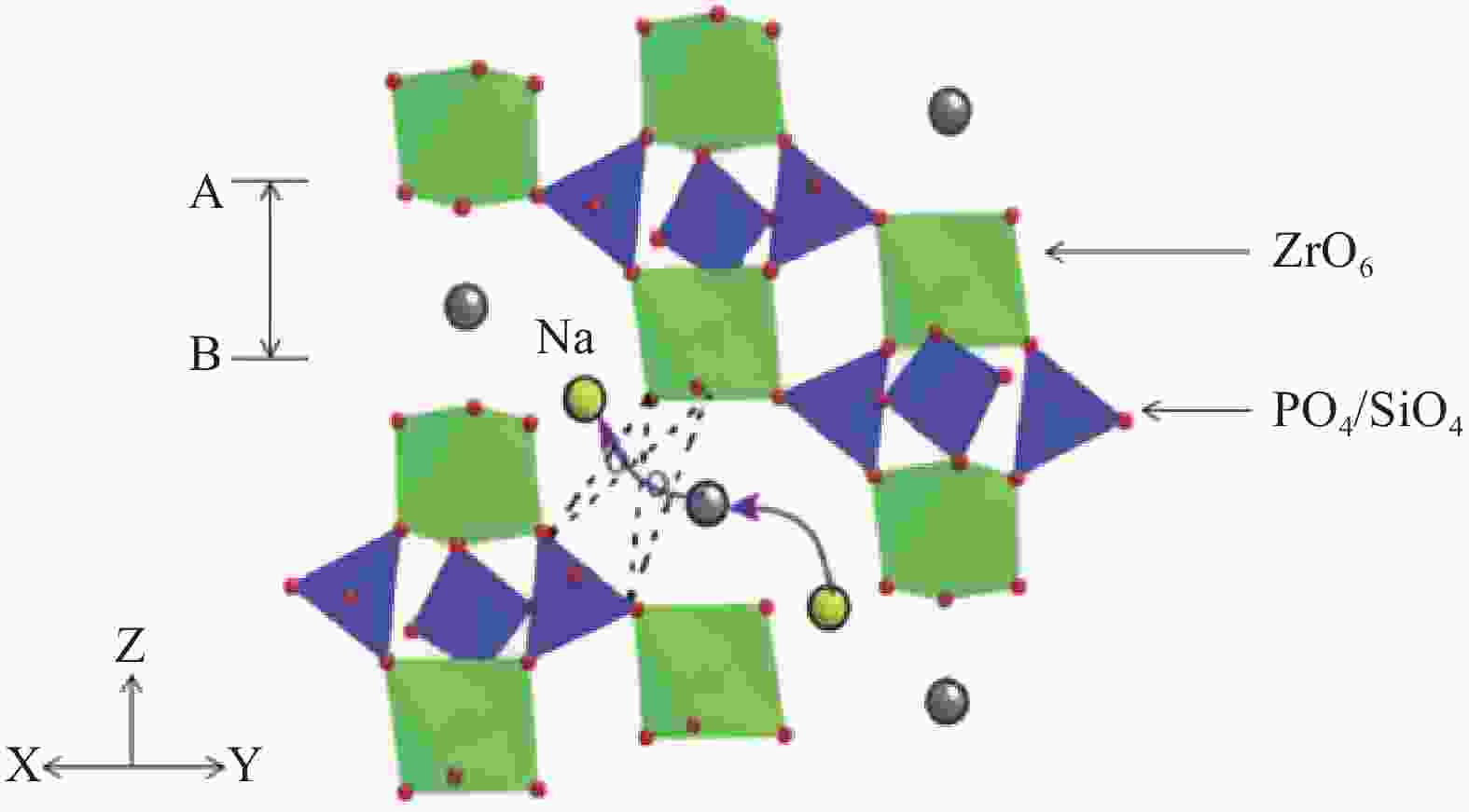
S·cm−11.89×10−6
S·cm−11.90×10−6
S·cm−14.88×10−5
S·cm−19.07×10−5
S·cm−11.88×10−4
S·cm−1 -
[1] WEI T, PENG P, Yi T, et al. Advancement of technology towards high-performance non-aqueous aluminum-ion batteries[J]. Journal of Energy Chemistry, 2021, 57: 169-188. doi: 10.1016/j.jechem.2020.08.035 [2] XIA L, YU L, CHEN G. Electrolytes for electrochemical energy storage[J]. Materials Chemistry Frontiers, 2017, 1: 584-618. doi: 10.1039/C6QM00169F [3] HAN X, BAI Y, WU C, et al. Electrolytes for rechargeable aluminum batteries[J]. Progress in Materials Science, 2022, 128: 100960. doi: 10.1016/j.pmatsci.2022.100960 [4] 谢乐琼, 王莉, 胡坚耀. 锂离子动力电池产业技术发展概述[J]. 新材料产业, 2019, 5(1): 38-44.XIE Leqiong, WANG Li, HU Jianyao. Technology development overview of lithium ion power battery industry[J]. Advanced Materials Industry, 2019, 5(1): 38-44(in Chinese). [5] 陈诺, 郭轲, 焦树强, 等. 铝离子电池电解质研究进展[J]. 有色金属科学与工程, 2023, 14(2): 189-201.CHENG Nuo, GUO Ke, JIAO Shuqiang, et al. Research progress in electrolytes of aluminum ion Batteries[J]. Nonferrous Metals Science and Engineering, 2023, 14(2): 189-201(in Chinese). [6] ZHANG L, ZHANG F, ALSHAREEF H N. Sodium-ion battery anodes: Status and future trends[J]. Energy Chem, 2019, 1(2): 100012. doi: 10.1016/j.enchem.2019.100012 [7] CHAYAMBUKA K, MULDER G, NOTTEN P H L. From Li-ion batteries toward Na-ion chemistries: challenges and opportunities[J]. Advanced Energy Materials, 2020, 10(38): 2001310. doi: 10.1002/aenm.202001310 [8] NING Z, MONROE C W, BRUCE P G. Dendrite initiation and propagation in lithium metal solid-state batteries[J]. Nature, 2023, 618: 287-293. doi: 10.1038/s41586-023-05970-4 [9] DAS S K, MAHAPATRA S, LAHAN H. Aluminium-ion batteries: developments and challenges[J]. Journal of Materials Chemistry A, 2017, 5(14): 6347-6367. doi: 10.1039/C7TA00228A [10] JIA B E, THANG A Q, YAN Q, et al. Rechargeable aqueous aluminum-ion battery: progress and outlook[J]. Small, 2022, 18(43): 2107773. doi: 10.1002/smll.202107773 [11] KOK L N, BROHATH A, GISELE A, Nonaqueous rechargeable aluminum batteries[J]. Joule, 2022, 6(1): 134-170. [12] HU Z, ZHANG H, LI H, et al. Nonaqueous Aluminum ion batteries: recent progress and prospects[J]. 2020, 2(8): 887-904. [13] ZHAO R, WU Y, ZHAO Y, et al. Metal–organic frameworks for solid-state electrolytes[J]. Energy & Environmental Science, 2020, 13: 2386. [14] 方亮, 张凯, 周丽敏. 铝离子电池电解液的研究进展[J]. 储能科学与技术, 2022, 11(4): 1236-1245.FANG Liang, ZHANG Kai, ZHOU Limin. Research progress of aluminum ion battery electrolyte[J]. Energy Storage Science and Technology, 2022, 11(4): 1236-1245(in Chinese). [15] LIANG X, WANG L, WANG J. Solid-state electrolytes for solid-state lithium-sulfur batteries: Comparisons, advances and prospects[J]. Journal of Energy Chemistry, 2022, 73: 370-386. doi: 10.1016/j.jechem.2022.06.035 [16] Ma D, Du Y, Pan J, et al. Current progress and future perspectives of electrolytes for rechargeable aluminum-ion batteries[J]. Energy & Environmental Materials, 2023, 6(1): e12301. [17] DAS S, MANNA S S, PATHAK B. Recent trends in electrode and electrolyte design for aluminum batteries[J]. 2021, 6(2): 1043-1053. [18] 余智静. 聚合物铝离子二次电池的基础研究 [D]. 北京: 北京科技大学, 2021.YU Zhijing. Basic research on polymer aluminum ion secondary battery [D]. Beijing: University of Science and Technology Beijing, 2021 (in Chinese). [19] JAYAPRAKASH N, DAS S K, ARCHER L A. The rechargeable aluminum-ion battery[J]. Chemical Communications, 2011, 47(47): 12610-12612. doi: 10.1039/c1cc15779e [20] LIN M, GONG M, DAI H, et al. An ultrafast rechargeable aluminium-ion battery[J]. Nature, 2015, 520(7547): 324-328. doi: 10.1038/nature14340 [21] PARK Y, LEE D B, KIM J. Fast charging with high capacity for aluminum rechargeable batteries using organic additive in anionic liquid electrolyte[J]. Physical Chemistry Chemical Physics, 2020, 22(47): 27525-27528. doi: 10.1039/D0CP05050D [22] DAMME V S, DECONINCK J. Relaxation effect on the onsager coefficients of mixed strong electrolytes in the mean spherical approximation[J]. The Journal of Physical Chemistry B, 2007, 111(19): 5308-5315. doi: 10.1021/jp071651l [23] LEE D, LEE G, TAK Y. Hypostatic instability of aluminum anode in acidic ionic liquid for aluminum-ion battery[J]. Nanotechnology, 2018, 29(36). [24] WANG H, GU S, WU C, WU F, et al. Anion-effects on electrochemical properties of ionic liquid electrolytes for rechargeable aluminum batteries[J]. Journal of Materials Chemistry A, 2015, 3(45): 22677-22686. doi: 10.1039/C5TA06187C [25] WANG H, GU S, WU C, WU F, et al. High-voltage and noncorrosive ionic liquid electrolyte used in rechargeable aluminum battery[J]. ACS Applied Materials & Interfaces, 2016, 8(41): 27444-27448. [26] YU H, HE S. Progresses and perspectives of aluminum ion batteries[J]. Journal of Beijing University of Technology, 2020, 46(6): 680-697. [27] ABBOTT A P, CAPPER G, TAMBYRAJAH V, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical Communications, 2003, (1): 70-71. doi: 10.1039/b210714g [28] HANSEN B B, SPITTLE S, CHEN B, SANGORO J R, et al. Deep eutectic solvents: a review of fundamentals and applications[J]. Chemical Reviews, 2021, 121(3): 1232-1285. doi: 10.1021/acs.chemrev.0c00385 [29] DYMEK C, WILLIAMS J, GROEGER D. An aluminum acid-base concentraion cell using room temperature chloroaluminate ionic liquids[J]. Journal of The Electrochemi cal Society, 1984, 131(12): 2887-2892. doi: 10.1149/1.2115436 [30] HAN X, BAI Y, WU C, et al. Electrolytes for rechargeable aluminum batteries[J]. Progress in Materials Science, 2022, 128: 100960. doi: 10.1016/j.pmatsci.2022.100960 [31] SHARMA A, SHARMA R, SINGH L, et al. An overview of deep eutectic solvents: Alternative for organic electrolytes, aqueous systems & ionic liquids for electrochemical energy storage[J]. Journal of Energy Chemistry, 2023, 82: 592-626. doi: 10.1016/j.jechem.2023.03.039 [32] SMITH E L, ABBOTT A P, RYDER K S, et al. Deep eutectic solvents (DESs) and their applications[J]. Chemical Reviews 2014, 114(21): 11060−11082. [33] ANGELL M, PAN C J, RONG Y, Dai H, et al. High coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(5): 834-839. [34] XU H, BAI T, CHEN H, LING J, GAO C. Low-cost AlCl3/Et3NHCl electrolyte for high-performance aluminum-ion battery[J]. Energy Storage Materials, 2019, 17: 38-45. doi: 10.1016/j.ensm.2018.08.003 [35] LONG K N, TONY D, JOHN A, GISELE A. High-performance aluminum ion battery using cost-effective AlCl3-Trimethylamine hydrochloride ionic liquid electrolyte[J]. advanced sustainable systems, 2020, 4(8). [36] FERRARA C, ASTA D V, BERBENNI V, QUARTARONE E, MUSTARELLI P. Physicochemical characterization of AlCl3-1-Ethyl-3-methylimidazolium chloride ionic liquid electrolytes for aluminum rechargeable batteries[J]. The Journal of Physical Chemistry C, 2017, 121(48): 26607-26614. doi: 10.1021/acs.jpcc.7b07562 [37] ZHENG Y, DONG K, WANG Q, LU X, et al. Density, viscosity, and conductivity of lewis acidic 1-butyl- and 1-hydrogen-3-methylimidazolium chloroaluminate ionic liquids[J]. The Journal of Physical Chemistry C, 2013, 58(1): 32-42. [38] LIN P C, SUN I W, CHANG J K, LIN J C. Corrosion characteristics of nickel, copper, and stainless steel in a Lewis neutral chloroaluminate ionic liquid[J]. Corrosion Science, 2011, 53(12): 4318-4323. doi: 10.1016/j.corsci.2011.08.047 [39] LI C, LI J, CHANG J K, et al. A novel moisture-insensitive and low-corrosivity ionic liquid electrolyte for rechargeable aluminum batteries[J]. Advanced Functional Materials, 2020, 30: 1909565. doi: 10.1002/adfm.201909565 [40] KȌHLER J, IMANAKA N, Adachi G Y. Multivalent cationic conduction in crystalline solids[J]. Chemistry of Materials, 1998, 10(12): 3790-3812. doi: 10.1021/cm980473t [41] KOBAYASHI Y, EGAWA T, IMANAKA N, ADACHI G Y, et al. Trivalent Al3+ ion conduction in aluminum tungstate solid[J]. Chemistry of Materials, 1997, 9(7): 1649-1654. doi: 10.1021/cm970004b [42] KOBAYASHI Y, TAMURA S, IMANAKA N, Adachi G. Quantitative demonstration of Al3+ ion conduction in Al2(WO4)3 solids[J]. Solid State Ionics, 1998, 113–115: 545–552. [43] IMANAKA N, TAMURA S. Development of multivalent ion conducting solid electrolytes[J]. Bulletin of the Chemical Society of Japan, 2011, 84(4): 353-362. doi: 10.1246/bcsj.20100178 [44] ÖZBILGIN C E, KOBAYASHI K, TAMURA S, SUZUKI T S. Enhanced ionic conductivity of aluminum tungstate by crystallographic orientation in a strong magnetic field[J]. Journal of the American Ceramic Society, 2021, 104(12): 6364-6372. doi: 10.1111/jace.18001 [45] 张含. 基于NASICON 和金属氧化物电极的有毒有害气体传感器的研究 [D]. 吉林: 吉林大学, 2015.ZHANG Han. Research on toxic and harmful gas sensor based on NASICON and metal oxide electrode [D]. Jilin: Jilin University, 2015(in Chinese). [46] IMANAKA N, HASEGAWA Y, YAMAGUCHI M, et al. Extraordinary high trivalent Al3+ ion conduction in solids[J]. Chemistry of Materials, 2002, 14(11): 4480-4483. [47] HASEGAWA Y, TAMURA S, IMANAKA N. Effect of low-melting oxide additives on the sinterability and ion conductivity of Al3+ ion conducting solid electrolytes with the NASICON type structure[J]. Journal of New Materials for Electrochemical Systems, 2005, 8(3): 203-207. [48] 史明. 新型高价阳离子导电固体电解质的制备及应用 [D]. 唐山: 华北理工大学, 2017.SHI Ming. Preparation and application of novel high-priced cationic conductive solid electrolytes [D]. Tangshan: North China University of Science and Technology, 2017(in Chinese). [49] FENTON D E, PARKER J M, WRIGHT P V. Complexes of alkali metal ions with poly(ethylene oxide)[J]. Polymer, 1973, 14(11): 589. [50] YUE L, MA J, CUI G, CHEN L, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries[J]. Energy Storage Materials, 2016, 5: 139-164. doi: 10.1016/j.ensm.2016.07.003 [51] SING N K, RAMESH S, RAMESH K, CHING J. A review of polymer electrolytes: fundamental, approaches and applications[J]. Ionics, 2016, 22(8): 1259-1279. doi: 10.1007/s11581-016-1756-4 [52] Wang Q, Zhang H, Cui G, et al. Siloxane-based polymer electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 23: 466-490. doi: 10.1016/j.ensm.2019.04.016 [53] GAELE M F, DI-PALMA T M. Polymer electrolytes for Al-Air batteries: current state and future perspectives[J]. Energy & Fuels, 2022, 36(21): 12875-12895. [54] YU Z, XIE Y, GE J, et al. Selection principles of polymeric frameworks for solid-state electrolytes of non-aqueous aluminum-ion batteries[J]. Front. Chem, 2023, 11: 1190102. doi: 10.3389/fchem.2023.1190102 [55] 康树森, 方金刚, 孟翠洲, 等. 铝离子聚合物固态电解质[J]. 化学学报, 2019, (7): 647-652KANG Shusen, FANG Jingang, MENG Cuizhou, et al. Aluminum ion polymer solid electrolyte[J]. Acta Chimica Sinica, 2019, (7): 647-652(in Chinese). [56] YAO T, BIRIA S, HOSEINA I D, et al. A solid polymer electrolyte for aluminum ion conduction[J]. Results in Physics, 2018, 10: 529-531. doi: 10.1016/j.rinp.2018.07.001 [57] KOTOBUKI M, Li L, ALDOSHIN S M, et al. Poly(vinylidene fluoride)-based Al-Ion conductive solid polymer electrolyte for Al battery[J]. Journal of The Electrochemical Society, 2017, 164(14): A3868 doi: 10.1149/2.1601714jes [58] SUN X, FANG Y, DAI S, et al. Polymer gel electrolytes for application in aluminum deposition and rechargeable aluminum ion batteries[J]. Chemical Communications, 2016, 52(2): 292-295. doi: 10.1039/C5CC06643C [59] YU Z, JIAO S, ZHANG G, et al. Flexible stable solid-state Al-ion batteries[J]. Advance Functional Materials, 2019, 29(1): 1806799. doi: 10.1002/adfm.201806799 [60] SHEN X, SUN T, WU Z, TAN L. Ultrafast charging and ultralong cycle life in solid-state Al-ion batteries[J]. Journal of Materials Chemistry A, 2022, 10(15): 8178-8185. doi: 10.1039/D2TA00630H [61] YU Z, JIAO S, FANG D, et al. Gel electrolytes with wide potential window for high-rate Al-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(35): 20348-20356. doi: 10.1039/C9TA06815E [62] LIU Z, DU H, CUI G, et al. A reliable gel polymer electrolyte enables stable cycling of rechargeable aluminum batteries in a wide-temperature range[J]. Journal of Power Sources, 2021, 497: 229839. doi: 10.1016/j.jpowsour.2021.229839 [63] 黄俊达, 朱宇辉, 冯煜等. 二次电池研究进展[J]. 物理化学学报, 2022, 38(12): 28-173.HUANG Junda, ZHU Yuhui, FENG Yu, et al. Research progress of secondary battery[J]. Journal of Physical Chemistry, 2022, 38(12): 28-173 (in Chinese). [64] WU F, ZHANG K, WU C, et al. Polymer electrolytes and interfaces toward solid-state batteries: Recent advances and prospects[J]. Energy Storage Materials, 2020, 33: 26-54. doi: 10.1016/j.ensm.2020.08.002 [65] KLIMPEL M, KOVALENKO M V, KRAVCHYK K V. Advances and challenges of aluminum–sulfur batteries[J]. Communications Chemistry, 2022, 5: 77. doi: 10.1038/s42004-022-00693-5 [66] HUANG Z, LI S, JIAO S, et al. A hundreds-milliampere-hour-scale solid-state aluminum–sulfur pouch cell[J]. Advanced Energy Materials, 2023, 13(43): 2302464. doi: 10.1002/aenm.202302464 [67] LIU S, ZHANG X, AMINE K. An advanced high energy-efficiency rechargeable aluminum-selenium battery[J]. Nano Energy, 2019, 66: 104159. doi: 10.1016/j.nanoen.2019.104159 [68] HUANG X, LIU Y, YU C. Rechargeable aluminum–selenium batteries with high capacity[J]. Chemical Science, 2018, 9: 5178-5182. doi: 10.1039/C8SC01054D [69] LEI H, TU J, JIAO S. MOF-based quasi-solid-state electrolyte for long-life Al-Se battery[J]. Journal of Energy Chemistry, 2023, 86: 237-245. doi: 10.1016/j.jechem.2023.07.026 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 100
- HTML全文浏览量: 78
- 被引次数: 0





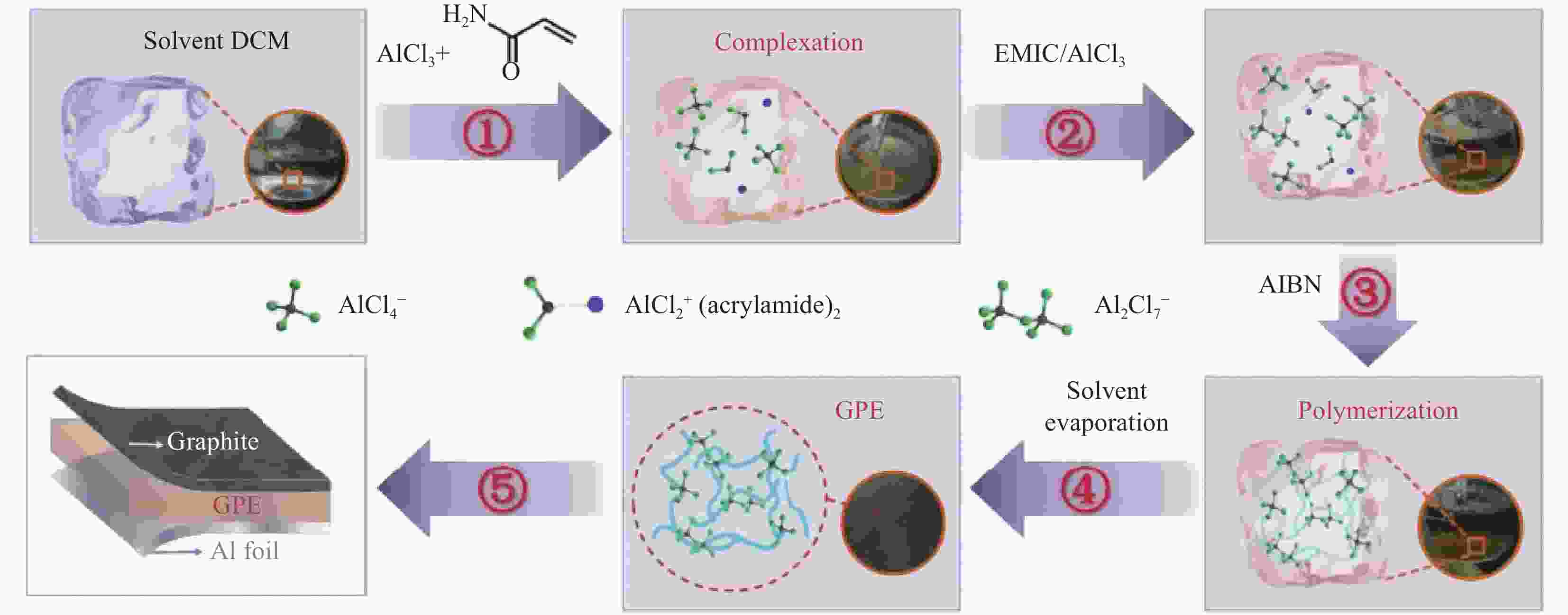
 下载:
下载: