Research progress of hydrogen permeation palladium composite membranes
-
摘要: 钯复合膜由于其特殊的透氢机制—溶解/扩散机制,对氢具有极高的选择渗透性,是膜反应器中氢气分离的理想材料。为促进高透氢性、高稳定性钯复合膜的研究与应用,本文综述了钯复合膜的化学镀制备法和化学镀与其他方式结合的复合制备法以及不同类型的复合膜。化学镀是钯复合膜最常用的制备方法,通过与真空、连续流结合可提高钯膜的质量;ⅤB族金属、多孔陶瓷和不锈钢作为化学镀钯膜的载体,可减小钯膜厚度,提高机械强度和透氢性能;在钯膜和多孔载体之间掺入难熔氧化物、沸石、天然矿物、聚合物,可进一步减小钯膜厚度,提高热稳定性和化学稳定性;与纯钯膜相比,Pd-Ag、Pd-Cu、Pd-Au二元合金膜和Pd-Au-Ag三元合金膜在低温下不发生氢脆,可不同程度地改善钯膜的透氢性和抗硫性。最后,对未来钯复合膜的研究方向进行了展望。Abstract: Palladium composite membranes have highly selective permeability to hydrogen due to their special dissolution/diffusion mechanism of hydrogen permeation, and are an ideal material for hydrogen separation in membrane reactors. In order to promote the research and application of palladium composite membranes with high hydrogen permeability and stability, the preparation method of the membranes via electroless plating and its combined with other methods, and different types of the membranes are reviewed in this paper. Electroless plating is the most common preparation method of palladium composite membranes, and the quality of the membranes can be improved by combining with vacuum and continuous flow. Using group ⅤB metals, porous ceramics and stainless steel as the matrix of electroless Pd membranes, the membrane thickness can be reduced, mechanical strength and hydrogen permeability can be improved; The thickness of Pd membranes can be further reduced by adding refractory oxide, zeolite, natural mineral and polymer between Pd membrane and porous matrix, and the thermal and chemical stability can be improved. Compared with pure Pd membrane, Pd-Ag, Pd-Cu, Pd-Au binary alloy membranes and Pd-Au-Ag ternary alloy membrane have no hydrogen embrittlement phenomenon at low temperature, and can improve the hydrogen permeability and sulfur resistance of Pd membranes to a certain degrees. Finally, the future research direction of palladium composite membranes is prospected.
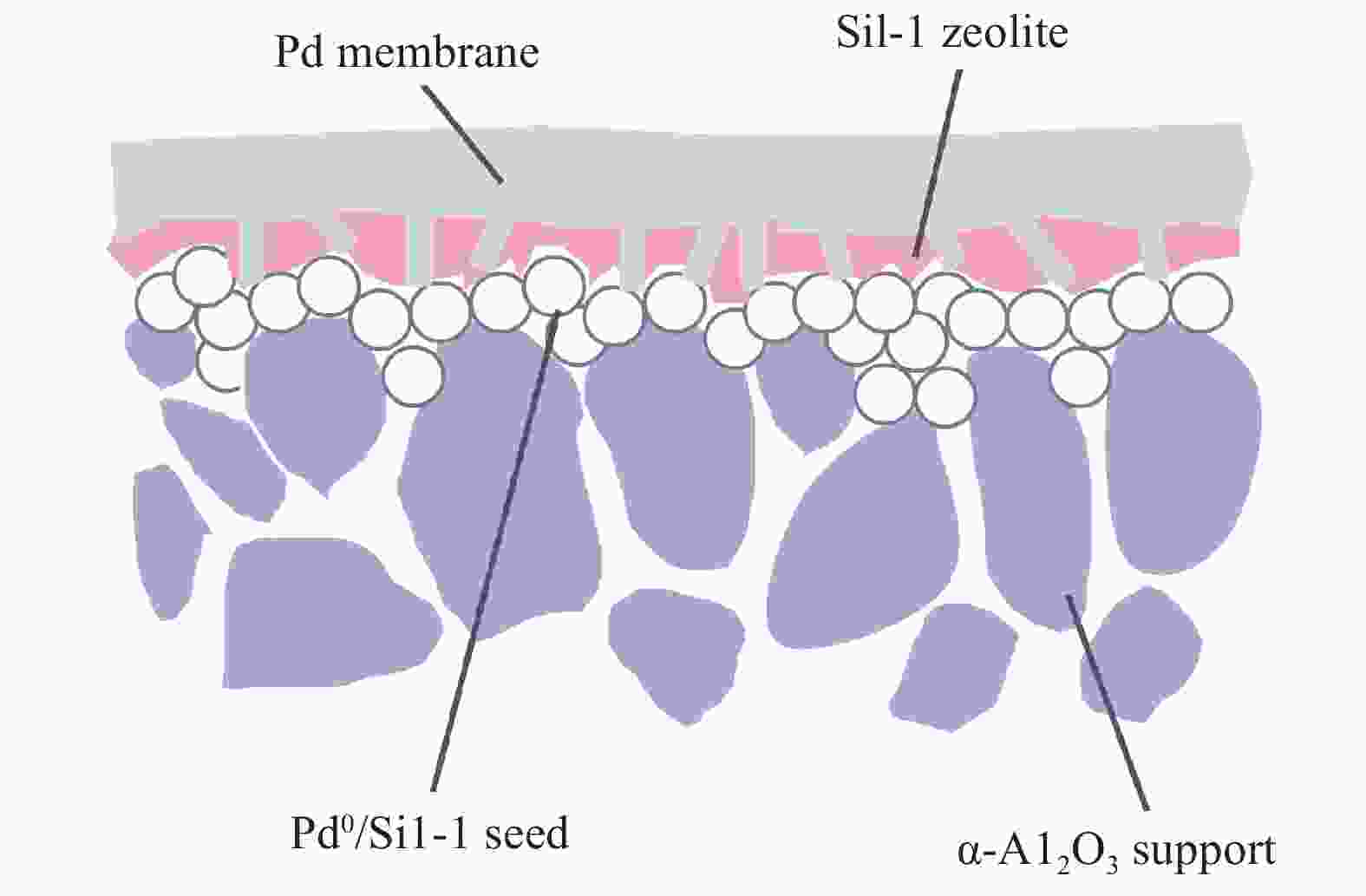
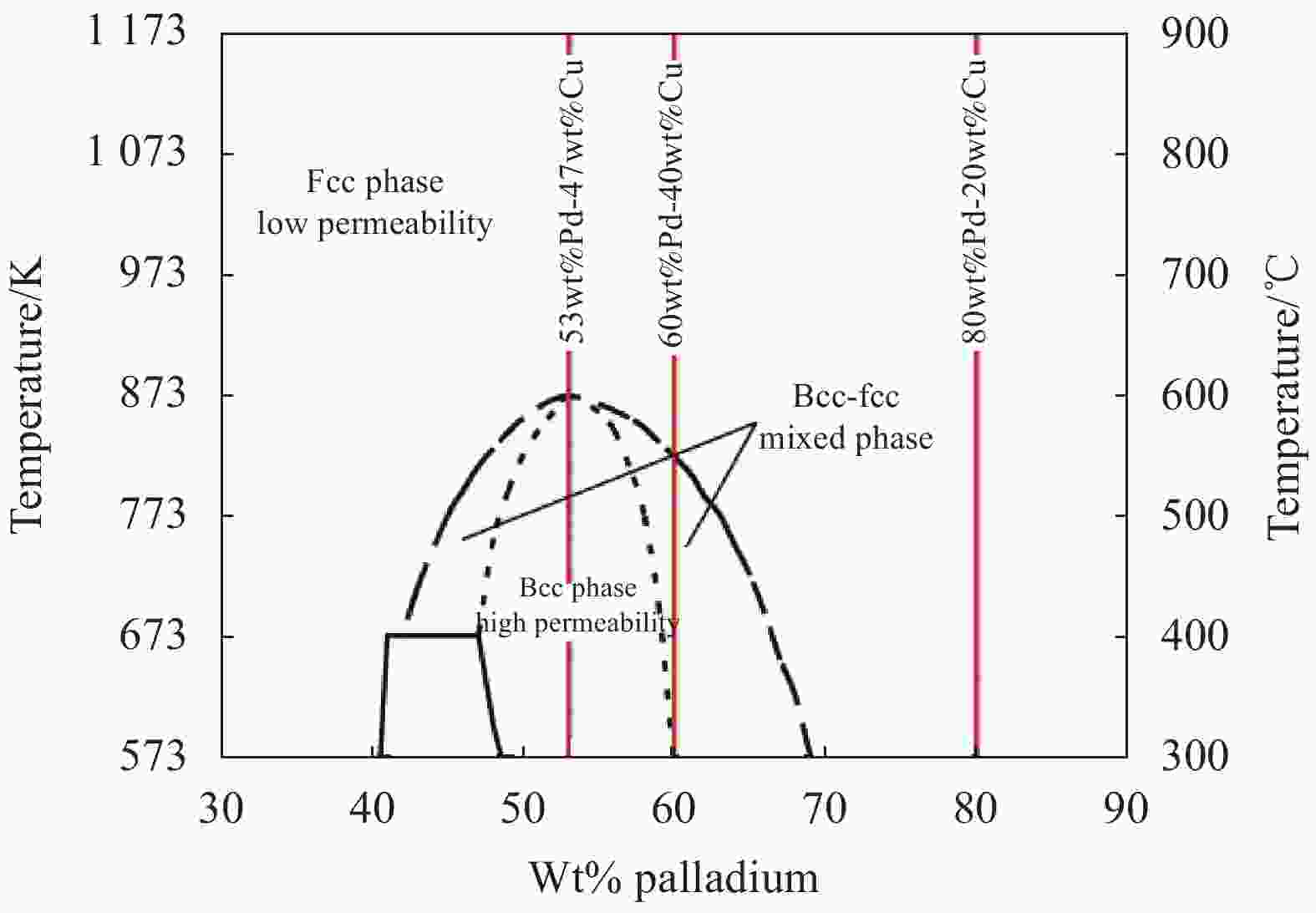
-
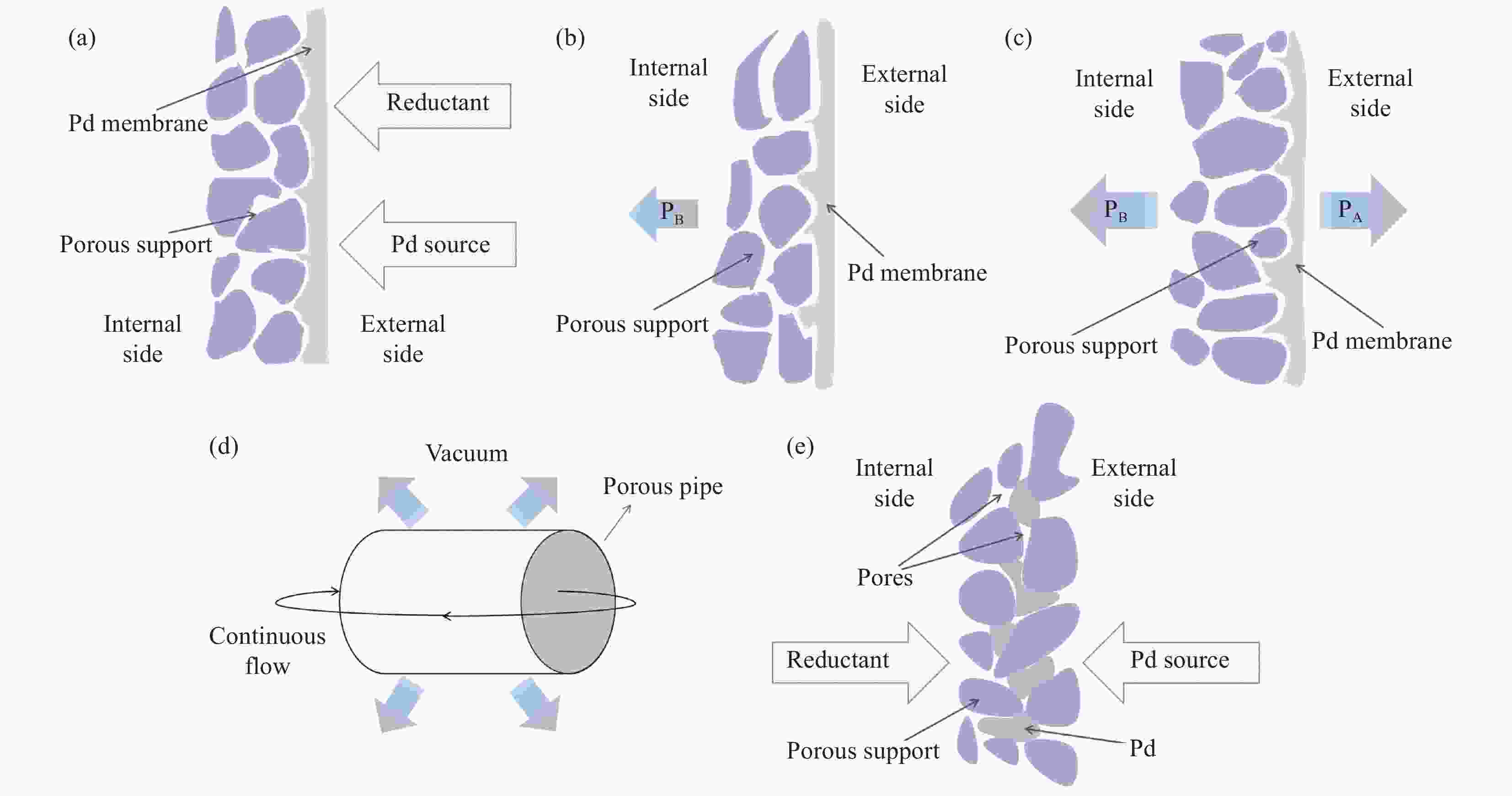
图 1 各种化学镀技术制备钯复合膜的示意图。(a) 化学镀;(b) 辅助抽吸化学镀;(c) 真空化学镀;(d) 真空辅助连续流化学镀;(e) 化学孔镀
Figure 1. Schematic of palladium composite membranes prepared by various electroless plating techniques. (a) Electroless plating; (b) Assisted suction electroless plating; (c) Vacuum electroless plating; (d) Vacuum-assisted continuous flow electroless plating; (e) Electroless pore-plating
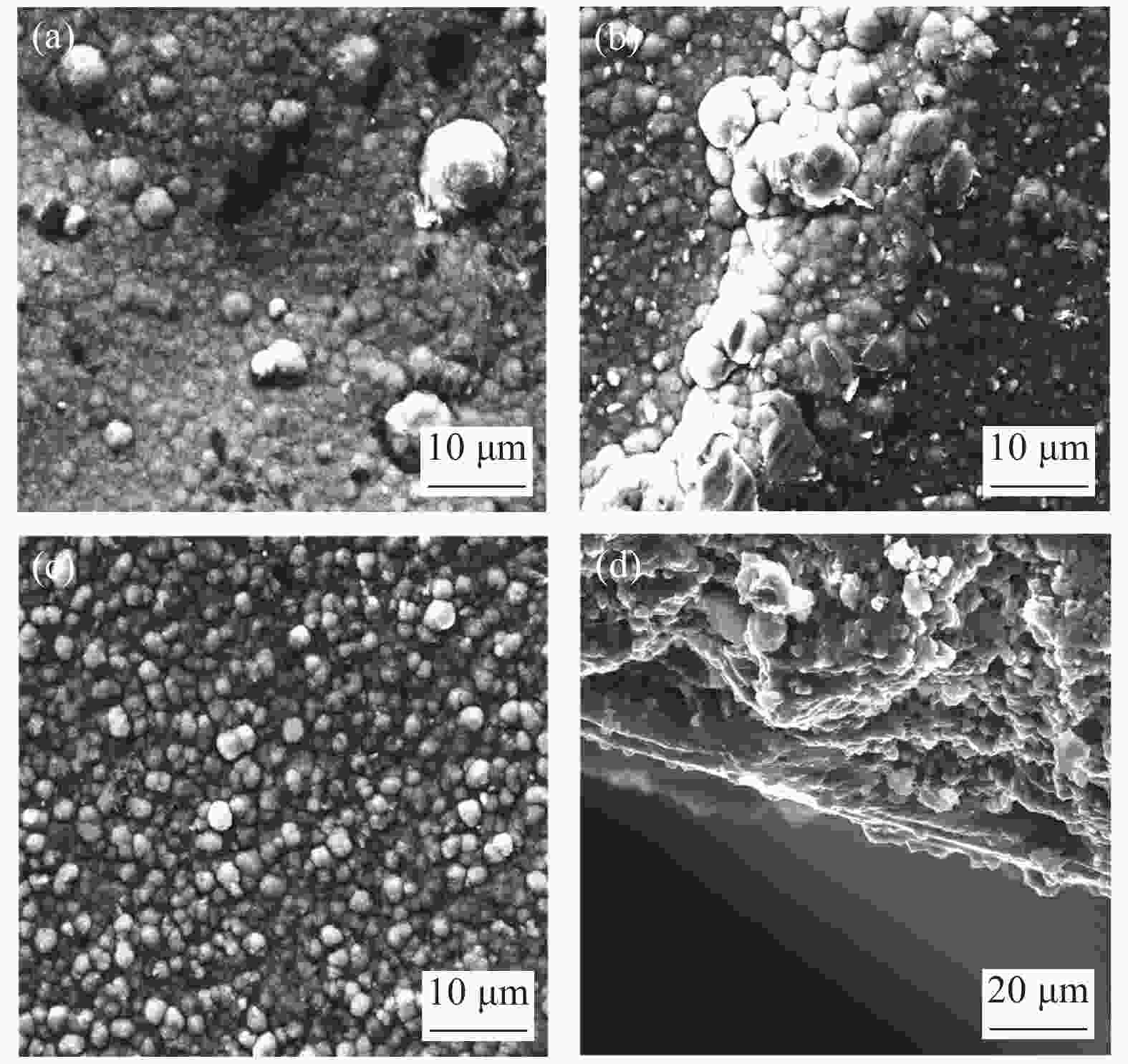
图 2 三种方法制备得到的钯复合膜的SEM图:(a) ELP(化学镀);(b) SELP(辅助抽吸化学镀);(c) VELP(真空化学镀)和(d) VELP得到的钯膜的截面形貌[10]
Figure 2. SEM images of palladium composite membranes prepared by three methods: (a) ELP(Electroless plating); (b) SELP(Assisted suction electroless plating); (c) VELP(Vacuum electroless plating) and (d) cross-section morphology of Pd membrane by VELP method[10]
表 1 各典型难熔氧化物修饰层组成的钯复合膜性能比较
Table 1. Comparison of the properties of palladium composite membranes composed of typical refractory oxide interlayers
Membranes Preparation method Thickness/
μmTemperature/℃ ΔP/kPa H2 permeability H2 flux H2 selectivity Ref. Pd/Fe2O3,Cr2O3/PSS ELP-PP 18 623-723 0-300 2.83-4.04×10−4 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}{\cdot \mathrm{P}\mathrm{a}}^{0.5}) $$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}} > 2000 $ [55] Pd/YSZ/PSS ELP 3 773 20 9.86×10−2 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}=595 $ [56] Pd/CeO2/PSS ELP-PP 15 623-723 100-200 4.74-6.35×10−4 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}} > 10000 $ [7] Pd/OMC/PSS ELP-PP 10 673 1.03×10−3 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}\ge 24000 $ [57] Pd/YSZ/Al2O3
hollow fiberVCFELP 4 773 100 0.075 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}=\mathrm{\infty } $ [6] Pd/TiO2/PSS ELP 5.0 723 500 1.58×10−3 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)0.355 mol/
($ \mathrm{m}\cdot \mathrm{s} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}} $=1700 [43] Pd/Al2O3/PSS ELP 4.4 773 800 2.94×10−3 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/\mathrm{H}\mathrm{e}}=1124 $ [58] Pd/SiO2/PSS ELP 2-6 773 50 2.7×10−6 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot \mathrm{P}\mathrm{a} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}=300-450 $ [60] Pd/Alumina sol/
γ-Al2O3/α-Al2O3ELP 4.5 723 100 0.16 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}=2072 $ [63] Pd/Pd-CeO2/PSS ELP-PP 9 350-450 100-200 4.46-6.39×10−4 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}} > 10000 $ [12] Pd/Pd-TiO2/PSS ELP-PP 9.7 623-723 50-250 2.80-4.17×10−4 mol/
($ {\mathrm{m}}^{2}\cdot \mathrm{s}\cdot {\mathrm{P}\mathrm{a}}^{0.5} $)$ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}}=\mathrm{\infty } $ [32] Notes:PSS is the porous stainless steel; $ {\mathrm{\alpha }}_{{\mathrm{H}}_{2}/{\mathrm{N}}_{2}},{\mathrm{\alpha }}_{{\mathrm{H}}_{2}/\mathrm{H}\mathrm{e}} $ is the ideal separation factor; YSZ is the yttria stabilized zirconia; OMC is the Ordered Mesoporous Ceria; ELP is Electroless plating; . 表 2 原位氧化PSS或加入不同难熔氧化物修饰层修饰的PSS的透氢量比较
Table 2. Comparison of hydrogen flux of PSS oxidized in situ or modified with different refractory oxide interlayers
Sample Temperature/K ΔP/kPa H2$ \mathrm{f} $lux/$ ({\mathrm{mol}}{\cdot \mathrm{m}}^{-2}\cdot {\mathrm{s}}^{-1} $) N2 flux/$({\mathrm{mol}} \cdot {\mathrm{m}}^{-2}\cdot {\mathrm{s}}^{-1} $) Ref. PSS 298 1 ~1.12 [32] TiO2/PSS(or Pd-TiO2/PSS) ~0.60 PSS 298 2 ~0.26 ~0.12 [56] YSZ/PSS ~0.11 ~0.03 PSS 673 1 3.61 1.48 [55] Fe2O3,Cr2O3(600℃)/PSS 2.36 0.82 表 3 各种钯基膜的H2S中毒及氢通量回收率比较
Table 3. poisoning and recovery of hydrogen flux for various Pd-based membranes
表 4 SMR或NG SR反应下钯复合膜的甲烷转化率比较
Table 4. Comparison of methane conversion for palladium composite membranes under SMR reaction
Membranes Thickness/
μmReactor
feed gasTemperature/
℃Reaction pressure/
kPaCatalyst
base metalSweep
gas rate/
(mL·min-1)$ {X}_{{\mathrm{C}\mathrm{H}}_{4}} $/
%S/C Active
area/cm2GHSV/
h−1Ref. Pd–Ag Tubular 50 CH4 450 300 Ni/Al2O3 98(N2) 50 2/1
(H2O/CH4)~ 3710 [85] Pd/PSS 13 CH4+
6 vol% CO2400 300 Ni 100 84 3.5/1 44 2600 [84] Pd/PSS 4-5 CH4 550 1013 Ru ~ 82 3 175 2000 [87] Pd/PSS 7 CH4 500 500 Ru/Al2O3 ~ 79.5 3 100 1700 [88] Pd/Al2O3 3.8 CH4 550 2500 Ni ~ 91 ~ 155.0 950 [86] Pd72Au28/YSZ/PSS 5.0 CH4 534 2800 Ru ~ 88 3 16.1 342 [90] Pd95.6-Au4.4/ZrO2/PSS 12 CH4 450 300 Ni 100 48 3.5/1 11.3 2600 [92] 86.63 wt%CH4
+5.86 wt%C2H6
+3.50 wt%C3H8、
+1.51 wt%C4H10
+2.50 wt%CO2450 300 Ni 100 37 3.5/1 11.3 2600 450 300 Ni 100 40%以上 3.5/1 11.3 2600 [93] Notes:$ {X}_{{\mathrm{C}\mathrm{H}}_{4}} $ is the methane conversion, S/C is the steam-to-carbon ratio; GHSV is the gas hourly space velocity. -
[1] ZHOU Q, LUO S F, ZHANG M, et al. Selective and efficient hydrogen separation of Pd–Au–Ag ternary alloy membrane[J]. International Journal of Hydrogen Energy, 2022, 47(26): 13054-13061. doi: 10.1016/j.ijhydene.2022.02.044 [2] 赵辰阳, 徐伟. 用于氢气纯化的负载型钯膜制备与性能研究[J]. 能源化工, 2019, 40(5): 31-36. doi: 10.3969/j.issn.1006-7906.2019.05.008ZHAO C Y, XU W. Research on preparation and properties of supported palladium membrane for hydrogen purification[J]. Energy Chemical Industry, 2019, 40(5): 31-36(in Chinese). doi: 10.3969/j.issn.1006-7906.2019.05.008 [3] KIADEHI D A, TAGHIZADEH M. Fabrication, characterization, and application of palladium composite membrane on porous stainless steel substrate with NaY zeolite as an intermediate layer for hydrogen purification[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2889-2904. doi: 10.1016/j.ijhydene.2018.12.058 [4] SANZ-VILLANUEVA D, ALIQUE D, VIZCAINO J A, et al. Pre-activation of SBA-15 intermediate barriers with Pd nuclei to increase thermal and mechanical resistances of pore-plated Pd-membranes[J]. International Journal of Hydrogen Energy, 2021, 46(38): 20198-20212. doi: 10.1016/j.ijhydene.2020.07.028 [5] 林定标, 唐春华, 李慧等. 多通道型高效钯复合膜在超高纯氢气提纯的应用[J]. 低温与特气, 2018, 36(2): 41-47.LIN D B, TANG C H, LI H, et al. The Application of Highly Efficient Multi-Channel Pd Composite Membranes in Ultra-pure Hydrogen Purification[J]. Low Temperature and Specialty Gases, 2018, 36(2): 41-47(in Chinese). [6] JI Y, SUN H F, WANG X B, et al. Vacuum-assisted continuous flow electroless plating approach for high performance Pd membrane deposition on ceramic hollow fiber lumen[J]. Journal of Membrane Science, 2022, 645: 120207. doi: 10.1016/j.memsci.2021.120207 [7] MARTINEZ-DIAZ D, SANZ R, CALLES A J, et al. H2 permeation increase of electroless pore-plated Pd/PSS membranes with CeO2 intermediate barriers[J]. Separation and Purification Technology, 2019, 216: 16-24. doi: 10.1016/j.seppur.2019.01.076 [8] 马玉钰, 李慧. 钯银合金膜制备研究进展[J]. 天然气化工(C1化学与化工), 2020, 45(5): 115-120.MA Y Y, LI H. Recent research progress in preparation of palladium-silver alloy films[J]. Natural Gas Chemical Industry, 2020, 45(5): 115-120(in Chinese). [9] MELENDEZ J, NOOIJER D N, COENEN K, et al. Effect of Au addition on hydrogen permeation and the resistance to H2S on Pd-Ag alloy membranes[J]. Journal of Membrane Science, 2017, 542: 329-341. doi: 10.1016/j.memsci.2017.08.029 [10] ZHANG X L, XIONG G X, YANG W S. A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability[J]. Journal of Membrane Science, 2008, 314(1-2): 226-237. doi: 10.1016/j.memsci.2008.01.051 [11] CALLES A J, SANZ R, ALIQUE D, et al. Influence of the selective layer morphology on the permeation properties for Pd-PSS composite membranes prepared by electroless pore-plating: Experimental and modeling study[J]. Separation and Purification Technology, 2018, 194: 10-18. doi: 10.1016/j.seppur.2017.11.014 [12] MARTINEZ-DIAZ D, ALIQUE D, CALLES A J, et al. Pd-thickness reduction in electroless pore-plated membranes by using doped-ceria as interlayer[J]. International Journal of Hydrogen Energy, 2020, 45(12): 7278-7289. doi: 10.1016/j.ijhydene.2019.10.140 [13] MARTINEZ-DIAZ D, LEO P, SANZ R, et al. Life cycle assessment of H2-selective Pd membranes fabricated by electroless pore-plating[J]. Journal of Cleaner Production, 2021, 316: 128229. doi: 10.1016/j.jclepro.2021.128229 [14] ALIQUE D, SANZ R, CALLES A J. 2 - Pd membranes by electroless pore-plating: synthesis and permeation behavior[J]. Current Trends and Future Developments on (Bio-) Membranes, 2020: 31-62. [15] LEE H Y, JANG Y, HAN H D, et al. Palladium-copper membrane prepared by electroless plating for hydrogen separation at low temperature[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106509. doi: 10.1016/j.jece.2021.106509 [16] MORREALE D B, CIOCCO V M, HOWARD H B, et al. Effect of hydrogen-sulfide on the hydrogen permeance of palladium–copper alloys at elevated temperatures[J]. Journal of Membrane Science, 2004, 241(2): 219-224. doi: 10.1016/j.memsci.2004.04.033 [17] HAN Z Z, XU K, LIAO N B, et al. Theoretical investigations of permeability and selectivity of Pd–Cu and Pd–Ni membranes for hydrogen separation[J]. 2021, 46(46): 23715-23722. [18] CHEN C H, MA Y H. The effect of H2S on the performance of Pd and Pd/Au composite membrane[J]. Journal of Membrane Science, 2010, 362(1-2): 535-544. doi: 10.1016/j.memsci.2010.07.002 [19] THOMAS G. Adsorption and separation of gases by colloid septa[J]. Philos Trans R Soc London, 1866, 156: 415-426. [20] KIM H D, KONG Y S, LEE G-H, et al. Effect of PBI-HFA surface treatments on Pd/PBI-HFA composite gas separation membranes[J]. International Journal of Hydrogen Energy, 2017, 42(36): 22915-22924. doi: 10.1016/j.ijhydene.2017.07.140 [21] MELENDEZ J, FERNANDEZ E, GALLUCCI F, et al. Preparation and characterization of ceramic supported ultra-thin (~1µm) Pd-Ag membranes[J]. Journal of Membrane Science, 2017, 528: 12-23. doi: 10.1016/j.memsci.2017.01.011 [22] HABIB A M, HARALE A, PAGLIERI S, et al. Palladium-alloy membrane reactors for fuel reforming and hydrogen production: a review[J]. Energy Fuels, 2021, 35(7): 5558-5593. doi: 10.1021/acs.energyfuels.0c04352 [23] LAURA BOSKO M, FONTANA D A, TARDITI A, et al. Advances in hydrogen selective membranes based on palladium ternary alloys[J]. International Journal of Hydrogen Energy, 2021, 46(29): 15572-15594. doi: 10.1016/j.ijhydene.2021.02.082 [24] OKAZAKI J, IKEDA T, PACHECO TANAKA A D, et al. An investigation of thermal stability of thin palladium–silver alloy membranes for high temperature hydrogen separation[J]. Journal of Membrane Science, 2011, 366(1-2): 212-219. doi: 10.1016/j.memsci.2010.10.011 [25] CHANDRASEKHAR N, SHOLL S D. Computational study of hydrogen induced lattice rearrangement and its influence on hydrogen permeance in Pd–Au alloys[J]. Journal of Alloys and Compounds, 2014, 609: 244-252. doi: 10.1016/j.jallcom.2014.04.156 [26] YUN S, TED OYAMA S. Correlations in palladium membranes for hydrogen separation: A review[J]. Journal of Membrane Science, 2011, 375(1-2): 28-45. doi: 10.1016/j.memsci.2011.03.057 [27] DOLAN D M. Non-Pd BCC alloy membranes for industrial hydrogen separation[J]. Journal of Membrane Science, 2010, 362(1-2): 12-28. doi: 10.1016/j.memsci.2010.06.068 [28] DOLAN D M, VIANO M D, LANGLEY J M, et al. Tubular vanadium membranes for hydrogen purification[J]. Journal of Membrane Science, 2018, 549: 306-311. doi: 10.1016/j.memsci.2017.12.031 [29] ORAKWE I, SHEHU H, GOBINA E. Preparation and characterization of palladium ceramic alumina membrane for hydrogen permeation[J]. International Journal of Hydrogen Energy, 2019, 44(20): 9914-9921. doi: 10.1016/j.ijhydene.2019.01.033 [30] FERNANDEZ E, MEDRANO A J, MELENDEZ J, et al. Preparation and characterization of metallic supported thin Pd–Ag membranes for hydrogen separation[J]. Chemical Engineering Journal, 2016, 305: 182-190. doi: 10.1016/j.cej.2015.09.119 [31] WEI L, YU J, HU X J, et al. Fabrication of H2-permeable palladium membranes based on pencil-coated porous stainless steel substrate[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13007-13012. doi: 10.1016/j.ijhydene.2012.05.064 [32] SANZ-VILLANUEVA D, ALIQUE D, VIZCAINO J A, et al. On the long-term stability of Pd-membranes with TiO2 intermediate layers for H2 purification[J]. International Journal of Hydrogen Energy, 2022, 47(21): 11402-11416. doi: 10.1016/j.ijhydene.2021.12.005 [33] 孟野, 江鹏, 史晓斌等. 抗氢脆-高通量氢分离钒合金膜研究进展[J]. 稀有金属材料与工程, 2021, 50(3): 1107-1112.MENG Y, JIANG P, SHI X B, et al. Research Progress on Hydrogen Separation Vanadium Alloy Membranes with High Flux and Hydrogen Embrittlement Resistance[J]. Rare Metal Materials and Engineering, 2021, 50(3): 1107-1112(in Chinese). [34] 康海涛, 姚春艳, 吴展华等. 透氢铌基钯合金膜[J]. 广东化工, 2022, 49(18): 28-30. doi: 10.3969/j.issn.1007-1865.2022.18.010KANG H T, YAO C Y, WU Z H, et al. Niobium-based Palladium Alloy Membranes for Hydrogen Purification[J]. Guangdong Chemical Industry, 2022, 49(18): 28-30(in Chinese). doi: 10.3969/j.issn.1007-1865.2022.18.010 [35] LI A, GRACE R J, JIM LIM C. Preparation of thin Pd-based composite membrane on planar metallic substrate: Part I: Pre-treatment of porous stainless steel substrate[J]. Journal of Membrane Science, 2007, 298(1-2): 175-181. doi: 10.1016/j.memsci.2007.04.016 [36] 钟博扬, 李芳芳, 陈长安等. 钯膜制备及渗氢性能研究[J]. 材料导报, 2016, 30(19): 63-69.ZHONG B Y, LI F F, CHEN C A, et al. Fabrication and Hydrogen Permeability of Palladium Membranes[J]. MATERIALS REPORTS, 2016, 30(19): 63-69(in Chinese). [37] JO S Y, LEE H C, KONG Y S, et al. Characterization of a Pd/Ta composite membrane and its application to a large scale high-purity hydrogen separation from mixed gas[J]. Separation and Purification Technology, 2018, 200: 221-229. doi: 10.1016/j.seppur.2017.12.019 [38] JO S Y, CHA J, LEE H C, et al. A viable membrane reactor option for sustainable hydrogen production from ammonia[J]. Journal of Power Sources, 2018, 400: 518-526. doi: 10.1016/j.jpowsour.2018.08.010 [39] KIM K, JUNG I. The permeability characteristics of non-porous membrane by C7H5F3/SiH4 plasma polymeric membrane[J]. Korean Journal of Chemical Engineering, 2000, 17(2): 149-155. doi: 10.1007/BF02707136 [40] ROA F, DOUGLAS WAY J, MCCORMICK L R, et al. Preparation and characterization of Pd–Cu composite membranes for hydrogen separation[J]. Chemical Engineering Journal, 2003, 93(1): 11-22. doi: 10.1016/S1385-8947(02)00106-7 [41] NORDIO M, SORESI S, MANZOLINI G, et al. Effect of sweep gas on hydrogen permeation of supported Pd membranes: Experimental and modeling[J]. International Journal of Hydrogen Energy, 2019, 44(8): 4228-4239. doi: 10.1016/j.ijhydene.2018.12.137 [42] KIADEHI D A, TAGHIZADEH M, RAMI D M. Preparation of Pd/SAPO-34/PSS composite membranes for hydrogen separation: Effect of crystallization time on the zeolite growth on PSS support[J]. Journal of Industrial and Engineering Chemistry, 2020, 81: 206-218. doi: 10.1016/j.jiec.2019.09.010 [43] 李梦珠, 李帅, 吕琴丽等. 多孔316L不锈钢管表面钯膜制备及性能表征[J]. 稀有金属, 2022, 46(4): 471-479.LI M Z, LI S, LV Q L, et al. Preparation and Characterization of Pd Membranes on Porous 316L Stainless Steel[J]. CHINESE JOURNAL OF RARE METALS, 2022, 46(4): 471-479(in Chinese). [44] GIL G A, REIS M H M, CHADWICK D, et al. A highly permeable hollow fibre substrate for Pd/Al2O3 composite membranes in hydrogen permeation[J]. Int. J. Hydrogen Energy, 2015, 40: 3249-3258. doi: 10.1016/j.ijhydene.2015.01.021 [45] 卢成壮, 张瑞云, 程健等. 用于燃料电池气体分离钯复合膜的制备与性能评价[J]. 应用化工, 2020, 49(S2): 53-56.LU C Z, ZHANG R Y, CHENG J, et al. Preparation and performance evaluation of palladium composite membrane used for fuel cell gas separation[J]. Applied Chemical Industry, 2020, 49(S2): 53-56(in Chinese). [46] WANG P W, THOMAS S, ZHANG L X, et al. H2/N2 gaseous mixture separation in dense Pd/α-Al2O3 hollow fiber membranes: Experimental and simulation studies[J]. Separation and Purification Technology, 2006, 52(1): 177-185. doi: 10.1016/j.seppur.2006.04.007 [47] LIM S, MAGNONE E, SHIN C M, et al. Simple scalable approach to advanced membrane module design and hydrogen separation performance using twelve replaceable palladium-coated Al2O3 hollow fibre membranes[J]. Journal of Industrial and Engineering Chemistry, 2022, 114: 391-401. doi: 10.1016/j.jiec.2022.07.028 [48] HU X J, HUANG Y, SHU S L, et al. Toward effective membranes for hydrogen separation: Multichannel composite palladium membranes[J]. Journal of Power Sources, 2008, 181(1): 135-139. doi: 10.1016/j.jpowsour.2008.02.091 [49] 刘金霞, 唐春华, 李慧等. 具有空隙结构的钯复合膜的制备与研究[J]. 天然气化工(C1化学与化工), 2019, 44(4): 1-5.LIU J X, TANG C H, LI H, et al. Fabrication and testing of Pd composite membranes with a novel gap structure[J]. Natural Gas Chemical Industry, 2019, 44(4): 1-5(in Chinese). [50] MOBARAKE D M, SAMIEE L. Preparation of palladium/NaX/PSS membrane for hydrogen separation[J]. International Journal of Hydrogen Energy, 2016, 41(1): 79-86. doi: 10.1016/j.ijhydene.2015.10.009 [51] UEMIYA S, Sep. Purif. Method, 1999, 28(51). [52] MARDILOVICH P I, ENGWALL E, MA H Y. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes[J]. Desalination, 2002, 144(1-3): 85-89. doi: 10.1016/S0011-9164(02)00293-X [53] PEREIRA I A, PEREZ P, RODRIGUES C S, et al. Deposition of Pd–Ag thin film membranes on ceramic supports for hydrogen purification/separation[J]. Materials Research Bulletin, 2015, 61: 528-533. doi: 10.1016/j.materresbull.2014.10.055 [54] MA Y H, AKIS B C, AYTURK M E, et al. Characterization of Intermetallic Diffusion Barrier and Alloy Formation for Pd/Cu and Pd/Ag Porous Stainless Steel Composite Membranes[J]. Ind. Eng. Chem. Res, 2004, 43: 2936-2945. doi: 10.1021/ie034002e [55] FURONES L, ALIQUE D. Interlayer Properties of In-Situ Oxidized Porous Stainless Steel for Preparation of Composite Pd Membranes[J]. ChemEngineering, 2017, 2: 1. doi: 10.3390/chemengineering2010001 [56] HAN J-Y, KIM C-H, LIM H, et al. Diffusion barrier coating using a newly developed blowing coating method for a thermally stable Pd membrane deposited on porous stainless-steel support[J]. International Journal of Hydrogen Energy, 2017, 42(17): 12310-12319. doi: 10.1016/j.ijhydene.2017.03.053 [57] MARTINEZ-DIAZ D, MARTINEZ DEL MONTE D, GARCIA-ROJAS E, et al. Comprehensive permeation analysis and mechanical resistance of electroless pore-plated Pd-membranes with ordered mesoporous ceria as intermediate layer[J]. Separation and Purification Technology, 2021, 258(Part 1): 118066. [58] CHI Y-H, YEN P-S, JENG M-S, et al. Preparation of thin Pd membrane on porous stainless steel tubes modified by a two-step method[J]. International Journal of Hydrogen Energy, 2010, 35(12): 6303-6310. doi: 10.1016/j.ijhydene.2010.03.066 [59] GESTEL V T, HAULER F, BRAM M, et al. Synthesis and characterization of hydrogen-selective sol–gel SiO2 membranes supported on ceramic and stainless steel supports[J]. Separation and Purification Technology, 2014, 121: 20-29. doi: 10.1016/j.seppur.2013.10.035 [60] SU C, JIN T, KURAOKA K, et al. Thin palladium film supported on SiO2-modified porous stainless steel for a high-hydrogen-flux membrane[J]. Ind Eng Chem Res, 2005, 44: 3053-3058. doi: 10.1021/ie049349b [61] BROGLIA M, PINACCI P, RADAELLI M, et al. Desalination, 2009, 245: 508. [62] BOTTINO A, BROGLIA M, CAPANNELLI G, et al. Sol–gel synthesis of thin alumina layers on porous stainless steel supports for high temperature palladium membranes[J]. International Journal of Hydrogen Energy, 2014, 39(9): 4717-4724. doi: 10.1016/j.ijhydene.2013.11.096 [63] ZHENG L, LI H, XU H Y. “Defect-free” interlayer with a smooth surface and controlled pore-mouth size for thin and thermally stable Pd composite membranes[J]. International Journal of Hydrogen Energy, 2016, 41(2): 1002-1009. doi: 10.1016/j.ijhydene.2015.09.024 [64] ZHANG L, REN Y L, LUO Q, et al. A Novel Method to from Well-adhered γ-Al2O3 Coating in 316L Stainless Steel Microchannels[J]. Energy Procedia, 2015, 75: 2044-2048. doi: 10.1016/j.egypro.2015.07.278 [65] MOBARAKE D M, JAFARI P, IRANI M. Preparation of Pd-based membranes on Pd/TiO2 modified NaX/PSS substrate for hydrogen separation: Design and optimization[J]. Microporous and Mesoporous Materials, 2016, 226: 369-377. doi: 10.1016/j.micromeso.2016.02.022 [66] GUO Y, ZHANG X F, DENG H, et al. A novel approach for the preparation of highly stable Pd membrane on macroporous α-Al2O3 tube[J]. Journal of Membrane Science, 2010, 362(1-2): 241-248. doi: 10.1016/j.memsci.2010.06.050 [67] HUANG Y, LIU Q, JIN X X, et al. Coating the porous Al2O3 substrate with a natural mineral of Nontronite-15A for fabrication of hydrogen-permeable palladium membranes[J]. International Journal of Hydrogen Energy, 2020, 45(12): 7412-7422. doi: 10.1016/j.ijhydene.2019.04.102 [68] TONG J H, SU L L, HARAYA K, et al. Thin Pd membrane on α-Al2O3 hollow fiber substrate without any interlayer by electroless plating combined with embedding Pd catalyst in polymer template[J]. Journal of Membrane Science, 2008, 310(1-2): 93-101. doi: 10.1016/j.memsci.2007.10.053 [69] JAMSHIDI S, BABALUO A A. Preparation and evaluation of Pd membrane on supports activated by PEG embedded Pd nanoparticles for ATR membrane reactor[J]. Chemical Engineering and Processing - Process Intensification, 2020, 147: 107736. doi: 10.1016/j.cep.2019.107736 [70] 郭宇, 吴红梅, 周立岱等. 聚乙烯醇修饰多孔氧化铝载体及其表面化学镀制备透氢钯膜[J]. 电镀与涂饰, 2017, 36(4): 194-197.GUO Y, WU H M, ZHOU L D, et al. Modification of porous alumina substrate by polyvinyl alcohol and preparation of palladium membrane on its surface by electroless plating[J]. ELERTROPATING & FINISHING, 2017, 36(4): 194-197(in Chinese). [71] YUN S, TED OYAMA S. Correlations in palladium membranes for hydrogen separation: A review[J]. Journal of Membrane Science, 2011, 375(1-2): 28-45. doi: 10.1016/j.memsci.2011.03.057 [72] IULIANELLI A, MANISCO M, BION N, et al. Sustainable H2 generation via steam reforming of biogas in membrane reactors: H2S effects on membrane performance and catalytic activity[J]. International Journal of Hydrogen Energy, 2021, 46(57): 29183-29197. doi: 10.1016/j.ijhydene.2020.10.038 [73] KULPRATHIPANJA A, ALPTEKIN O G, FALCONER L J, et al. Pd and Pd–Cu membranes: inhibition of H2 permeation by H2S[J]. Journal of Membrane Science, 2005, 254(1-2): 49-62. doi: 10.1016/j.memsci.2004.11.031 [74] KAMAKOTI P, MORREALE B D, CIOCCO M V, et al. Prediction of hydrogen flux through sulfur-tolerant binary alloy membranes[J]. Science, 2005, 307(5709): 569-573. doi: 10.1126/science.1107041 [75] DEEPTI, KUMAR H, TRIPATHI A, et al. Improved hydrogen sensing behaviour in ion-irradiated Pd-Au alloy thin films[J]. Sensors and Actuators B:Chemical, 2019, 301: 127006. doi: 10.1016/j.snb.2019.127006 [76] MUNDSCHAU M V, XIE X, EVENSON C R, et al. Dense inorganic membranes for production of hydrogen from methane and coal with carbon dioxide sequestration[J]. Catalysis Today, 2006, 118(1-2): 12-23. doi: 10.1016/j.cattod.2006.01.042 [77] GADE K S, DEVOSS J S, COULTER E K, et al. Palladium–gold membranes in mixed gas streams with hydrogen sulfide: Effect of alloy content and fabrication technique[J]. Journal of Membrane Science, 2011, 378(1-2): 35-41. doi: 10.1016/j.memsci.2010.11.044 [78] JAZANI O, BENNETT J, LIGUORI S. Carbon-low, renewable hydrogen production from methanol steam reforming in membrane reactors – a review[J]. Chemical Engineering and Processing - Process Intensification, 2023, 189: 109382. doi: 10.1016/j.cep.2023.109382 [79] WIM ELSEVIERS W M, HASSETT F P, NAVARRE J-L. 50 Years of PSA technology for H2 purification, UOP. [80] NAQUASH A, QYYUM A M, CHANIAGO D Y, et al. Separation and purification of syngas-derived hydrogen: A comparative evaluation of membrane- and cryogenic-assisted approaches[J]. Chemosphere, 2023, 313: 137420. doi: 10.1016/j.chemosphere.2022.137420 [81] SARI R, DEWI R, PARDI, et al. Morphology of one-time coated palladium-alumina composite membrane prepared by sol-gel process and electroless plating technique[J]. IOP Conference Series: Materials Science and Engineering, 2018, 334. [82] HABIB A M, HAQUE A M, HARALE A, et al. Palladium-alloy membrane reactors for fuel reforming and hydrogen production: Hydrogen Production Modeling[J]. Case Studies in Thermal Engineering, 2023, 49: 103359. doi: 10.1016/j.csite.2023.103359 [83] ARRATIBEL A, TANAKA P A, LASO I, Martin van Sint Annaland, et al. Development of Pd-based double-skinned membranes for hydrogen production in fluidized bed membrane reactors[J]. Journal of Membrane Science, 2018, 550: 536-544. doi: 10.1016/j.memsci.2017.10.064 [84] ANZELMO B, WILCOX J, LIGUORI S. Natural gas steam reforming reaction at low temperature and pressure conditions for hydrogen production via Pd/PSS membrane reactor[J]. Journal of Membrane Science, 2017, 522: 343-350. doi: 10.1016/j.memsci.2016.09.029 [85] IULIANELLI A, MANZOLINI G, DE FALCO M, et al. H2 production by low pressure methane steam reforming in a Pd–Ag membrane reactor over a Ni-based catalyst: Experimental and modeling[J]. International Journal of Hydrogen Energy, 2010, 35(20): 11514-11524. doi: 10.1016/j.ijhydene.2010.06.049 [86] SARIC M, VAN DELFT C Y, SUMBHARAJU R, et al. Steam reforming of methane in a bench-scale membrane reactor at realistic working conditions[J]. Catalysis Today, 2012, 193(1): 74-80. doi: 10.1016/j.cattod.2012.04.009 [87] KIM C-H, HAN J-Y, KIM S, et al. Hydrogen production by steam methane reforming in a membrane reactor equipped with a Pd composite membrane deposited on a porous stainless steel[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7684-7692. doi: 10.1016/j.ijhydene.2017.11.176 [88] KIM C-H, HAN J-Y, LIM H, et al. Methane steam reforming using a membrane reactor equipped with a Pd-based composite membrane for effective hydrogen production[J]. International Journal of Hydrogen Energy, 2018, 43(11): 5863-5872. doi: 10.1016/j.ijhydene.2017.10.054 [89] UPADHYAY M, LEE H, KIM A, et al. CFD simulation of methane steam reforming in a membrane reactor: Performance characteristics over range of operating window[J]. International Journal of Hydrogen Energy, 2021, 46(59): 30402-30411. doi: 10.1016/j.ijhydene.2021.06.178 [90] ABU EI HAWA W H, LUNDIN B S-T, PATKI S N, et al. Steam methane reforming in a PdAu membrane reactor: Long-term assessment[J]. International Journal of Hydrogen Energy, 2016, 41(24): 10193-10201. doi: 10.1016/j.ijhydene.2016.04.244 [91] NAYEBOSSADRI S, SPEIGHT D J, BOOK D. Hydrogen separation from blended natural gas and hydrogen by Pd-based membranes[J]. International Journal of Hydrogen Energy, 2019, 44(55): 29092-29099. doi: 10.1016/j.ijhydene.2019.03.044 [92] ANZELMO B, WILCOX J, LIGUORI S. Hydrogen production via natural gas steam reforming in a Pd-Au membrane reactor. Comparison between methane and natural gas steam reforming reactions[J]. Journal of Membrane Science, 2018, 568: 113-120. doi: 10.1016/j.memsci.2018.09.054 [93] ANZELMO B, WILCOX J, LIGUORI S. Hydrogen production via natural gas steam reforming in a Pd-Au membrane reactor. Investigation of reaction temperature and GHSV effects and long-term stability[J]. Journal of Membrane Science, 2018, 565: 25-32. doi: 10.1016/j.memsci.2018.07.069 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 62
- HTML全文浏览量: 67
- 被引次数: 0




 下载:
下载: