Preparation of lignin surface-functionalized MXene nanosheets and its U(VI)adsorption properties
-
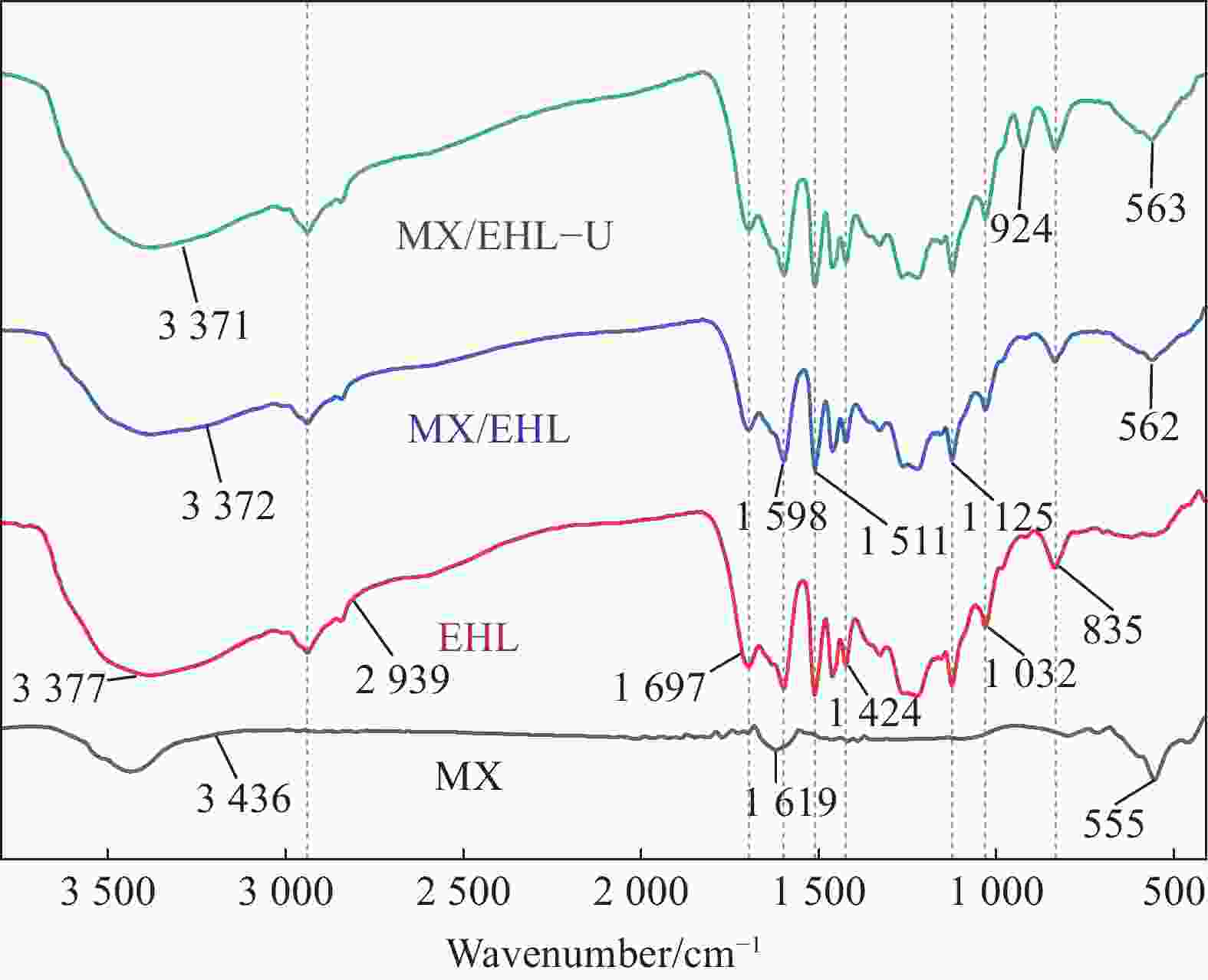
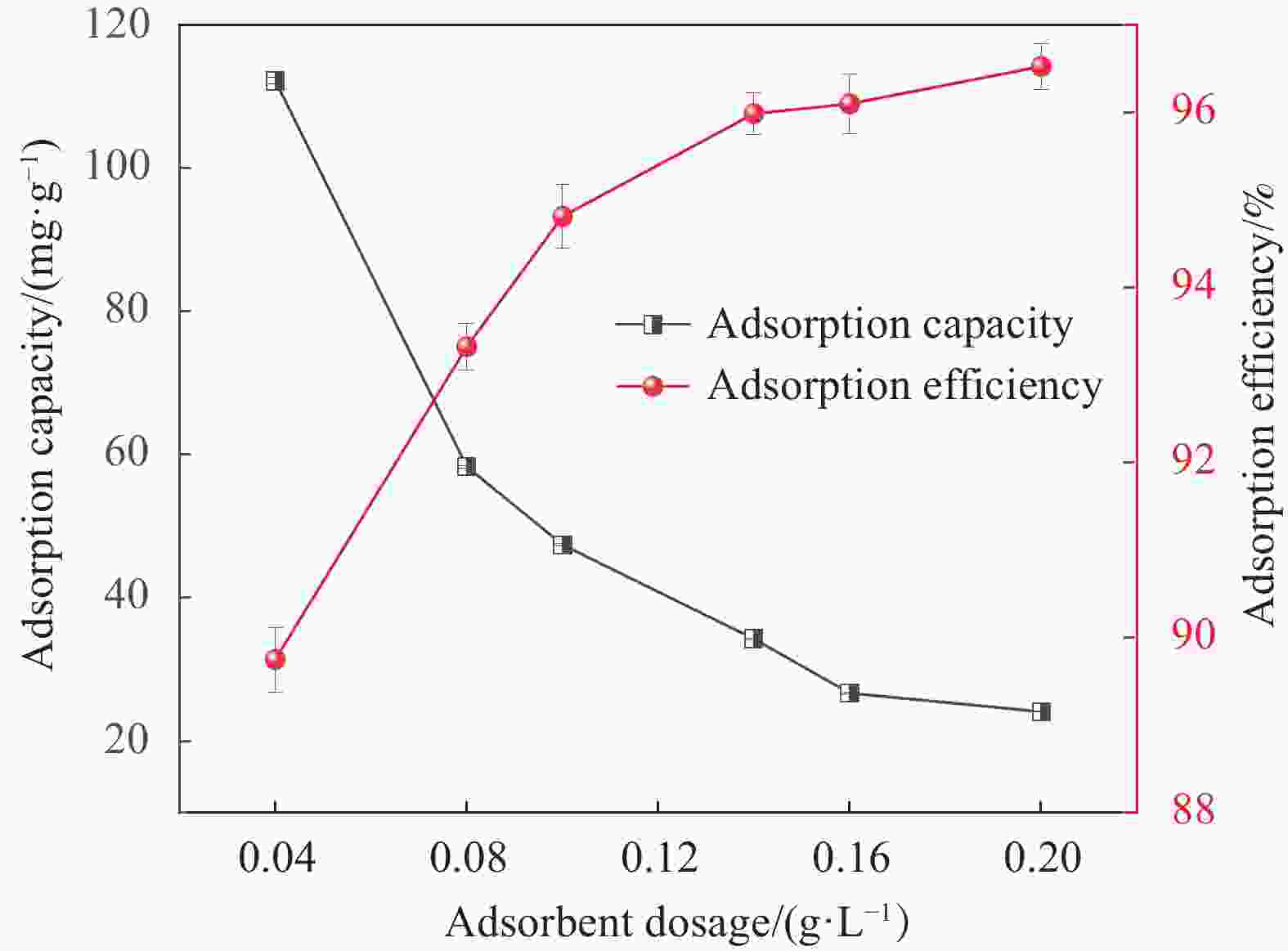
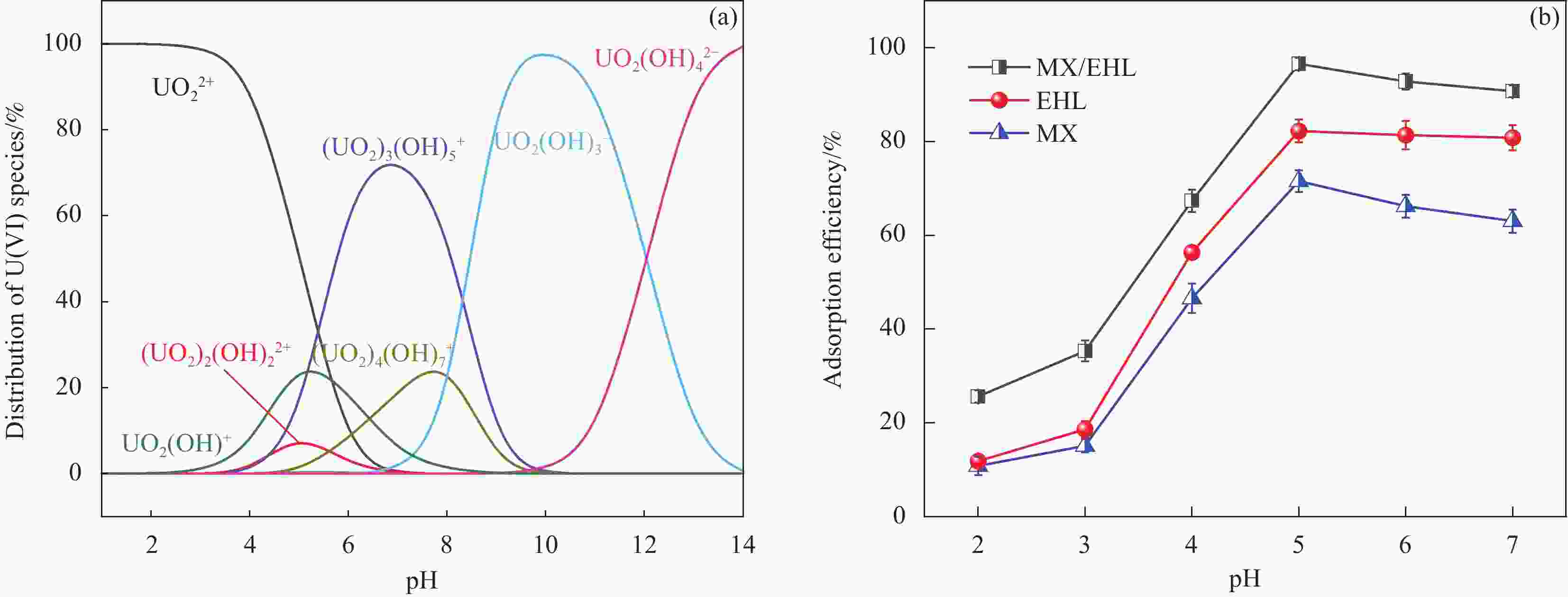
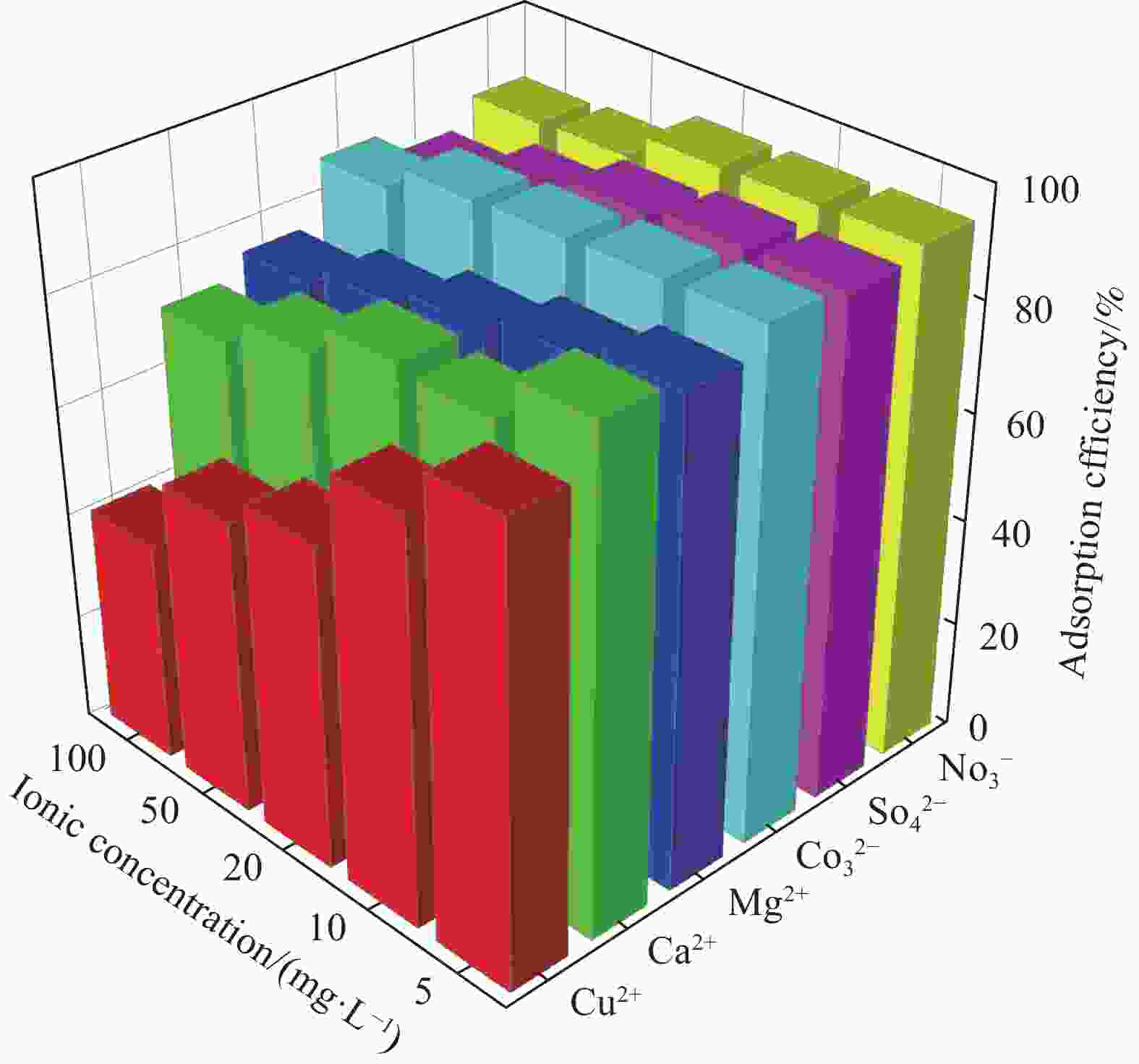
摘要: 为了进一步改善MXene纳米材料对模拟放射性废水中U(Ⅵ)的吸附性能,利用天然资源酶水解木质素(EHL)作为生物表面活性剂对MXene进行表面功能化处理,采用SEM-EDS、XRD及FTIR对改性前后的材料进行了表征分析,并在吸附实验中探究了pH、温度、反应时间、干扰离子及不同初始U(Ⅵ)浓度等因素对除U(Ⅵ)效果的影响。结果表明,EHL阻止了MXene纳米片的聚集堆叠,并且引入了大量活性官能团,提高了EHL功能化MXene纳米片的吸附性能。在MXene与EHL的质量比为1∶5、投加量为0.1 g·L−1、pH为5、温度为303 K时,对U(VI)的最大吸附容量为231.95 mg·g−1。此外,吸附动力学和等温线分析表明,拟二阶动力学模型和Frendlich等温线模型能很好的拟合此吸附过程,热力学分析表明其吸附过程是自发吸热的。经历5次循环再生后,对U(VI)的去除率仍在80%以上。表征分析结果表明,MX/EHL与U(VI)之间相互作用机制包括离子交换、静电吸引以及与含氧官能团之间的络合作用。基于此研究,MX/EHL作为一种环境友好型吸附材料,对去除废水中的U(VI)具有巨大潜力。Abstract: In order to further improve the adsorption performance of MXene nanomaterials on U(Ⅵ) in simulated radioactive wastewater, the surface functionalization of MXene was carried out by using natural resources of enzymatically hydrolyzed lignin (EHL) as a biosurfactant, and the materials before and after the modification were characterized and analyzed by using SEM-EDS, XRD, and FTIR, and the effects of pH, temperature, and the adsorption experiments were explored, reaction time, interfering ions and different initial U(VI) concentrations on the effect of U(VI) removal. The results show that EHL prevents the re-stacking of MXene nanosheets and introduces a large number of active functional groups, which improves the adsorption performance of EHL-functionalized MXene nanosheets. The maximum adsorption capacity for U(VI) is 231.95 mg·g−1 at the mass ratio of MXene to EHL of 1∶5, the dosage of 0.1 g·L−1, pH = 5, and the temperature of 303 K. In addition, the adsorption kinetic and isotherm analyses show that the proposed second-order kinetic model and the Frendlich isotherm model fit this adsorption process well, and the thermodynamic analyses indicate that its adsorption process is spontaneous heat absorption. After five cycles of regeneration, the removal rate of U(VI) is still above 80%. Characterization results reveals that the interaction mechanisms between MX/EHL and U(VI) involve ion exchange, electrostatic attraction, and complexation with oxygen-containing functional groups. Based on this study, MX/EHL has great potential as an environmentally friendly adsorbent material for the removal of U(VI) from wastewater.
-
Key words:
- MXene /
- lignin /
- nanomaterials /
- U(VI) /
- adsorption performance
-
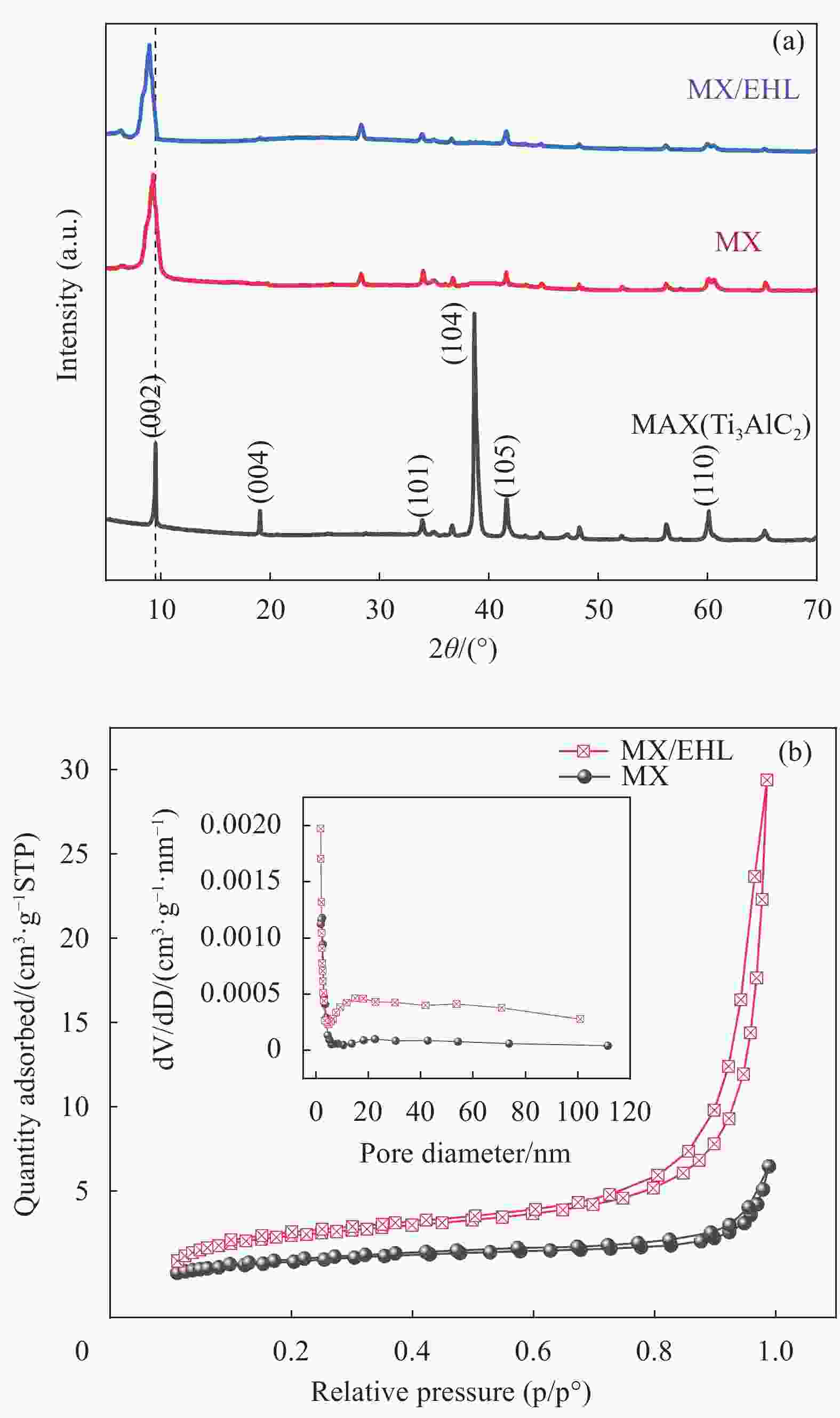
表 1 MX和MX/EHL的孔隙结构参数
Table 1. Pore structure parameters of MX and MX/EHL
Material Surface area/
(m2·g−1)Pore volume/
(cm3·g−1)Pore diameter/
nmMX 3.8297 0.0100 10.4628 MX/EHL 8.7751 0.0455 20.7320 表 2 MX/EHL对U(VI)的吸附动力学参数
Table 2. The adsorption kinetic parameters of U(VI) on MX/EHL
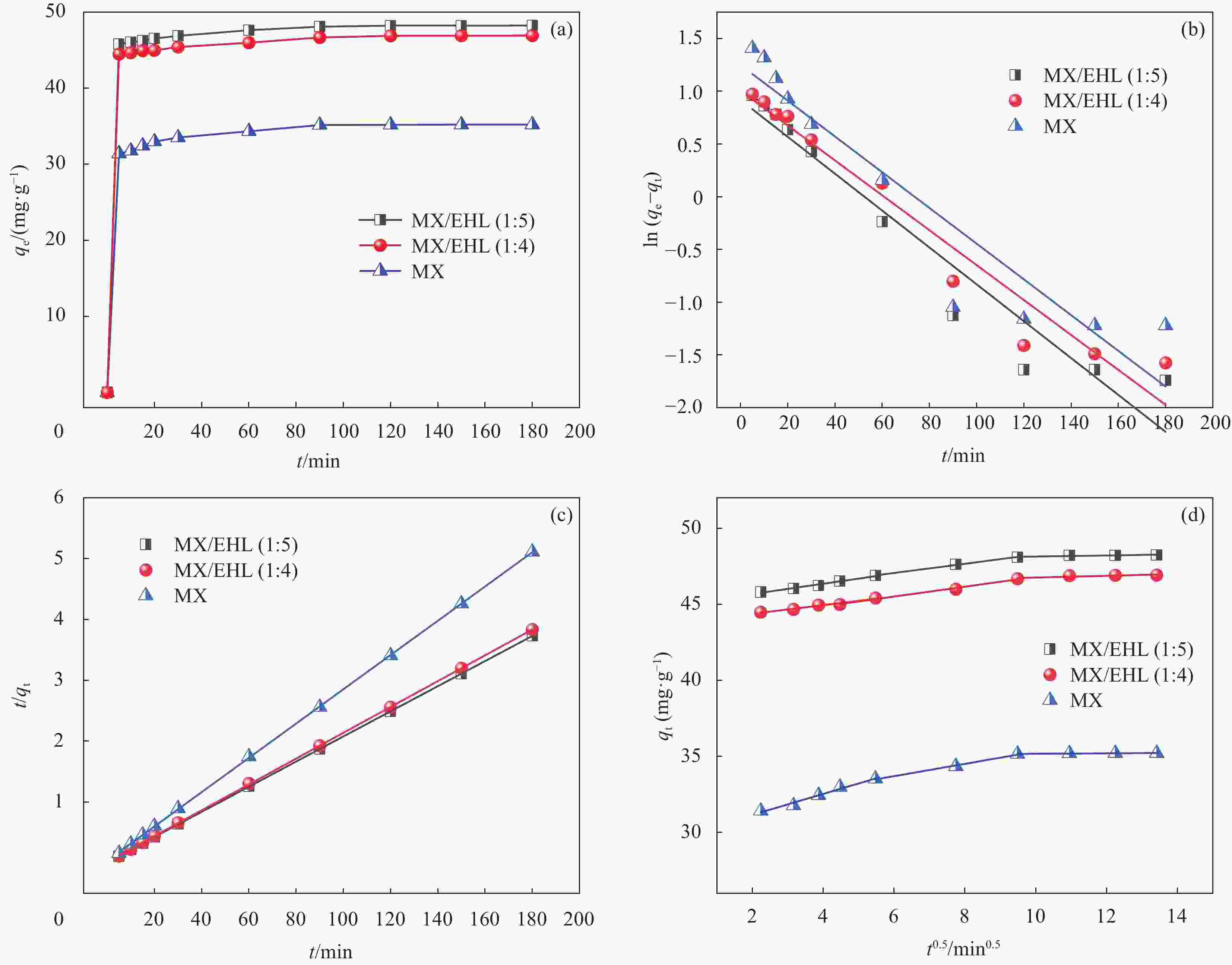
Kinetic model Name of sample MX MX/EHL(1∶4) MX/EHL(1∶5) qe,exp/(mg·g−1) 35.22 46.92 48.24 Pseudo-first-order k1/min−1 0.017 0.017 0.018 qe,cal/(mg·g−1) 3.487 2.737 2.502 R2 0.882 0.949 0.930 Pseudo-second-order k2/min−1 0.021 0.027 0.030 qe,cal/(mg·g−1) 35.51 47.13 48.43 R2 0.999 0.999 0.999 Intraparticle diffusion kp1/(mg·(g·min0.5)−1) 0.688 0.282 0.338 C1 29.782 43.819 45.002 R12 0.973 0.958 0.981 Intraparticle diffusion kp2/(mg·(g·min0.5)−1) 0.406 0.324 0.303 C2 31.266 43.568 45.243 R22 0.989 0.986 0.994 kp3/(mg·(g·min0.5)−1) 0.015 0.058 0.035 C3 35.029 46.175 47.794 R32 0.804 0.653 0.615 Notes: qe,exp is the actual adsorption capacity at adsorption equilibrium; qe,cal is the calculated adsorption capacity at adsorption equilibrium; k1 and k2 are the adsorption rate constants of the pseudo-first and pseudo-second, respectively ; R2 is the correlation coefficient; kp1, kp2, kp3 are the adsorption rate constants of intraparticle diffusion. 表 3 Langmuir、Freundlich和Dubinin‒Radushkevich吸附等温线模型的相关参数
Table 3. Parameters associated with Langmuir, Freundlich and Dubinin-Radushkevich adsorption isotherm models
T/K Langmuir Freundlich Dubinin‒Radushkevich qmax/(mg·g−1) KL/(L∙mg−1) R2 KF 1/n R2 qDR E R2 293 K 205.493 0.164 0.890 48.175 0.399 0.982 115.99 1.879 0.554 298 K 217.057 0.221 0.925 58.802 0.378 0.989 129.09 2.077 0.607 303 K 231.947 0.251 0.924 65.565 0.379 0.997 138.33 2.337 0.627 Notes: qmax is the maximum adsorption capacity; KL is the Langmuir adsorption equilibrium constant; KF and n are the constants that are related to the adsorption capacity and the adsorption intensity, respectively; R is the universal gas constant (8.312 J·mol−1·K−1), T is the temperature (K); E is the average free energy of adsorption. 表 5 不同吸附剂对U(VI)的吸附去除效果对比
Table 5. Comparison of adsorption and removal effects of different adsorbents on U(VI)
Adsorbent pH T/K qmax/(mg·g−1) Reference C-TC 5 308 165.43 [20] MXene/SA 4 298 126.82 [34] C-TC-CS 6 313 141.96 [36] PANI/Ti3C2Tx 5 298 102.8 [37] PAO/Ti3C2Tx 4 298 98.04 [38] Ti3C2-AO-PA 8.3 298 81.1 [47] MX/EHL 5 303 231.95 This work Notes: C-TC is Chloroacetic acid modified-Ti3C2Tx; MXene/SA is MXene composite sodium alginate gel microsphere; C-TC-CS is chloroacetic acid-modified MXene-CS gel microspheres; PANI/Ti3C2Tx is polyaniline modified Mxene composites; PAO/Ti3C2Tx is Polyamidoxime functionalized MXene composite; Ti3C2-AO-PA is polyamide enhanced amidoxime-functionalized Ti3C2 nanosheet; T is the reaction temperature; qmax is the maximum adsorption capacity. 表 4 MX/EHL吸附U(VI)的热力学参数
Table 4. Thermodynamic parameters of MX/EHL adsorption of U(VI)
T/K lnK0 ΔG0/(kJ·mol−1) ΔH0/(kJ·mol−1) ΔS0/(J·(mol·K)−1) 293 K 4.69 −11.43 38.89 175.26 298 K 5.00 −12.39 303 K 5.23 −13.18 Notes: T is the thermodynamic temperature; K0 is the equilibrium constant at different temperatures; ΔH0 is the standard enthalpy change; ΔG0 is the standard free energy change; ΔS0 is the standard entropy change. -
[1] YUAN D, ZHANG S, XIANG Z, et al. Highly Efficient Removal of Uranium from Aqueous Solution Using a Magnetic Adsorbent Bearing Phosphine Oxide Ligand: A Combined Experimental and Density Functional Theory Study[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9619-27. [2] HUANG S, JIANG S, PANG H, et al. Dual functional nanocomposites of magnetic MnFe2O4 and fluorescent carbon dots for efficient U(VI) removal[J]. Chemical Engineering Journal, 2019, 368: 941-50. doi: 10.1016/j.cej.2019.03.015 [3] He Z, Huang D, Yue G, et al. Ca2+ induced 3D porous MXene gel for continuous removal of phosphate and uranium[J]. Applied Surface Science, 2021, 570: 150804. doi: 10.1016/j.apsusc.2021.150804 [4] ZHANG S, YUAN D, ZHANG Q, et al. Highly efficient removal of uranium from highly acidic media achieved using a phosphine oxide and amino functionalized superparamagnetic composite polymer adsorbent[J]. Journal of Materials Chemistry A, 2020, 8(21): 10925-34. doi: 10.1039/D0TA01633K [5] ZAHERI P, DAVARKHAH R. Selective separation of uranium from sulfuric acid media using a polymer inclusion membrane containing alamine336[J]. Chem-ical Papers, 2020, 74(8): 2573-81. doi: 10.1007/s11696-019-01029-9 [6] ORREGO P, HERNáNDEZ J, REYES A. Uranium and molybdenum recovery from copper leaching solutions using ion exchange[J]. Hydrometallurgy, 2019, 184: 116-22. doi: 10.1016/j.hydromet.2018.12.021 [7] CHEN J, HUANG Q, HUANG H, et al. Recent progress and advances in the environmental applications of MXene related materials[J]. Nanoscale, 2020, 12(6): 3574-92. doi: 10.1039/C9NR08542D [8] YU H, WANG Y, JING Y, et al. Surface Modified MXene-Based Nanocomposites for Electrochemical Energy Conversion and Storage[J]. Small, 2019, 15(25): 1901503. doi: 10.1002/smll.201901503 [9] ZHOU Z, LIU J, ZHANG X, et al. Ultrathin MXene/calcium alginate aerogel film for highper-formance electromagnetic interference shielding[J]. Advanced Materials Interfaces, 2019, 6(6): 1802040. doi: 10.1002/admi.201802040 [10] SINHA A, DHANJAI, ZHAO H, et al. MXene: An emerging material for sensing and biosensing[J]. TrAC Trends in Analytical Chemistry, 2018, 105: 424-35. doi: 10.1016/j.trac.2018.05.021 [11] ZHANG Y, WANG L, ZHANG N, et al. Adsorptive environmental applications of MXene nanomaterials: a review[J]. RSC Adv, 2018, 8(36): 19895-905. doi: 10.1039/C8RA03077D [12] YING Y, LIU Y, WANG X, et al. Two-dimensional titanium carbide for efficiently reductive removal of highly toxic chromium(VI) from water[J]. ACS Appl Mater Interfaces, 2015, 7(3): 1795-803. doi: 10.1021/am5074722 [13] SHAHZAD A, RASOOL K, MIRAN W, et al. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water[J]. ACS Sustainable Che-mistry & Engineering, 2017, 5(12): 11481-8. [14] ZHANG P, WANG L, DU K, et al. Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets[J]. J Hazard Mater, 2020, 396: 122731. doi: 10.1016/j.jhazmat.2020.122731 [15] ZHANG F, LI S, ZHANG Q, et al. Adsorption of different types of surfactants on graphene oxide[J]. Journal of Molecular Liquids, 2019, 276: 338-46. doi: 10.1016/j.molliq.2018.12.009 [16] MENG Y, LU J, CHENG Y, et al. Lignin-based hydrogels: A review of preparation, properties, and application[J]. Int J Biol Macromol, 2019, 135: 1006-19. doi: 10.1016/j.ijbiomac.2019.05.198 [17] LUO R, ZHANG W, HU X, et al. Preparation of sodium ligninsulfonate functionalized MXene using hexach-lorocyclotriphosphazene as linkage and its adsorption applications[J]. Applied Surface Science, 2022, 602: 154197 doi: 10.1016/j.apsusc.2022.154197 [18] WANG S, LIU Y, Lü Q-F, et al. Facile preparation of biosurfactant-functionalized Ti2CTx MXene nanosheets with an enhanced adsorption performance for Pb(II) ions[J]. Journal of Molecular Liquids, 2020, 297: 111810. doi: 10.1016/j.molliq.2019.111810 [19] ZHANG K N, WANG C Z, LU Q F, et al. Enzymatic hydrolysis lignin functionalized Ti(3)C(2)T(x) nanosh-eets for effective removal of MB and Cu(2+) ions[J]. Int J Biol Macromol, 2022, 209(Pt A): 680-91. [20] XIE L, YAN J, LIU Z, et al. Synthesis of a Two-Dimensional MXene Modified by Chloroacetic Acid and Its Adsorption of Uranium[J]. ChemistrySelect, 2022, 7(1): e202103583. doi: 10.1002/slct.202103583 [21] Lü Q-F, LUO J-J, LIN T-T, et al. Novel Lignin–Poly(N-methylaniline) Composite Sorbent for Silver Ion Removal and Recovery[J]. ACS Sustainable Chemistry & Enginee-ring, 2013, 2(3): 465-71. [22] HU Y, ZHUO H, LUO Q, et al. Biomass polymerassisted fabrication of aerogels from MXenes with ultrahigh com-pression elasticity and pressure sensitivity[J]. Journal of Materials Chemistry A, 2019, 7(17): 10273-81. doi: 10.1039/C9TA01448A [23] SALEH T A. Carbon nanotube-incorporated alumina as a support for MoNi catalysts for the efficient hydrode-sulfurization of thiophenes[J]. Chemical Engineering Journal, 2021, 404: 126987. doi: 10.1016/j.cej.2020.126987 [24] WANG Q-M, LIU Z-H, Lü Q-F. Lignin modified Ti3C2Tx assisted construction of functionalized interface for separation of oil/water mixture and dye wastewater[J]. Colloids and Surfaces A:Physicochemical and Engine-ering Aspects, 2023, 656: 130371. [25] DING L, WEI Y, WANG Y, et al. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks[J]. Angew Chem Int Ed Engl, 2017, 56(7): 1825-9. doi: 10.1002/anie.201609306 [26] HAN R, MA X, XIE Y, et al. Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux[J]. RSC Advances, 2017, 7(89): 56204-10. doi: 10.1039/C7RA10318B [27] LI S, WANG L, PENG J, et al. Efficient thorium(IV) removal by two-dimensional Ti2CTx MXene from aqueous solution[J]. Chemical Engineering Journal, 2019, 366: 192-9. doi: 10.1016/j.cej.2019.02.056 [28] GUO Y, GONG Z, LI C, et al. Efficient removal of uranium (VI) by 3D hierarchical Mg/Fe-LDH supported nanoscale hydroxyapatite: A synthetic experimental and mechanism studies[J]. Chemical Engineering Journal, 2020, 392: 123682. doi: 10.1016/j.cej.2019.123682 [29] DONG X, WANG Y, JIA M, et al. Sustainable and scalable insitu synthesis of hydrochar-wrapped Ti3AlC2-derived nanofibers as adsorbents to remove heavy metals[J]. Bioresour Technol, 2019, 282: 222-7. doi: 10.1016/j.biortech.2019.03.010 [30] LEVITT A S, ALHABEB M, HATTER C B, et al. Electrospun MXene/carbon nanofibers as supercapacitor electrodes[J]. Journal of Materials Chemistry A, 2019, 7(1): 269-77. doi: 10.1039/C8TA09810G [31] MA Z, LI S, FANG G, et al. Modification of chemical reactivity of enzymatic hydrolysis lignin by ultrasound treatment in dilute alkaline solutions[J]. Int J Biol Macromol, 2016, 93(Pt A): 1279-84. [32] AN L, WANG G, JIA H, et al. Fractionation of enzymatic hydrolysis lignin by sequential extraction for enhancing antioxidant performance[J]. Int J Biol Macromol, 2017, 99: 674-81. doi: 10.1016/j.ijbiomac.2017.03.015 [33] KONG L, RUAN Y, ZHENG Q, et al. Uranium extraction using hydroxyapatite recovered from phosphorus contain-ing wastewater[J]. J Hazard Mater, 2020, 382: 120784. doi: 10.1016/j.jhazmat.2019.120784 [34] 李仕友, 胡俊毅, 贺俊钦, et al. MXene/SA凝胶微球的制备及对U(Ⅵ)的吸附性能[J]. 复合材料学报, 2022, 39(10): 4868-78.LI Shiyou, HU Junyi, HE Junqin, et al. Preparation of MXene/SA gel microspheres and its adsorption perfor-mance for U(VI)[J]. Acta Materiae Compositae Sinica, 2022, 39(10): 4868-4878(in Chinese). [35] REN X, WANG S, YANG S, et al. Influence of contact time, pH, soil humic/fulvic acids, ionic strength and temperature on sorption of U(VI) onto MX-80 bentonite[J]. Journal of Radioanalytical and Nuclear Chemistry, 2009, 283(1): 253-9. [36] LI S, HE J, WANG Y, et al. Adsorption characteristics of U(VI) in aqueous solution by chloroacetic acid-modified MXene-CS gel microspheres[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2023, 674: 131983. doi: 10.1016/j.colsurfa.2023.131983 [37] 顾鹏程, 宋爽, 张塞, et al. 聚苯胺改性Mxene复合材料对U(VI)的高效富集及机理研究[J]. 化学学报, 2018, 76(09): 701-8.Gu Pengcheng, Song Shuang, Zhang Sai, et al. Enrichment of U(VI) on Polyaniline Modified Mxene Composites Studied by Batch Experiment and Mechan-ism Investigation[J]. Acta Chim. Sinica, 2018, 76(9): 701-708(in Chinese). [38] ZHOU Y, HAO H-X, DONG T-H, et al. Efficient enrichment of U(VI) by two-dimensional layered transi-tion metal carbide composite[J]. Radiochimica Acta, 2022, 110(5): 311-22. doi: 10.1515/ract-2021-1130 [39] SHAHZAD A, NAWAZ M, MOZTAHIDA M, et al. Ti3C2Tx MXene core-shell spheres for ultrahigh removal of mercuric ions[J]. Chemical Engineering Journal, 2019, 368: 400-8. doi: 10.1016/j.cej.2019.02.160 [40] FENG X, YU Z, LONG R, et al. Self-assembling 2D/2D (MXene/LDH) materials achieve ultra-high adsorption of heavy metals Ni2+ through terminal group modification[J]. Separation and Puri-fication Technology, 2020, 253: 117525. doi: 10.1016/j.seppur.2020.117525 [41] ZAHAKIFAR F, KESHTKAR A R, TALEBI M. Performance evaluation of sodium alginate/polyvinyl alcohol/poly-ethylene oxide/ZSM5 zeolite hybrid adsorb-ent for ion uptake from aqueous solutions: a case study of thorium (IV)[J]. Journal of Radioanalytical and Nuclear Chemistry, 2020, 327(1): 65-72. [42] WU J, ZHENG Z, ZHU K, et al. Adsorption performance and mechanism of g-C3N4/UiO-66 composite for U(VI) from aqueous solution[J]. Journal of Radioanalytical and Nuclear Chemistry, 2022, 331(1): 469-81. doi: 10.1007/s10967-021-08116-w [43] WU L, LIN X, ZHOU X, et al. Removal of uranium and fluorine from wastewater by double-functional microsph-ere adsor-bent of SA/CMC loaded with calcium and aluminum[J]. Applied Surface Science, 2016, 384: 466-79. doi: 10.1016/j.apsusc.2016.05.056 [44] 张鹏丽, 武莉娅, 杨宗政, et al. MXene改性材料的制备及其吸附除Sr2+性能[J]. 复合材料学报, 2023, 40(10): 5678-91.ZHANG Pengli, WU Liya, YANG Zongzheng, et al. Preparation of modified MXene material and its adsorption performance for Sr2+[J]. Acta Materiae Comp-ositae Sinica, 2023, 40(10): 5678-5691(in Chinese). [45] WANG L, SONG H, YUAN L, et al. Efficient U(VI) Reduction and Sequestra-tion by Ti2CTx MXene[J]. Environ Sci Technol, 2018, 52(18): 10748-56. doi: 10.1021/acs.est.8b03711 [46] Zhang P, Wang L, Huang Z, et al. Aryl diazonium-assisted amidoximation of MXene for boosting water stability and uranyl sequestration via electrochemical sorption[J]. ACS applied materials & interfaces, 2020, 12(13): 15579-15587. [47] ZHANG D, LIU L, ZHAO B, et al. Highly efficient extraction of uranium from seawater by polyamide and amidoxime cofunctionalized MXene[J]. Environ Pollut, 2023, 317: 120826. doi: 10.1016/j.envpol.2022.120826 [48] HALIM J, COOK K M, NAGUIB M, et al. X-ray photoelectron spectroscopy of select multilayered tra-nsition metal carbides (MXenes)[J]. Applied Surface Science, 2016, 362: 406-17. doi: 10.1016/j.apsusc.2015.11.089 [49] RETHINASABAPATHY M, HWANG S K, KANG S M, et al. Amino-functionalized POSS nanocage-intercalated titanium carbide (Ti3C2Tx) MXene stacks for eff-icient cesium and strontium radionuclide sequestration[J]. J Hazard Mater, 2021, 418: 126315. doi: 10.1016/j.jhazmat.2021.126315 [50] MISHRA V, SURESHKUMAR M K, GUPTA N, et al. Study on Sorption Characteristics of Uranium onto Biochar Derived from Eucalyptus Wood[J]. Water, Air, & Soil Pollution, 2017, 228: 1-14. -

 点击查看大图
点击查看大图
计量
- 文章访问数: 140
- HTML全文浏览量: 48
- 被引次数: 0





 下载:
下载: