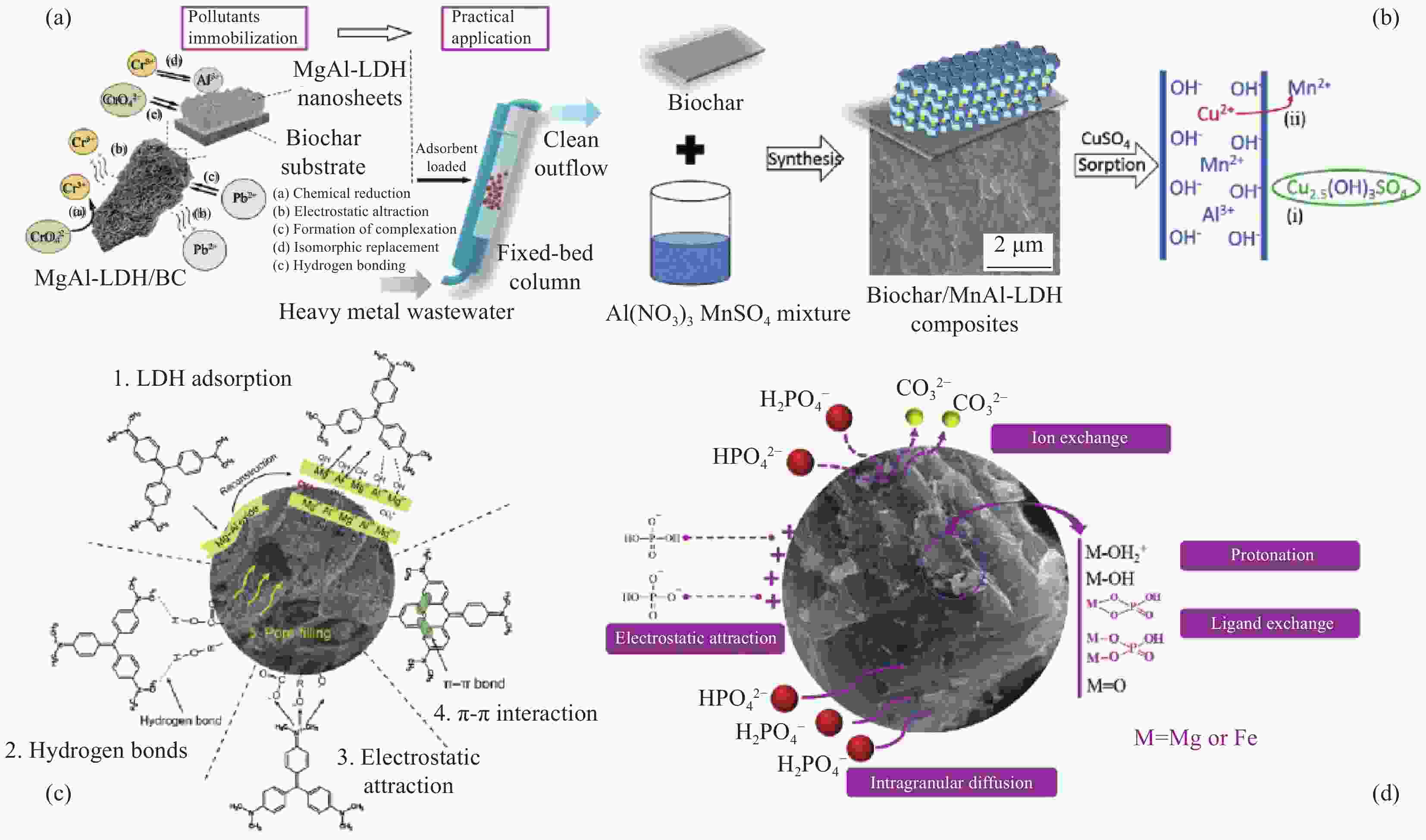
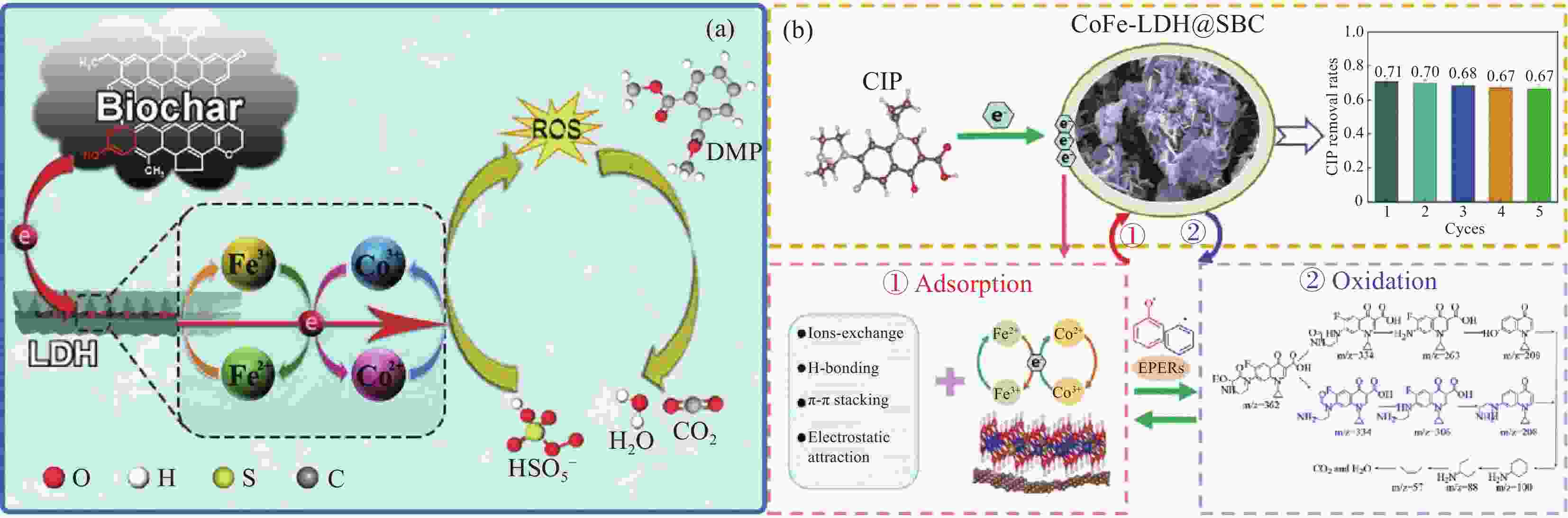
Application of layered double hydroxide-biochar composite in wastewater treatment
-
摘要: 层状双氢氧化物-生物炭(LDH-BC)复合材料,作为一种新型生物炭基复合纳米材料,在废水处理中展现了优异的污染物吸附和催化降解性能。本篇综述全面系统地总结LDH-BC复合材料的相关研究进展,为日后研究方向提供指导。研究内容综述了LDH-BC复合材料合成方法,改性策略和复合材料在废水处理中的应用及机制。展望部分提出LDH-BC复合材料存在具体组分降解路径不清晰、复合污染物体系应用较少、缺少现场规模试验等问题,接下来应针对以上问题进行深化拓展以推动LDH-BC复合材料的研究与应用。Abstract: Layered double hydroxide-biochar (LDH-BC) composites, novel biochar-based composite nanomaterials that have been shown to be highly effective in adsorbing pollutants and catalyzing degradation in wastewater treatment. This review provides a thorough and organized overview of the current research on LDH-BC composites, which can be used to inform future studies. The research content provides a summary of the synthesis method of LDH-BC composites, the modification strategy, and the application and mechanism of the composites in wastewater treatment. It is suggested in the outlook section that LDH-BC composites have problems inadequate research such as unclear degradation paths of specific components, less application of composite pollutant systems, and lack of field scale tests. The next step is to explore the above shortages of research further in order to promote the research and application of LDH-BC composites.
-
表 1 LDH-BC复合材料在废水处理中的应用
Table 1. Application of LDH-BC composites in wastewater treatment
Composites Biochar raw material Pollutant Adsorption capacity/
Removal rateSynthetic method Ageing condition References MgAl-LDH/BC Ramie stem Crystal violet 374.686 mg/g Co-precipitation 100℃,24 h [44] MgAl-LDH/BC Bovine bone Methylene blue 406.47 mg/g Co-precipitation 60℃,16 h [36] CuAl-LDH@BC Rice
huskCongo red 61.35 mg/g Co-precipitation 80℃,72 h [45] MgAl-LDH/BC Bovine bone Caffeine 26.219 mg/g Co-precipitation 60℃,15 h [46] Mg/Fe-LDH@BC Rice straw Zn+,Ni+,Pb2+,Cd2+ 141.70 mg/g,75.59 mg/g,
1264.10 mg/g,126.30 mg/gCo-precipitation 70℃,24 h [47] MnMgFe-LDHs/BC Rape stalks Cd2+ 118 mg/g Co-precipitation 60℃,24 h [48] MgAl-LDH/BC Pinewood sawdust Cr6+,Pb2+ 330.8 mg/g,591.2 mg/g Hydrothermal 60℃ [49] MnAl-LDH/BC Oil-tea Camellia Shells Cu2+ 74.07 mg/g Hydrothermal 80℃,12 h [50] Mg-Al LDH/BC Corn straw Cu2+ 94.7 mg/g Co-precipitation 55℃,12 h [51] Mg-Al LDH/BC Coconut shell Cu2+,Pb2+ 38.6 mg/g,294 mg/g Co-precipitation 60℃,6 h [52] MgAl-LDH/BC Wheat-straw Nitrate 24.8 mg/g Co-precipitation 70℃,72 h [53] MgAl-LDH/BC Sugarcane leaves Phosphates 81.83 mg/g Co-precipitation 105℃,24 h [20] MgAl-LDH/BC Date-palm Phosphates, nitrates 177.97 mg/g,28.06 mg/g Co-precipitation 60℃,48 h [14] MgAl-LDH/BC Cotton Wood Phosphates 410 mg/g Co-precipitation 80℃,72 h [54] Ni-Fe LDH/BC Loblolly pine As5+ 4.38 mg/g Co-precipitation 80℃,12 h [55] FeAl-LDH@BC Rice husk Phenol 85.28% Hydrothermal / [56] CoFe-LDH@SBC Municipal sludge Ciprofloxacin 71% Co-precipitation 80℃,24 h [57] CuCo-LDH/BC Pine needles Ciprofloxacin 84.7% Co-precipitation 65℃,24 h [58] Zn-Co-LDH@BC Wheat husks and paper sludge Jimifloxacin 92.7% Hydrothermal 90℃,24 h [59] ZnO/ZnFe-LDH@BC Rice husks Tetracycline 87.7% Hydrothermal 100℃,4 h [60] Notes: CoFe-LDH@SBC—Sludge biochar loaded with layered double hydroxides -
[1] 边颢昊. LDH/BC的绿色合成方法及其对沼液中氮磷的资源化利用研究[D]. 浙江科技学院, 2023.BIAN HaoHao. Green synthesis method of LDH / BC and its resource utilization of nitrogen and phosphorus in biogas slurry [D]. Zhejiang University of Science and Technology, 2023(in Chinese) [2] 陈强. FeCu-LDH/BC复合材料的合成及降解废水性能的研究[D]. 郑州大学, 2021.ZHENG Qiang Study on the synthesis of FeCu-LDH/BC composites and the performance of wastewater degradation [D]. Zhengzhou University, 2021(in Chinese). [3] BIAN H, WANG M, HAN J, et al. MgFe-LDH@biochars for removing ammonia nitrogen and phosphorus from biogas slurry: Synthesis routes, composite performance, and adsorption mechanisms[J]. Chemosphere, 2023, 324: 138333. doi: 10.1016/j.chemosphere.2023.138333 [4] LEE S Y, CHOI J, SONG K G, et al. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis[J]. Composites Part B:Engineering, 2019, 176: 107209. doi: 10.1016/j.compositesb.2019.107209 [5] WANG J, WANG S. Preparation, modification and environmental application of biochar: A review[J]. Journal of Cleaner Production, 2019, 227: 1002-1022. doi: 10.1016/j.jclepro.2019.04.282 [6] 姜志翔, 郑浩, 李锋民, 等. 生物炭碳封存技术研究进展[J]. 环境科学, 2013, 34(08): 3327-3333. doi: 10.13227/j.hjkx.2013.08.036JIANG Zhixiang, Zheng Hao, LI Fengmin, et al. Research progress of biochar carbon sequestration technology[J]. Environmental science, 2013, 34(08): 3327-3333(in Chinese). doi: 10.13227/j.hjkx.2013.08.036 [7] LYU H, ZHANG Q, SHEN B. Application of biochar and its composites in catalysis[J]. Chemosphere, 2020, 240: 124842. doi: 10.1016/j.chemosphere.2019.124842 [8] Huang L, Liu H, Wang Y, et al. Construction of ternary Bi2O3/biochar/g-C3N4 heterojunction to accelerate photoinduced carrier separation for enhanced tetracycline photodegradation[J]. Applied Surface Science, 2023, 616: 156509. doi: 10.1016/j.apsusc.2023.156509 [9] WAN S, WANG S, LI Y, et al. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions[J]. Journal of Industrial and Engineering Chemistry, 2017, 47: 246-253. doi: 10.1016/j.jiec.2016.11.039 [10] JIN Z L, LI Y J, DONG H R, et al. A comparative study on the activation of persulfate by mackinawite@biochar and pyrite@biochar for sulfamethazine degradation: The role of different natural ironsulfur minerals doping[J]. Chemical Engineering Journal. 2022, 448. [11] KUMAR A, BHATTACHARYA T, SHAIKH W A, et al. Biochar modification methods for augmenting sorption of contaminants[J]. Current Pollution Reports, 2022, 8(4): 519-555. doi: 10.1007/s40726-022-00238-3 [12] YANG Z, WANG F, ZHANG C, et al. Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: a review[J]. RSC Advances, 2016, 6(83): 79415-79436. doi: 10.1039/C6RA12727D [13] ZHOU H, JIANG Z, WEI S. A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water[J]. Applied Clay Science, 2018, 153: 29-37. doi: 10.1016/j.clay.2017.11.033 [14] ALAGHA O, MANZAR M S, ZUBAIR M, et al. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies[J]. Nanomaterials, 2020, 10(2): 336. doi: 10.3390/nano10020336 [15] ALAGHA O, MANZAR M S, ZUBAIR M, et al. Magnetic Mg-Fe/LDH Intercalated Activated Carbon Composites for Nitrate and Phosphate Removal from Wastewater: Insight into Behavior and Mechanisms[J]. Nanomaterials, 2020, 10(7): 1361. doi: 10.3390/nano10071361 [16] ZHANG T, YUE X, GAO L, et al. Hierarchically porous bismuth oxide/layered double hydroxide composites: Preparation, characterization and iodine adsorption[J]. Journal of Cleaner Production, 2017, 144: 220-227. doi: 10.1016/j.jclepro.2017.01.030 [17] TANG Z, QIU Z, LU S, et al. Functionalized layered double hydroxide applied to heavy metal ions absorption: A review[J]. 2020, 9(1): 800-819. [18] SUN Y, GAO B, YAO Y, et al. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties[J]. Chemical Engineering Journal, 2014, 240: 574-578. doi: 10.1016/j.cej.2013.10.081 [19] YAMAN S. Pyrolysis of biomass to produce fuels and chemical feedstocks[J]. Energy Conversion and Management, 2004, 45(5): 651-671. doi: 10.1016/S0196-8904(03)00177-8 [20] LI R, WANG J J, ZHOU B, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of The Total Environment, 2016, 559: 121-129. doi: 10.1016/j.scitotenv.2016.03.151 [21] ZHANG J, HUANG W, YANG D, et al. Removal and recovery of phosphorus from secondary effluent using layered double hydroxide-biochar composites[J]. Science of The Total Environment, 2022, 844: 156802. doi: 10.1016/j.scitotenv.2022.156802 [22] ZHANG M, GAO B, FANG J, et al. Self-assembly of needle-like layered double hydroxide (LDH) nanocrystals on hydrochar: characterization and phosphate removal ability[J]. RSC advances, 2014, 4(53): 28171-28175. doi: 10.1039/c4ra02332c [23] GENG C, XU T, LI Y, et al. Effect of synthesis method on selective adsorption of thiosulfate by calcined MgAl-layered double hydroxides[J]. Chemical Engineering Journal, 2013, 232: 510-518. doi: 10.1016/j.cej.2013.08.010 [24] CONTEROSITO E, GIANOTTI V, PALIN L, et al. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques[J]. Inorganica Chimica Acta, 2018, 470: 36-50. doi: 10.1016/j.ica.2017.08.007 [25] BUKHTIYAROVA M V. A review on effect of synthesis conditions on the formation of layered double hydroxides[J]. Journal of Solid State Chemistry, 2019, 269: 494-506. doi: 10.1016/j.jssc.2018.10.018 [26] LV Q, WANG H, ZHANG M, et al. Synthesis of magnetic biochar/carbonate intercalated Mg-Al layered double hydroxides for enhanced Cd(II) removal from aqueous solution[J]. Desalination and Water Treatment, 2020, 207: 258-269. doi: 10.5004/dwt.2020.26428 [27] JIA Y, ZHANG Y S, FU J G, et al. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics[J]. Colloids And Surfaces A-Physicochemical And Engineering Aspects, 2019, 567: 278-287. [28] Yi Y, Huang Z, Lu B, et al. Magnetic biochar for environmental remediation: A review[J]. Bioresource Technology, 2020, 298: 122468. doi: 10.1016/j.biortech.2019.122468 [29] CUI Q, JIAO G, ZHENG J, et al. Synthesis of a novel magnetic Caragana korshinskii bio-char/Mg-Al layered double hydroxide compo-site and its strong adsorption of phosphate in aqueous solutions[J]. RSC Advances, 2019, 9(32): 18641-18651. doi: 10.1039/C9RA02052G [30] ZOROUFCHI BENIS K, MOTALEBI DAMUCHALI A, SOLTAN J, et al. Treatment of aqueous arsenic – A review of biochar modification methods[J]. Science of The Total Environment, 2020, 739: 139750. doi: 10.1016/j.scitotenv.2020.139750 [31] GHOLAMI P, DINPAZHOH L, KHATAEE A, et al. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium[J]. Journal of Hazardous Materials, 2020, 381: 120742. doi: 10.1016/j.jhazmat.2019.120742 [32] XIAO B, DAI Q, YU X, et al. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge[J]. Journal of Hazardous Materials, 2018, 343: 347-355. doi: 10.1016/j.jhazmat.2017.10.001 [33] SIZMUR T, FRESNO T, AKGÜL G, et al. Biochar modification to enhance sorption of inorganics from water[J]. Bioresource Technology, 2017, 246: 34-47. doi: 10.1016/j.biortech.2017.07.082 [34] AHMED M B, ZHOU J L, NGO H H, et al. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater[J]. Bioresource Technology, 2016, 214: 836-851. doi: 10.1016/j.biortech.2016.05.057 [35] JIN H, CAPAREDA S, CHANG Z, et al. Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation[J]. Bioresource Technology, 2014, 169: 622-629. doi: 10.1016/j.biortech.2014.06.103 [36] MEILI L, LINS P V, ZANTA C L P S, et al. MgAl-LDH/Biochar composites for methylene blue removal by adsorption[J]. Applied Clay Science, 2019, 168: 11-20. doi: 10.1016/j.clay.2018.10.012 [37] FORANO C, COSTANTINO U, PRÉVOT V, et al. Chapter 14[J]. 1 - Layered Double Hydroxides (LDH)[M]. Developments in Clay Science, Bergaya F, Lagaly G, Elsevier, 2013, 5: 745-782. [38] WANG H, LIU R, YUE J, et al. Removal of methylene blue by Zn-Al layered double oxide/magnetic biochar derived from waste pineapple peel[J]. Biomass Conversion and Biorefinery. 2022. [39] AZALOK K A, OLADIPO A A, GAZI M. Hybrid MnFe-LDO–biochar nanopowders for degradation of metronidazole via UV-light-driven photocatalysis: Characterization and mechanism studies[J]. Chemosphere, 2021, 268: 128844. doi: 10.1016/j.chemosphere.2020.128844 [40] WANG C, WANG H. Pb(II) sorption from aqueous solution by novel biochar loaded with nano-particles[J]. Chemosphere, 2018, 192: 1-4. doi: 10.1016/j.chemosphere.2017.10.125 [41] LI J, YAN L, YANG Y, et al. Insight into the adsorption mechanisms of aqueous hexavalent chromium by EDTA intercalated layered double hydroxides: XRD, FTIR, XPS, and zeta potential studies[J]. New Journal of Chemistry, 2019, 43(40): 15915-15923. doi: 10.1039/C9NJ03479J [42] YU W, LIAN F, CUI G, et al. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution[J]. Chemosphere, 2018, 193: 8-16. doi: 10.1016/j.chemosphere.2017.10.134 [43] ZHU J, LI T, WANG S, et al. Lattice-distortion active sites of Ni-doped CuMgFe LDH for benzotraizole degradation[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107903. doi: 10.1016/j.jece.2022.107903 [44] TAN X, LIU Y, GU Y, et al. Biochar pyrolyzed from MgAl-layered double hydroxides pre-coated ramie biomass (Boehmeria nivea (L. ) Gaud. ): Characterization and application for crystal violet removal[J]. Journal of Environmental Management, 2016, 184: 85-93. [45] PALAPA N R, NOVIE JULEANTI N J, NORMAH N, et al. Adsorptive Performance of Congo Red using Copper-Aluminum LDHs Load to Rice Husk Biochar[J]. Sains Malaysiana, 2022, 51(1): 161-173. doi: 10.17576/jsm-2022-5101-13 [46] DOS SANTOS LINS P V, HENRIQUE D C, IDE A H, et al. Evaluation of caffeine adsorption by MgAl-LDH/biochar composite[J]. Environmental Science and Pollution Research, 2019, 26: 31804-31811. doi: 10.1007/s11356-019-06288-3 [47] ZHANG L, TANG S, JIANG C, et al. Simultaneous and efficient capture of inorganic nitrogen and heavy metals by polyporous layered double hydroxide and biochar composite for agricultural nonpoint pollution control[J]. ACS applied materials & interfaces, 2018, 10(49): 43013-43030. [48] YU Y, YANG W, WANG H, et al. In situ synthesis of MnMgFe-LDH on biochar for electrochemical detection and removal of Cd2+ in aqueous solution[J]. Molecules, 2022, 27(22): 7875. doi: 10.3390/molecules27227875 [49] WANG H, WANG S, CHEN Z, et al. Engineered biochar with anisotropic layered double hydroxide nanosheets to simultaneously and efficiently capture Pb2+ and CrO42− from electroplating wastewater[J]. Bioresource technology, 2020, 306: 123118. doi: 10.1016/j.biortech.2020.123118 [50] WANG T, LI C, WANG C, et al. Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2018, 538: 443-450. doi: 10.1016/j.colsurfa.2017.11.034 [51] PENG Y, SUN Y, HANIF A, et al. Design and fabrication of exfoliated Mg/Al layered double hydroxides on biochar support[J]. Journal of Cleaner Production, 2021, 289: 125142. doi: 10.1016/j.jclepro.2020.125142 [52] SU X, CHEN Y, LI Y, et al. Enhanced adsorption of aqueous Pb (II) and Cu (II) by biochar loaded with layered double hydroxide: Crucial role of mineral precipitation[J]. Journal of Molecular Liquids, 2022, 357: 119083. doi: 10.1016/j.molliq.2022.119083 [53] XUE L, GAO B, WAN Y, et al. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 63: 312-317. doi: 10.1016/j.jtice.2016.03.021 [54] ZHANG M, GAO B, YAO Y, et al. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition[J]. Chemosphere, 2013, 92(8): 1042-1047. doi: 10.1016/j.chemosphere.2013.02.050 [55] WANG S, GAO B, LI Y, et al. Sorption of arsenic onto Ni/Fe layered double hydroxide (LDH)-biochar composites[J]. Rsc Advances, 2016, 6(22): 17792-17799. doi: 10.1039/C5RA17490B [56] FAN X, CAO Q, MENG F, et al. A Fenton-like system of biochar loading Fe–Al layered double hydroxides (FeAl-LDH@ BC)/H2O2 for phenol removal[J]. Chemosphere, 2021, 266: 128992. doi: 10.1016/j.chemosphere.2020.128992 [57] ZHENG D, WU M, ZHENG E, et al. Adsorption and oxidation of ciprofloxacin by a novel layered double hydroxides modified sludge biochar[J]. Journal of Colloid and Interface Science, 2022, 625: 596-605. doi: 10.1016/j.jcis.2022.06.080 [58] LI L, CHENG M, QIN L, et al. Enhancing hydrogen peroxide activation of CuCo layered double hydroxide by compositing with biochar: Performance and mechanism[J]. Science of The Total Environment, 2022, 828: 154188. doi: 10.1016/j.scitotenv.2022.154188 [59] GHOLAMI P, KHATAEE A, SOLTANI R D C, et al. Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@ biochar nanocomposite[J]. Journal of hazardous materials, 2020, 382: 121070. doi: 10.1016/j.jhazmat.2019.121070 [60] LI M, LI P, ZHANG L, et al. Facile fabrication of ZnO decorated ZnFe-layered double hydroxides@ biochar nanocomposites for synergistic photodegradation of tetracycline under visible light[J]. Chemical Engineering Journal, 2022, 434: 134772. doi: 10.1016/j.cej.2022.134772 [61] BADRI A F, JULEANTI N, MOHADI R, et al. The Efficiency of Mg-Al/Biochar for Methyl Orange and Methyl Red Removal[J]. Ecological Engineering & Environmental Technology, 2022, 23(1): 202-211. [62] LIU H, SHAN J, CHEN Z, et al. Efficient recovery of phosphate from simulated urine by Mg/Fe bimetallic oxide modified biochar as a potential resource[J]. Science of The Total Environment, 2021, 784: 147546. doi: 10.1016/j.scitotenv.2021.147546 [63] YE Q, WU J, WU P, et al. Enhancing peroxymono-sulfate activation by Co-Fe layered double hydroxide catalysts via compositing with biochar[J]. Chemical Engineering Journal, 2021, 417: 129111. doi: 10.1016/j.cej.2021.129111 -





 下载:
下载: