Research progress of specific structural composites derived catalysts in dry reforming of methane
-
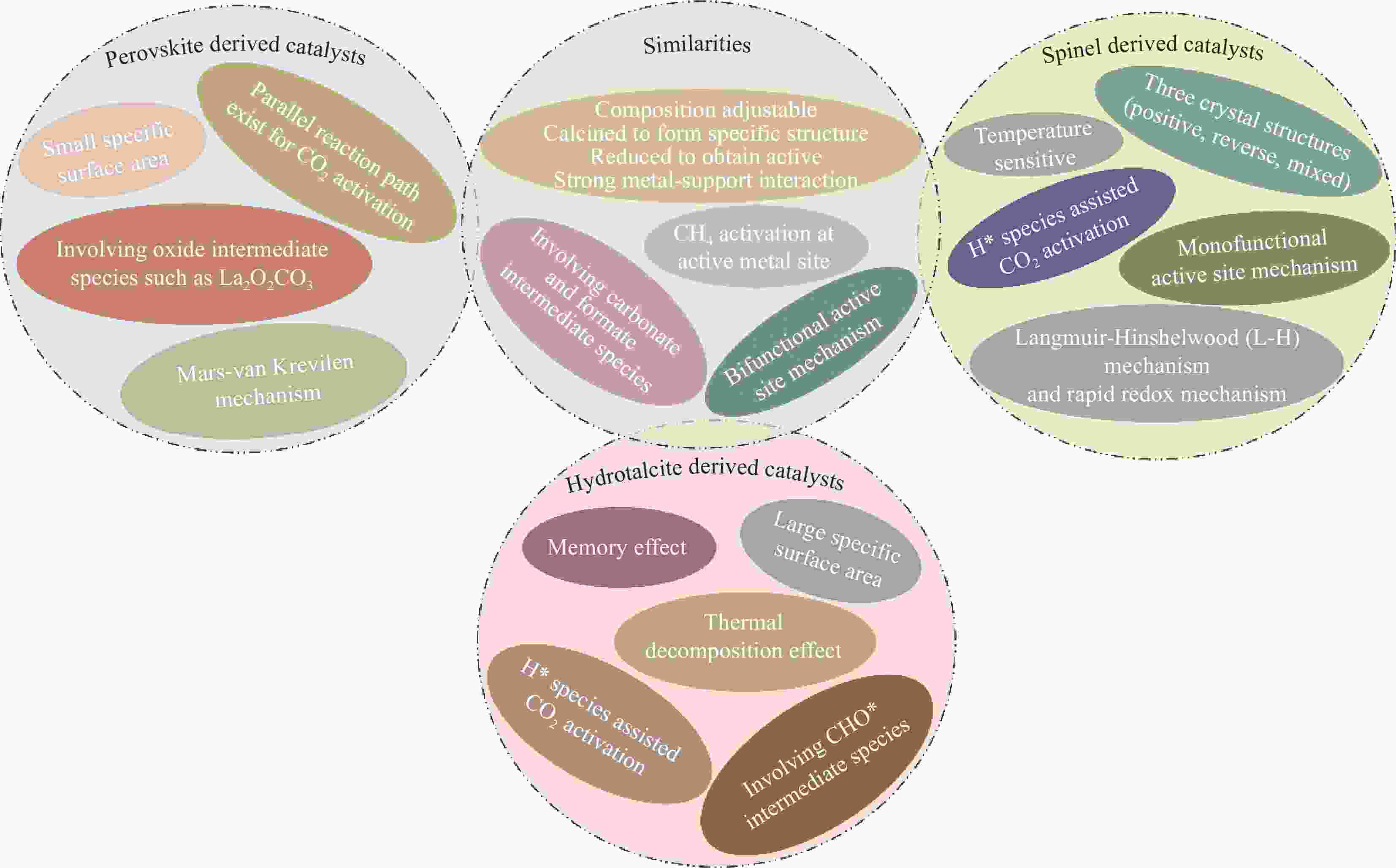
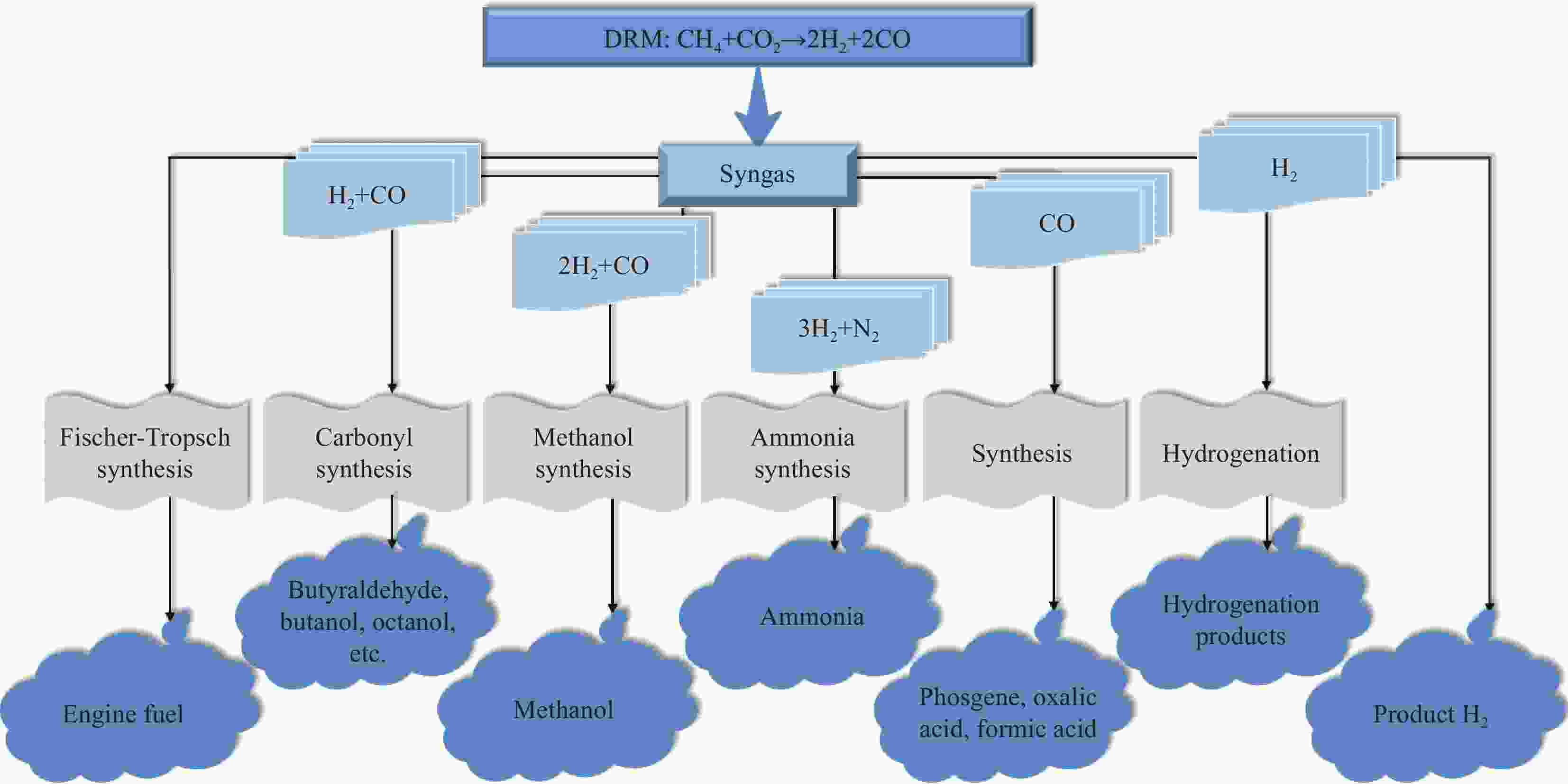
摘要: 钙钛矿、尖晶石和水滑石这类特定结构复合材料组成灵活、结构可控、热稳定性较好,在催化应用中吸引了广泛的研究兴趣。甲烷干重整是一项可同时将CH4和CO2转化为低H2/CO摩尔比合成气的极具应用前景技术,常规负载型催化剂在高温重整条件下易面临由积炭和活性组分烧结导致催化剂失活的难题,而由特定结构复合材料衍生的负载型催化剂因在催化活性和稳定性方面表现出一定的优越性而备受关注。本文先简述了甲烷干重整反应特征、面临的挑战以及反应机制研究现状,并阐述钙钛矿、尖晶石和水滑石这三种复合材料的结构特性,作为催化剂前体应用于该反应的优缺点,性能以及催化路径研究现状。文中指出:钙钛矿结构相对更稳定,但高煅烧温度易导致衍生催化剂表面积较低;水滑石衍生催化剂通常具有较高比表面积,且在特定情况下能恢复部分有序层状结构;水滑石与尖晶石对温度相对更敏感一些,存在的反尖晶石结构有利于提高衍生催化剂还原性。此外,还总结了这三种特定结构复合材料衍生催化剂的催化机制,明确CH4是在活性金属位点上活化,因催化剂和操作条件的影响,目前研究人员对于催化剂表面反应机制的细节暂时还没有达成明确共识。最后,本文对特定结构复合材料衍生催化剂在甲烷干重整中的应用提出了建议。Abstract: Specific structural composites such as perovskite, spinel and hydrotalcite have attracted widespread research interest in catalytic applications due to their flexible composition, controllable structure, and better thermal stability. Dry reforming of methane is a technology with great application prospect for converting CH4 and CO2 into syngas with low H2/CO molar ratio simultaneously. Conventional supported catalysts are susceptible to face the challenge of catalyst deactivation caused by carbon deposition and active component sintering under high-temperature reforming conditions, whereas supported catalysts derived from specific structural composites have attracted much attention owing to their superiority in terms of catalytic activity and stability. In this paper, the characteristics of dry reforming of methane, the challenges faced and the current research status of the reaction mechanism are first briefly outlined, and then elaborates on the structural characteristics of perovskite, spinel and hydrotalcite these three composites, the advantages and disadvantages of applying them as catalyst precursors in this reaction, their performance and the current status of research on the catalytic pathway. Perovskite structure is relatively more stable, but high calcination temperatures may easily lead to a lower surface area of its derived catalyst; hydrotalcite-derived catalysts usually have a high specific surface area and can restore partially ordered layered structures when calcined under certain circumstances; hydrotalcite and spinel are relatively more sensitive to temperature, and the presence of inverse spinel structure is beneficial for improving the reducibility of the derived catalysts. Additionally, the catalytic mechanisms of these three specific structural composites derived catalysts are summarized. The clear one is that CH4 is activated at the active metal sites, and due to the influence of catalysts and operating conditions, researchers have not reached a clear consensus on the details of the reaction mechanism at the catalyst surface for the time being. Finally, some advice is put forward on the application of these specific structural composites derived catalysts in dry reforming of methane.
-
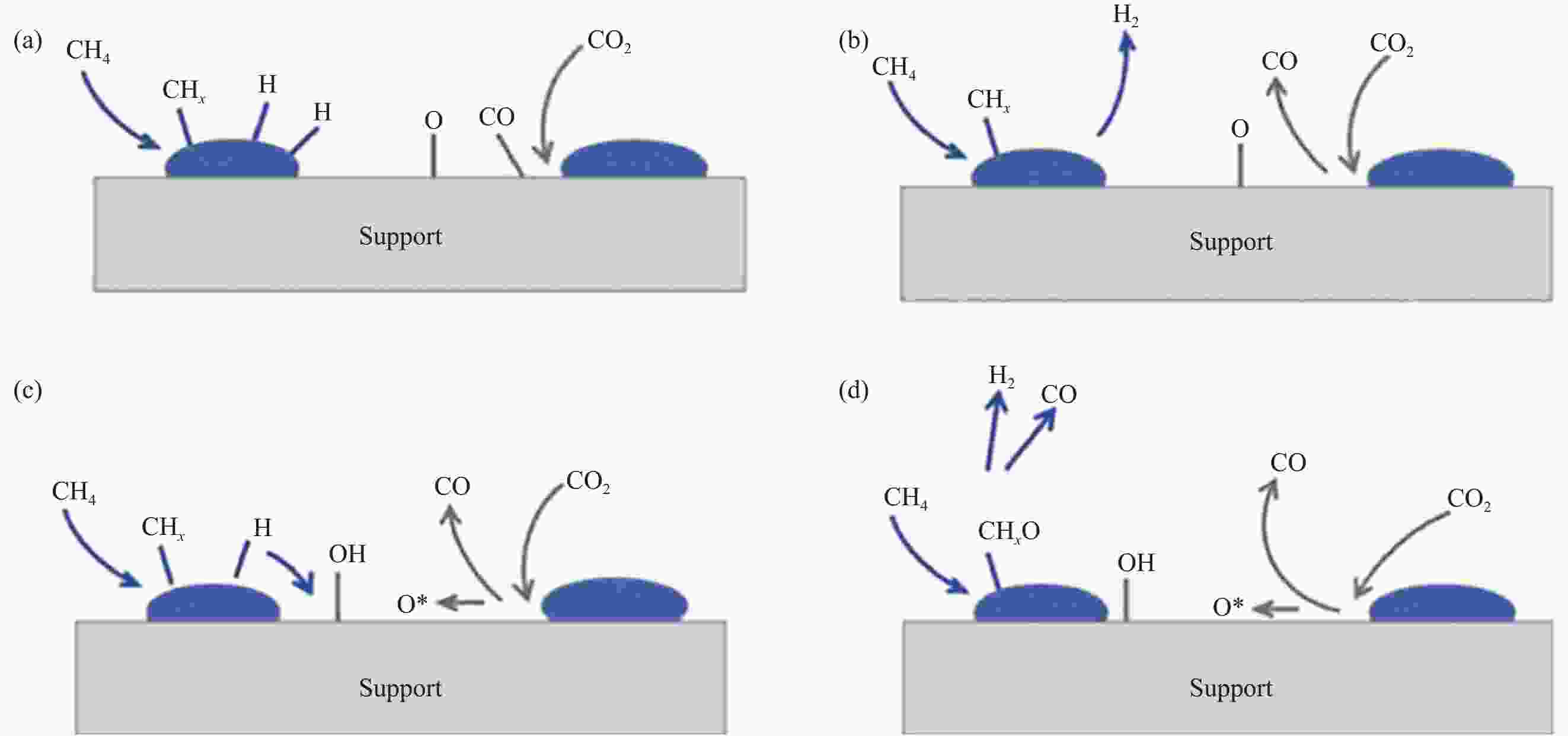
图 4 甲烷干重整反应步骤示意图[44]:(a) CH4和CO2分别在金属和金属-载体界面上的吸附和解离;(b) CO和H2的解吸属于快速步骤;(c)表面羟基由氢和氧溢出形成;(d)表面氧物种或羟基氧化贫氢表面类甲基物种(M—CHx),形成M—CHxO物种,最后形成CO和H2,其中“M”表示金属活性位点
Figure 4. Schematic diagram for dry reforming of methane reaction steps [44] : (a) Adsorption and dissociation of CH4 and CO2 at metal and metal-support interfaces, respectively; (b) Desorption of CO and H2 belongs to the rapid step; (c) Surface hydroxyl groups are formed by the overflow of hydrogen and oxygen; (d) Surface oxygen species or hydroxyl oxidation of hydrogen-poor surface methyl-like species (M—CHx) to form M—CHxO species, and ultimately forming CO and H2
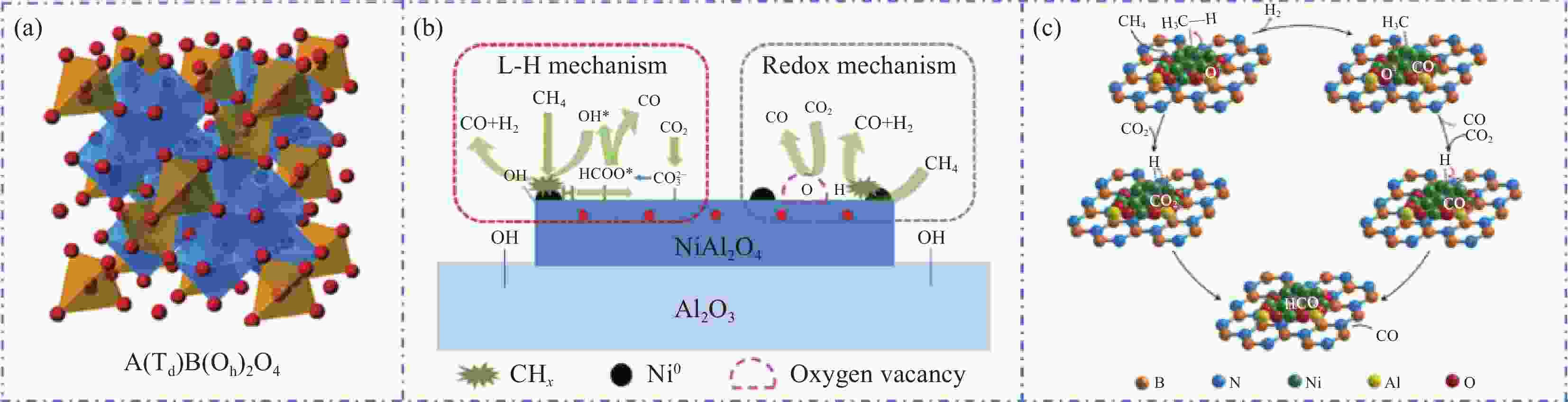
图 5 (a) ABO3和A2BO4钙钛矿结构的理想模型[46];CaZr0.8Ni0.2O3-δ (b-I)和BaZr0.8Ni0.2O3-δ[9] (b-II)以及La0.9Sr0.13Ni0.5Fe0.5O3 (c-I)和La0.9Sr0.1NiO3 (c-II)[57]催化剂催化甲烷干重整反应机制示意图
Figure 5. (a) Ideal model for ABO3 and A2BO4 perovskite structure[46]; Schematic diagram for the reaction mechanism of dry reforming of methane catalyzed by CaZr0.8Ni0.2O3-δ (b-I) and BaZr0.8Ni0.2O3-δ (b-II) catalysts[9], as well as La0.9Sr0.13Ni0.5Fe0.5O3 (c-I) and La0.9Sr0.1NiO3 (c-II) catalysts[57]
图 7 水滑石八面体单元(a-I)及整体结构(a-II)示意图[66];(b)溶胶-凝胶法所制备NiMgAl水滑石衍生催化剂上的甲烷干重整反应路径示意图[78];(c)多核@壳催化剂LDH@SiO2形成示意图[81];(d) 氮化硼界面约束层状双氢氧化物(Ni,Mg)Al2O4衍生Ni催化剂(NiMA-BN-M-R)催化甲烷干重整反应机制示意图[82]
Figure 7. Schematic diagram for hydrotalcite octahedral unit (a-I) and overall structure (a-II) [67]; (b) Schematic diagram for dry reforming of methane reaction pathway over NiMgAl hydrotalcite derived catalyst prepared by sol-gel method [79]; (c) Schematic diagram for the formation of multicore@shell catalyst LDH@SiO2 [82]; (d) Schematic diagram for the reaction mechanism of dry reforming of methane catalyzed by boron nitride interface-confined and layered double hydroxides (Ni,Mg)Al2O4 derived Ni cataltst(NiMA-BN-M-R)[83]
Ni0—Zero-valent nickel; CH*—Adsorbed CH species; *—Adsorbed state of species; SM—Metal-carrier interface site
-
[1] GAO W L, LIANG S Y, WANG R J, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges[J]. Chemical Society Reviews, 2020, 49: 8584-686. doi: 10.1039/D0CS00025F [2] FOUQUET R. Historical energy transitions: Speed, prices and system transformation[J]. Energy Research & Social Science, 2016, 22: 7-12. [3] SIANG T J, JALIL A A, ABDULRAHMAN A, et al. Enhanced carbon resistance and regenerability in methane partial oxidation to syngas using oxygen vacancy-rich fibrous Pd, Ru and Rh/KCC-1 catalysts[J]. Environmental Chemistry Letters, 2021, 19: 2733-2742. doi: 10.1007/s10311-021-01192-0 [4] HUSSAIN I, JALIL A A, HASSAN N S, et al. Fabrication and characterization of highly active fibrous silica-mordenite (FS@SiO2-MOR) cockscomb shaped catalyst for enhanced CO2 methanation[J]. Chemical Engineering Science, 2020, 228: 115978. doi: 10.1016/j.ces.2020.115978 [5] COOPER S A, RAMAN K K, YIN J. Halo effect or fallen angel effect? Firm value consequences of greenhouse gas emissions and reputation for corporate social responsibility[J]. Journal of Accounting and Public Policy, 2018, 37(3): 226-240. [6] LIU W M, LI L, LIN S X, et al. Confined Ni-In intermetallic alloy nanocatalyst with excellent coking resistance for methane dry reforming[J]. Journal of Energy Chemistry, 2022, 65: 34-47. doi: 10.1016/j.jechem.2021.05.017 [7] 邵斌, 孙哲毅, 章云, 等. 二氧化碳转化为合成气及高附加值产品的研究进展[J]. 化工进展, 2022, 41(3): 1136-1151.SHAO Bin, SUN Zheyi, ZHANG Yun, et al. Recent progresses in CO2 to syngas and high value-added products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1136-1151(in Chinese). [8] 周伟, 成康, 张庆红, 等. 合成气转化中的接力催化[J]. 科学通报, 2021, 66(10): 1157-1169. doi: 10.1360/TB-2020-1309ZHOU Wei, CHENG Kang, ZHANG Qinghong, et al. Relay catalysis in the conversion of syngas[J]. Chinese Science Bulletin, 2021, 66(10): 1157-1169(in Chinese). doi: 10.1360/TB-2020-1309 [9] DAMA S, GHODKE S R, BOBADE R, et al. Active and durable alkaline earth metal substituted perovskite catalysts for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2018, 224: 146-158. doi: 10.1016/j.apcatb.2017.10.048 [10] AL-FATESH A S, KUMAR R, KASIM S O, et al. The effect of modifier identity on the performance of Ni-based catalyst supported on γ-Al2O3 in dry reforming of methane[J]. Catalysis Today, 2020, 348: 236-242. doi: 10.1016/j.cattod.2019.09.003 [11] BARAKA S, BOUEARAN K, CANER L, et al. Catalytic performances of natural Ni-bearing clay minerals for production of syngas from dry reforming of methane[J]. Journal of CO2 Utilization, 2021, 52: 101696. [12] KONG W B, FU Y, SHI L, et al. Nickel nanoparticles with interfacial confinement mimic noble metal catalyst in methane dry reforming[J]. Applied Catalysis B: Environmental, 2021, 285: 119837. doi: 10.1016/j.apcatb.2020.119837 [13] PENG R F, CHEN Y M, ZHANG B X, et al. Tailoring the stability of Ni-Fe/mayenite in methane–carbon dioxide reforming[J]. Fuel, 2021, 284: 118909. doi: 10.1016/j.fuel.2020.118909 [14] YANG E, NAM E, LEE J H, et al. Al2O3-coated Ni/CeO2 nanoparticles as coke-resistant catalyst for dry reforming of methane[J]. Catalysis Science & Technology, 2020, 10(24): 8283-8294. [15] ARAMOUNI N A K, ZEAITER J, KWAPINSKI W, et al. Thermodynamic analysis of methane dry reforming: Effect of the catalyst particle size on carbon formation[J]. Energy Conversion and Management, 2017, 150: 614-622. doi: 10.1016/j.enconman.2017.08.056 [16] JEON O S, LEE H S, LEE K-S, et al. Harnessing strong metal–support interaction to proliferate the dry reforming of methane performance by in situ reduction[J]. ACS Applied Materials & Interfaces, 2022, 14(10): 12140-12148. [17] ROGERS J L, MANGARELLA M C, D’AMICO A D, et al. Differences in the nature of active sites for methane dry reforming and methane steam reforming over nickel aluminate catalysts[J]. ACS Catalysis, 2016, 6(9): 5873-5886. [18] ZHANG M, ZHANG J F, ZHOU Z L, et al. Effects of the surface adsorbed oxygen species tuned by rare-earth metal doping on dry reforming of methane over Ni/ZrO2 catalyst[J]. Applied Catalysis B: Environmental, 2020, 264: 118522. doi: 10.1016/j.apcatb.2019.118522 [19] XIE X L, LIANG D F, CHEN M Q, et al. Dry reforming of methane over silica zeolite-encapsulated Ni-based catalysts: Effect of preparation method, support structure and Ni content on catalytic performance[J]. International Journal of Hydrogen Energy, 2023, 48(20): 7319-7336. doi: 10.1016/j.ijhydene.2022.11.167 [20] LIANG D F, WANG Y S, CHEN M Q, et al. Dry reforming of methane for syngas production over attapulgite-derived MFI zeolite encapsulated bimetallic Ni-Co catalysts[J]. Applied Catalysis B: Environmental, 2023, 322: 122088. doi: 10.1016/j.apcatb.2022.122088 [21] ZHANG H, HE X, DONG K, et al. Selenate promoted stability improvement of nickel selenide nanosheet array with an amorphous NiOOH layer for seawater oxidation[J]. Materials Today Physics, 2023, 38: 101249. doi: 10.1016/j.mtphys.2023.101249 [22] ZHANG H, HAN H, YANG X, et al. Reversing Mg suppression effect on Co-site water oxidation of MgCo2O4 based on vanadium-atom electronic affinity synergy with Mg sites toward electronic redistribution[J]. Catalysis Science & Technology, 2023. [23] BHATTAR S, ABEDIN M A, KANITKAR S, et al. A review on dry reforming of methane over perovskite derived catalysts[J]. Catalysis Today, 2021, 365: 2-23. doi: 10.1016/j.cattod.2020.10.041 [24] LU Y, GUO D, ZHAO Y F, et al. Enhanced catalytic performance of Nix-V@HSS catalysts for the DRM reaction: The study of interfacial effects on Ni-VO x structure with a unique yolk-shell structure[J]. Journal of Catalysis, 2021, 396: 65-80. doi: 10.1016/j.jcat.2021.02.005 [25] PAKHAREA D, SPIVEY J. A review of dry (CO2) reforming of methane over noble metal catalysts[J]. Chemical Society Reviews, 2014, 43(22): 7813-7837. doi: 10.1039/C3CS60395D [26] WANG M, ZHAO T T, DONG X L, et al. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2018, 224: 214-221. doi: 10.1016/j.apcatb.2017.10.022 [27] DJINOVIC' J P, ČRNIVEC I G O, BOŠTJAN E, et al. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts[J]. Applied Catalysis B: Environmental, 2012, 125: 259-270. doi: 10.1016/j.apcatb.2012.05.049 [28] ZHANG G J, LIU J W, XU Y, et al. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017)[J]. International Journal of Hydrogen Energy, 2018, 43(32): 15030-15054. doi: 10.1016/j.ijhydene.2018.06.091 [29] JANG W-J, SHIM J-O, KIM H-M, et al. A review on dry reforming of methane in aspect of catalytic properties[J]. Catalysis Today, 2019, 324: 15-26. doi: 10.1016/j.cattod.2018.07.032 [30] BIAN Z F, DAS S L, WAI M H, et al. A Review on bimetallic nickel-based catalysts for CO2 Reforming of methane[J]. ChemPhysChem, 2017, 18(22): 3117-3134. doi: 10.1002/cphc.201700529 [31] KWEON S J, AN H J, SHIN C-H, et al. Nitrided Ni/N-zeolites as efficient catalysts for the dry reforming of methane[J]. Journal of CO2 Utilization, 2021, 46: 101478. doi: 10.1016/j.jcou.2021.101478 [32] KATHIRASER Y, OEMAR U, SAW E T, et al. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts[J]. Chemical Engineering Journal, 2015, 278: 62-78. doi: 10.1016/j.cej.2014.11.143 [33] BRADFORD M C J, VANNICE M A. CO2 reforming of CH4[J]. Catalysis Reviews, 1999, 41(1): 1-42. [34] YABE T, SEKINE Y. Methane conversion using carbon dioxide as an oxidizing agent: A review[J]. Fuel Processing Technology, 2018, 181: 187-198. doi: 10.1016/j.fuproc.2018.09.014 [35] ERDÖHELYI A, CSERÉNYI J, PAPP E, et al. Catalytic reaction of methane with carbon dioxide over supported palladium[J]. Applied Catalysis A: General, 1994, 108: 205–219. [36] ROSTRUP-NIELSEN J R, HANSEN J H B. CO2-reforming of methane over transition metals[J]. Journal of Catalysis, 1993, 144: 38–49. [37] TEH L P, SETIABUDI H D, TIMMIATI S N, et al. Recent progress in ceria-based catalysts for the dry reforming of methane: A review[J]. Chemical Engineering Science, 2021, 242: 116606. [38] GOKON N, OSAWA Y, NAKAZAWA D,et al. Kinetics of CO2 reforming of methane by catalytically activated metallic foam absorber for solar receiver-reactors[J]. International Journal of Hydrogen Energy, 2009, 34(4): 1787-1800. doi: 10.1016/j.ijhydene.2008.12.018 [39] ZHANG S S, YING M, YU J, et al. Ni xAl1O2- δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism[J]. Applied Catalysis B: Environmental, 2021, 291: 120074. doi: 10.1016/j.apcatb.2021.120074 [40] JIANG S P, LU Y, WANG S P, et al. Insight into the reaction mechanism of CO2 activation for CH4 reforming over NiO-MgO: A combination of DRIFTS and DFT study[J]. Applied Surface Science, 2017, 416: 59-68. doi: 10.1016/j.apsusc.2017.04.142 [41] NIU J T, DU X S, RAN J Y, et al. Dry (CO2) reforming of methane over Pt catalysts studied by DFT and kinetic modeling[J]. Applied Surface Science, 2016, 376: 79-90. doi: 10.1016/j.apsusc.2016.01.212 [42] FOPPA L, SILAGHI M-C, LARMIER K, et al. Intrinsic reactivity of Ni, Pd and Pt surfaces in dry reforming and competitive reactions: Insights from first principles calculations and microkinetic modeling simulations[J]. Journal of Catalysis, 2016, 343: 196-207. doi: 10.1016/j.jcat.2016.02.030 [43] ABDULRASHEED A, JALIL A A, GAMBO Y, et al. A review on catalyst development for dry reforming of methane to syngas: Recent advances[J]. Renewable and Sustainable Energy Reviews, 2019, 108: 175-193. doi: 10.1016/j.rser.2019.03.054 [44] PAPADOPOULOU C, MATRALIS H, VERYKIOS X. Catalysis for Alternative Energy Generation[M]. New York: Springer, 2012: 57-128. [45] FREUND H J, MESSMER R P. On the bonding and reactivity of CO2 on metal surfaces[J]. Surface Science, 1986, 172(1): 1-30. doi: 10.1016/0039-6028(86)90580-7 [46] ZHU J J, LI H L, ZHONG L Y, et al. Perovskite oxides: Preparation, characterizations, and applicationsin heterogeneous catalysis[J]. ACS Catalysis, 2014, 4(9): 2917-2940. doi: 10.1021/cs500606g [47] ZHU J J, THOMAS A. Perovskite-type mixed oxides as catalytic material for NO removal[J]. Applied Catalysis B: Environmental, 2009, 92(3-4): 225-233. doi: 10.1016/j.apcatb.2009.08.008 [48] MOOGI S, KO C H, RHEE G H, et al. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production[J]. Chemical Engineering Journal, 2022, 437(1): 135348. [49] ROYER S, DUPREZ D, CAN F, et al. Perovskites as substitutes of noble metals for heterogeneous catalysis: Dream or reality[J]. Chemical Reviews, 2014, 114(20): 10292-10368. doi: 10.1021/cr500032a [50] HUANG F, SUN X C, ZHENG Y, et al. Facile coprecipitation synthesis of La0.6Sr0.4MnO3 perovskites with high surface area[J]. Materials Letters, 2018, 210: 287-290. [51] BATIOT-DUPEYRAT C, VALDERRAMA G, MENESES A, et al. Pulse study of CO2 reforming of methane over LaNiO3[J]. Applied Catalysis A:General, 2003, 248(1-2): 143-151. doi: 10.1016/S0926-860X(03)00155-8 [52] SINGH S, ZUBENKO D, ROSEN B A. Influence of LaNiO3 shape on its solid-phase crystallization into coke-free reforming catalysts[J]. ACS Catalysis, 2016, 6(7): 4199-4205. doi: 10.1021/acscatal.6b00673 [53] MORADI G R, RAHMANZADEH M, SHARIFNIA S. Kinetic investigation of CO2 reforming of CH4 over La–Ni based perovskite[J]. Chemical Engineering Journal, 2010, 162(2): 787-791. [54] GALLEGO G S, BATIOT-DUPEYRAT C, BARRAULT J, et al. Dual active-site mechanism for dry methane reforming over Ni/La2O3 produced from LaNiO3 Perovskite[J]. Industrial & Engineering Chemistry Research, 2008, 47(23): 9272-9278. [55] TSIPOURIARI V A, VERYKIOS X E. Kinetic study of the catalytic reforming of methane with carbon dioxide to synthesis gas over Ni/La2O3 catalyst[J]. Catalysis Today, 2001, 64(1-2): 83-90. doi: 10.1016/S0920-5861(00)00511-3 [56] PINO L, ITALIANO C, VITA A, et al. Ce0.70La0.20Ni0.10O2- δ catalyst for methane dry reforming: Influence of reduction temperature on the catalytic activity and stability[J]. Applied Catalysis B: Environmental, 2017, 218: 779-792. doi: 10.1016/j.apcatb.2017.06.080 [57] DAS S, BHATTAR S, LIU L, et al. Effect of partial Fe substitution in La0.9Sr0.1NiO3 perovskite-derived catalysts on the reaction mechanism of methane dry reforming[J]. ACS Catalysis, 2020, 10(21): 12466-12486. doi: 10.1021/acscatal.0c01229 [58] SUEN N-T, HUNG S-F, QUAN Q, et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives[J]. Chemical Society Reviews, 2017, 46: 337-365. doi: 10.1039/C6CS00328A [59] ADVANI J H, MOREA G S, SRIVASTAVA R. Spinel-based catalysts for the biomass valorisation of platform molecules via oxidative and reductive transformations[J]. Green Chemistry, 2022, 24: 3574-3604. [60] Dennis W, Katja M W, Maximilian M H, et al. Modifying spinel precursors for highly active and stable Ni-based CO2 methanation catalysts[J]. ChemCatChem, 2022, 14: e202200563. [61] LI D L, LU M M, CAI Y B, et al. Synthesis of high surface area MgAl2O4 spinel as catalyst support via layered double hydroxides-containing precursor[J]. Applied Clay Science, 132–133, 2016: 243-250. [62] MARGOSSIA T, KIM L, KIM S M, et al. Molecularly tailored nickel precursor and support yield a stable methane dry reforming catalyst with superior metal utilization[J]. Journal of the American Chemical Society, 2017, 139(20): 6919-6927. doi: 10.1021/jacs.7b01625 [63] ZIELIŃSKI J. Morphology of nickel/alumina catalysts[J]. Journal of Catalysis, 1982, 76: 157-163. doi: 10.1016/0021-9517(82)90245-7 [64] ZIELIŃSKI J. Effect of water on the reduction of nickel/alumina catalysts Catalyst characterization by temperature-programmed reduction[J]. Journal of the Chemical Society, Faraday Transactions, 1997, 93: 3577-3580. doi: 10.1039/a703392c [65] ROELOFSEN J N, PETERSON R C. Structural variation in nickel aluminate spinel (NiAl2O4)[J]. American Mineralogist, 1992, 77(5-6): 522-528. [66] DENG J, YANG B, LIU Y Y, et al. Sintering- and coking-resistant nickel catalysts embedded in boron nitride supported nickel aluminate spinels for dry reforming of methane[J]. Applied Catalysis A: General, 2022, 642: 118706. doi: 10.1016/j.apcata.2022.118706 [67] DĘBEK R, MOTAK M, GRZYBEK T, et al. A short review on the catalytic activity of hydrotalcite-derived materials for dry reforming of methane[J]. Catalysts, 2017, 7(1): 32. [68] LIU H R, WIERZBICKI D, DEBEK R, et al. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures[J]. Fuel, 2016, 182: 8-16. doi: 10.1016/j.fuel.2016.05.073 [69] FAN G L, LI F, EVANSA D G, et al. Catalytic applications of layered double hydroxides: recent advances and perspectives[J]. Chemical Society Reviews, 2014, 43: 7040-7066. doi: 10.1039/C4CS00160E [70] FENG J T, HE Y F, LIU Y N, et al. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: general functionality and promising application prospects[J]. Chemical Society Reviews, 2015, 44: 5291-5319. doi: 10.1039/C5CS00268K [71] 黎晓杰, 成晓琼, 段书谦, 等. 改性水滑石调控聚乳酸-马来酸酐接枝聚丙烯复合材料及其性能[J]. 复合材料学报, 2022, 39(12): 5711-5726.LI Xiaojie, CHENG Xiaoqiong, DUAN Shuqian, et al. Properties of polylactic acid/maleic anhydride grafted polypropylene composites regulated by modified hydrotalcite[J]. Acta Materiae Compositae Sinica, 2022, 39(12): 5711-5726(in Chinese). [72] 阮勇哲, 卢遥, 王胜平. 甲烷干重整Ni基催化剂失活及抑制失活研究进展[J]. 化工进展, 2018, 37(10): 3850-3857.RUAN Yongzhe, LU Yao, WANG Shengping. Progress in deactivation and anti-deactivation of nickel-based catalysts for methane dry reforming[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3850-3857(in Chinese). [73] COSTA K Ś D, ZHANG H L, LI S S, et al. Carbon-resistant NiO-Y2O3-nanostructured catalysts derived from double-layered hydroxides for dry reforming of methane[J]. Catalysis Today, 2021, 366: 103-113. doi: 10.1016/j.cattod.2020.03.032 [74] 孙金陆, 甄卫军, 李进. LDHs材料的结构、性质及其应用研究进展[J]. 化工进展, 2013, 32(03): 610-616.SUN Jinlu, ZHEN Weijun, LI Jin. Structure, properties and applications of LDHs[J]. Chemical Industry and Engineering Progress, 2013, 32(03): 610-616(in Chinese). [75] XI Y Z, DAVIS R J. Influence of water on the activity and stability of activated MgAl hydrotalcites for the transesterification of tributyrin with methanol[J]. Journal of Catalysis, 2008, 254(2): 190-197. doi: 10.1016/j.jcat.2007.12.008 [76] KWON D, KANG J Y, AN S, et al. Tuning the base properties of Mg–Al hydrotalcite catalysts using their memory effect[J]. Journal of Energy Chemistry, 2020, 46: 229-236. doi: 10.1016/j.jechem.2019.11.013 [77] 方露, 何青青, 胡吉明等. 二维层状双金属氢氧化物(LDHs)在金属腐蚀防护中应用的研究进展[J]. 腐蚀科学与防护技术, 2019, 31(06): 665-671.FANG Lu, HE Qingqing, HU Jiming, ZHANG Jianqing. Research Advance in Application of 2D Layered Double Hydroxides (LDHs) in Corrosion Protection of Metals[J]. Corrosion Science and Protection Technology, 2019, 31(06): 665-671(in Chinese). [78] ABDELSADEK Z, SEHAILIA M, HALLICHE D, et al. In-situ hydrogasification/regeneration of NiAl-hydrotalcite derived catalyst in the reaction of CO2 reforming of methane: A versatile approach to catalyst recycling[J]. Journal of CO2 Utilization, 2016, 14: 98-105. [79] CARANTON A R G, STAVALE F, ANNESE E, et al. Enhancing catalytic performance with sol-gel hydrotalcite-derived nickel oxide catalysts for dry reforming of methane: An examination on reactivity and coke formation[J]. chemCatchem, 2023, 15(12): e202300280. doi: 10.1002/cctc.202300280 [80] DĘBEK R, MOTAK M, DURACZYSKA D, et al. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability[J]. Catalysis Science & Technology, 2016, 6: 6705-6715. [81] ABBAS M, SIKANDER U, MEHRAN M T, et al. Exceptional stability of hydrotalcite derived spinel Mg(Ni)Al2O4 catalyst for dry reforming of methane[J]. Catalysis Today, 2022, 403: 74-85. doi: 10.1016/j.cattod.2021.08.029 [82] BIAN Z F, DENG S B, SUN Z K, et al. Multi-core@Shell catalyst derived from LDH@SiO2 for low-temperature dry reforming of methane[J]. Renewable Energy, 2022, 200: 1362-1370. doi: 10.1016/j.renene.2022.10.046 [83] BU K K, KUBOON S C, DENG J, et al. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts[J]. Applied Catalysis B:Environmental, 2019, 252: 86-97. doi: 10.1016/j.apcatb.2019.04.007 -





 下载:
下载: