Performance and mechanism of biochar loaded magnetic nanocarbon hydroxyapatite(CHAP-γ-Fe2O3/BC) for the removal of U(VI) from water
-
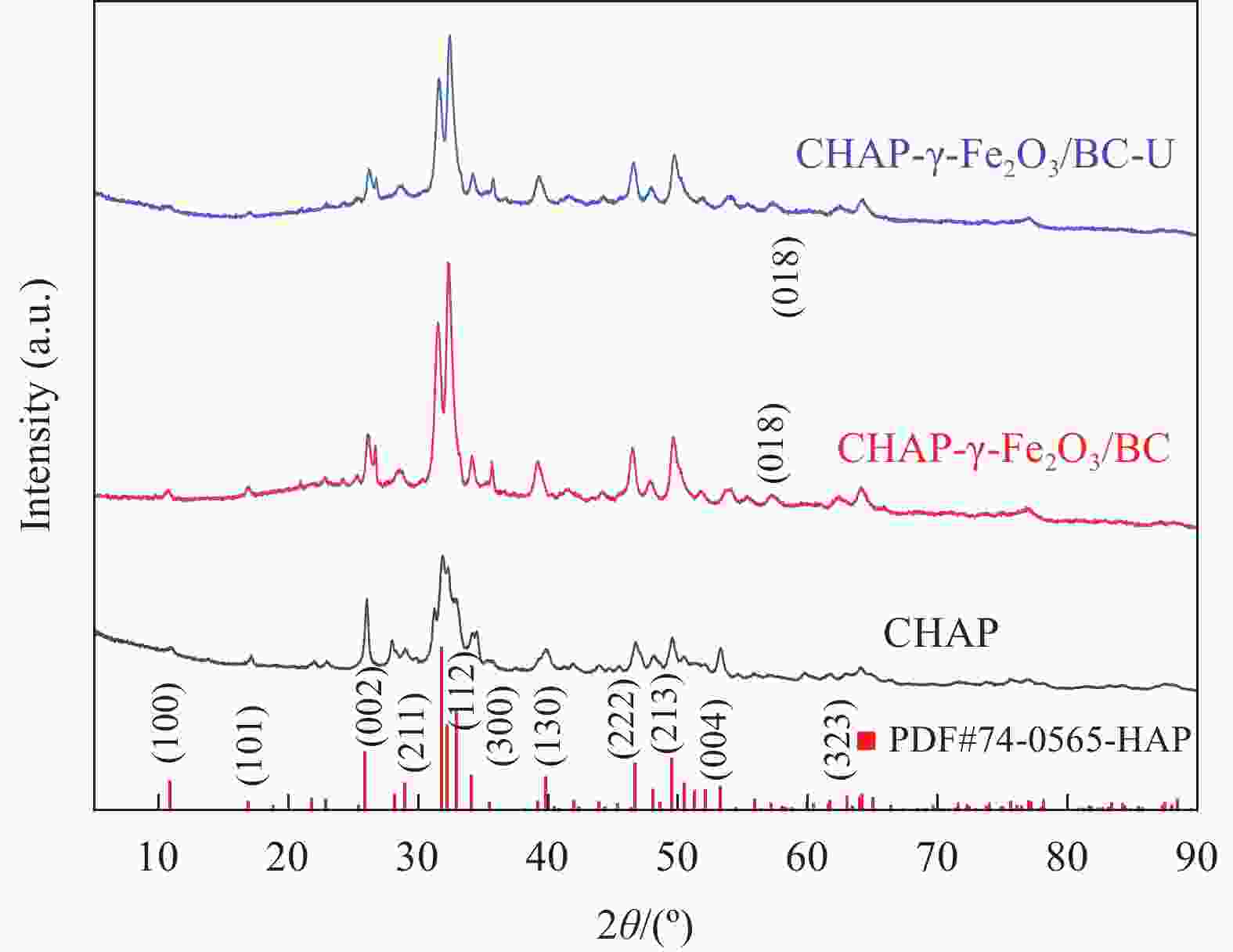
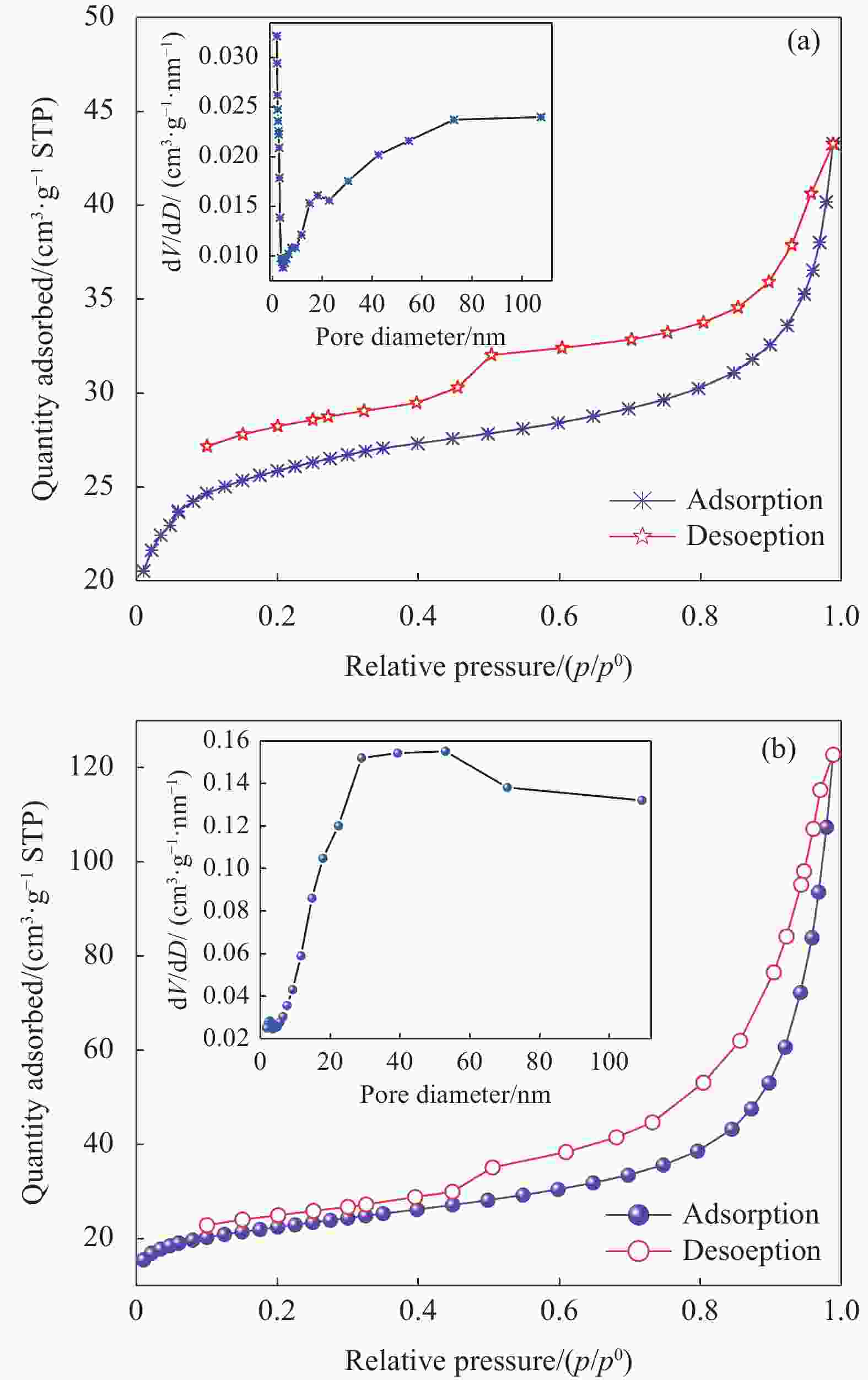
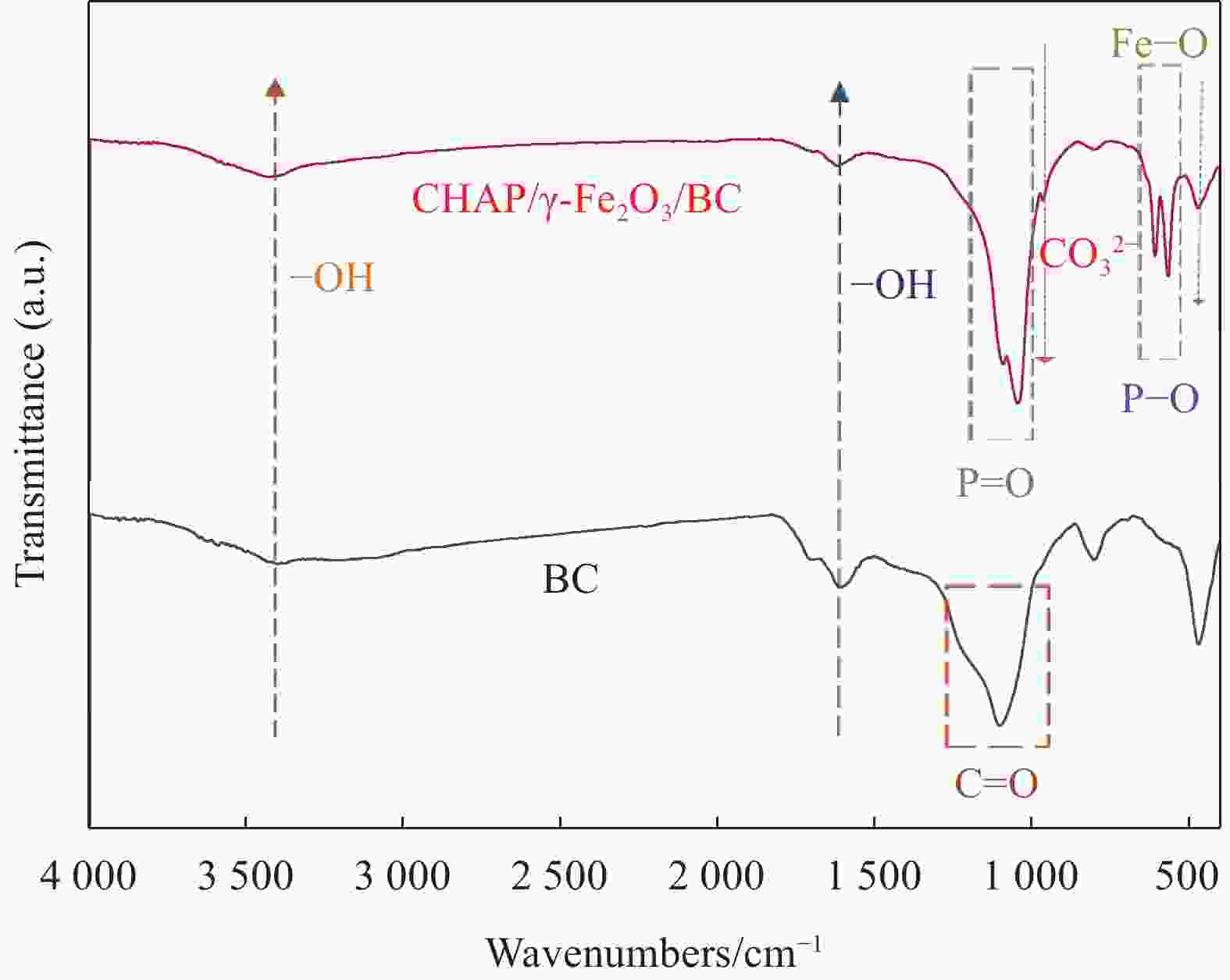
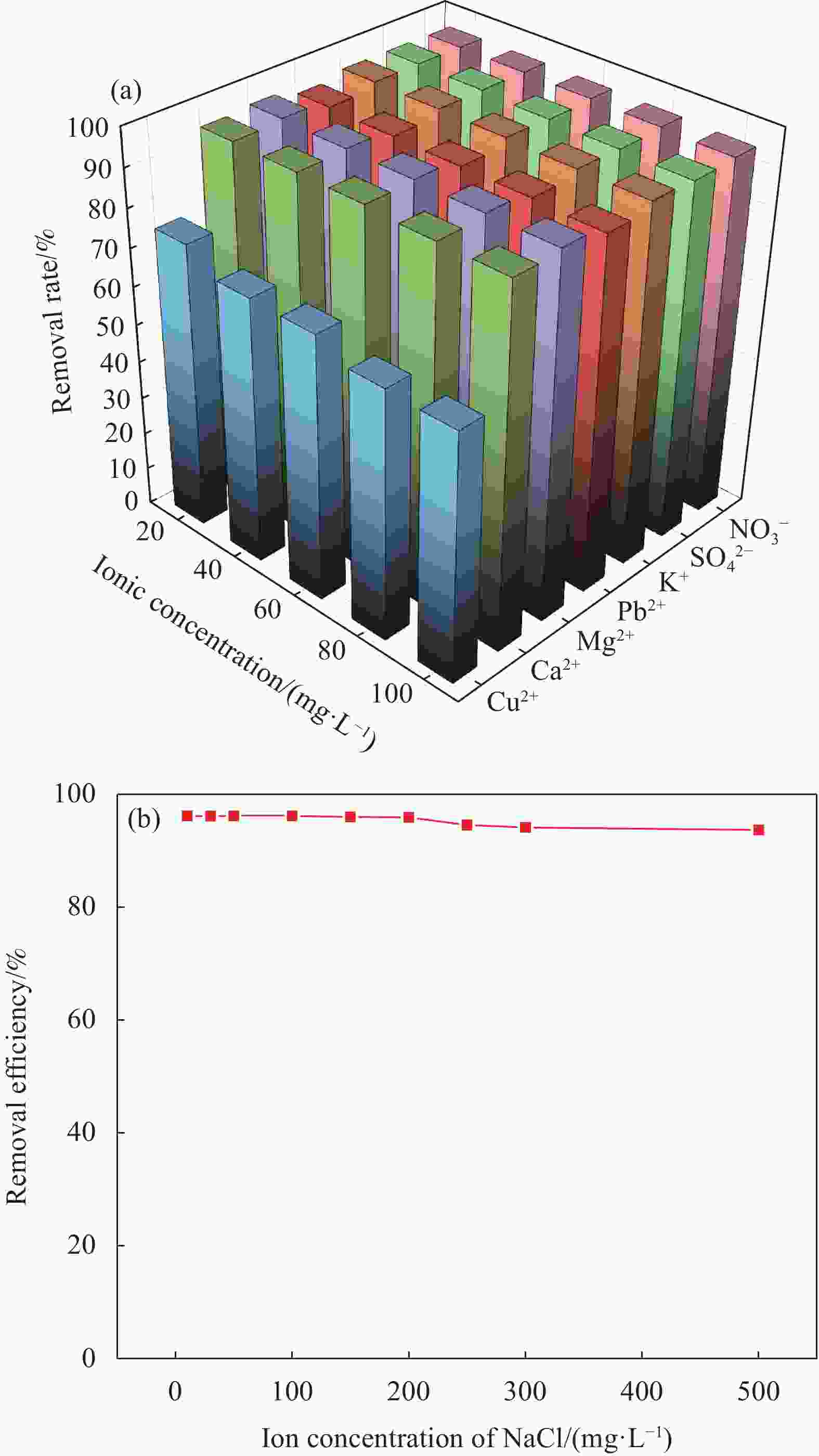
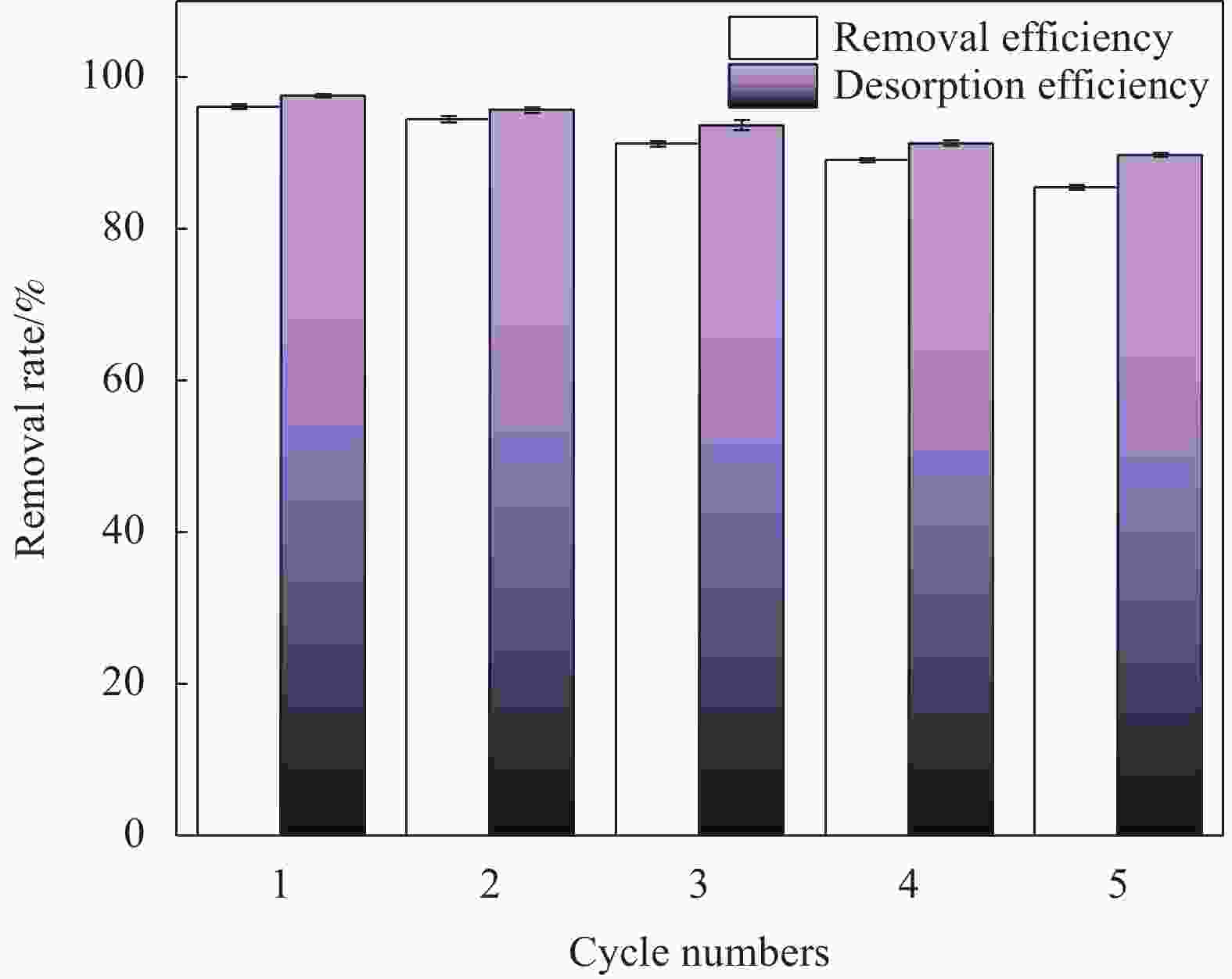
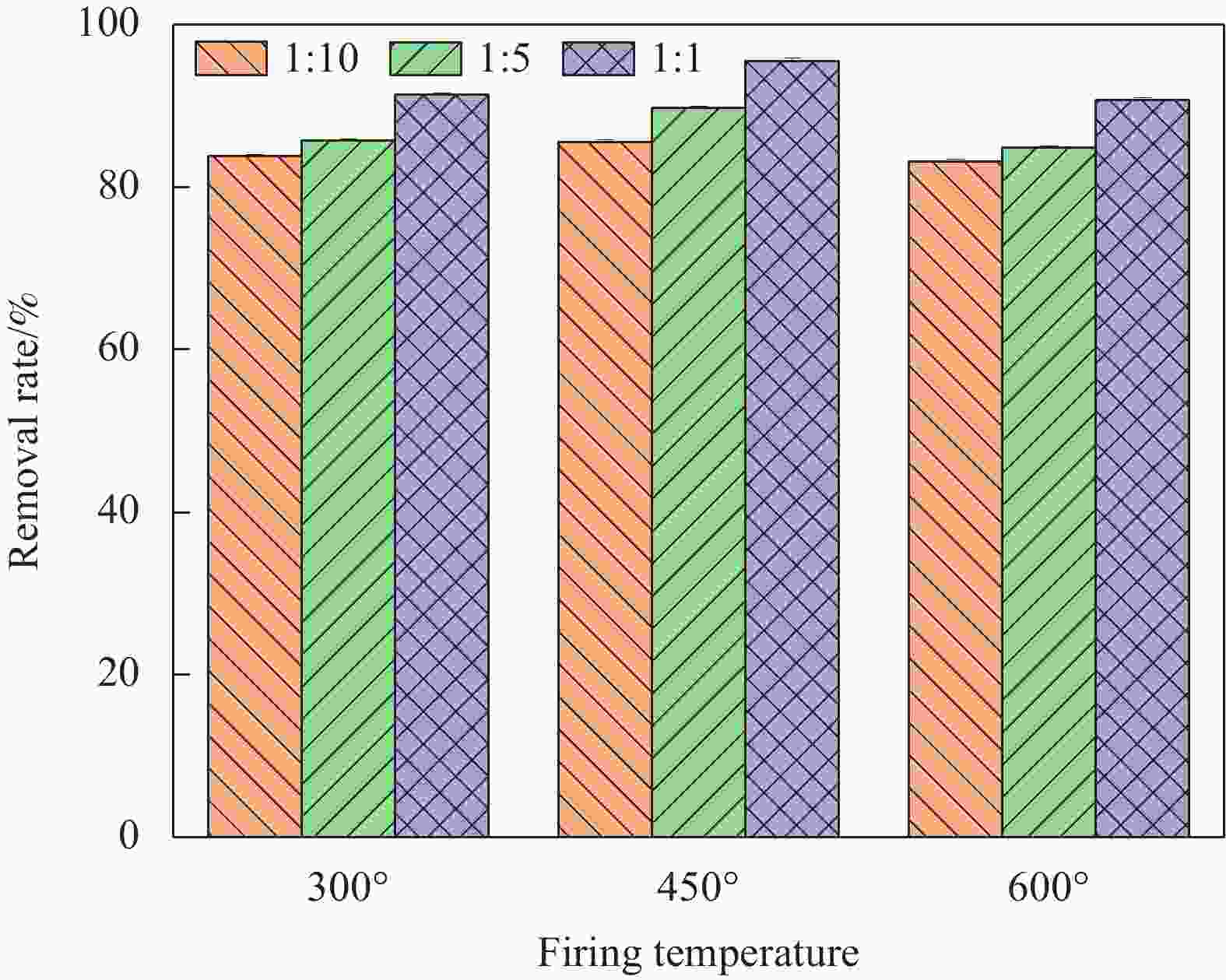
摘要: 针对利用功能材料去除水中U(Ⅵ)的效率与纳米颗粒易团聚的问题,利用玉米秸秆、蛋壳以及磁性γ-Fe2O3,采用动态油热法和浸渍法,制备了生物炭负载磁性纳米碳羟基磷灰石(CHAP-γ-Fe2O3/BC)复合材料,试验考察了其性能并用于水中U(Ⅵ)的去除。当U(Ⅵ)初始浓度为5 mg/L,CHAP-γ-Fe2O3/BC投加量为0.1 g/L,pH值为6,温度30°,反应时间1 h时,试验结果表明:CHAP-γ-Fe2O3/BC对U(Ⅵ)的最大吸附容量达324.4 mg/g,去除率达95.93%。拟二级动力学模型和Langmuir模型可较好拟合CHAP-γ-Fe2O3/BC对U(Ⅵ)的吸附过程,表明以单分子层化学吸附为主。材料通过表面改性技术,实现了减弱团聚的目的。复合材料在磁场中其表现出良好的分离回收和循环利用性。FTIR、XPS等表征结果证明该材料对铀的去除机制主要包括离子交换、溶解-沉淀的化学吸附作用和表面络合作用。Abstract: To address the efficiency of removing U(VI) from water using functional materials and the susceptibility of nanoparticles to agglomeration, biochar-loaded magnetic nanocarbon hydroxyapatite (CHAP-γ-Fe2O3/BC ) composites were prepared by dynamic oil-heating and impregnation methods using corn stover, egg shells, and magnetic γ-Fe2O, and the experiments were carried out to investigate the performances and used for the removal of U(Ⅵ) from water. When the initial concentration of U(Ⅵ) is 5 mg/L, the dosage of CHAP-γ-Fe2O3/BC is 0.1 g/L, the pH value is 6, the temperature is 30°, and the reaction time is 1 h, the experimental results show that the maximum adsorption capacity of CHAP-γ-Fe2O3/BC for U(Ⅵ) reaches 324.4 mg/g, and the removal rate reaches 95.93%. The proposed secondary kinetic model and Langmuir model could fit the adsorption process of CHAP-γ-Fe2O3/BC on U(Ⅵ) better, indicating that the monomolecular layer chemisorption is dominated. The materials are realized to attenuate the agglomeration by surface modification technique. The composite material shows good separation recovery and recyclability in the magnetic field. The characterization results of FTIR and XPS prove that the removal mechanism of uranium by this material mainly includes ion exchange, dissolution-precipitation chemisorption and surface complexation.
-
Key words:
- uranium(U(Ⅵ)) /
- biochar(BC) /
- carbon hydroxyapatite(CHAP) /
- γ-Fe2O3 /
- chemical adsorption /
- magnetic nanocomposite
-
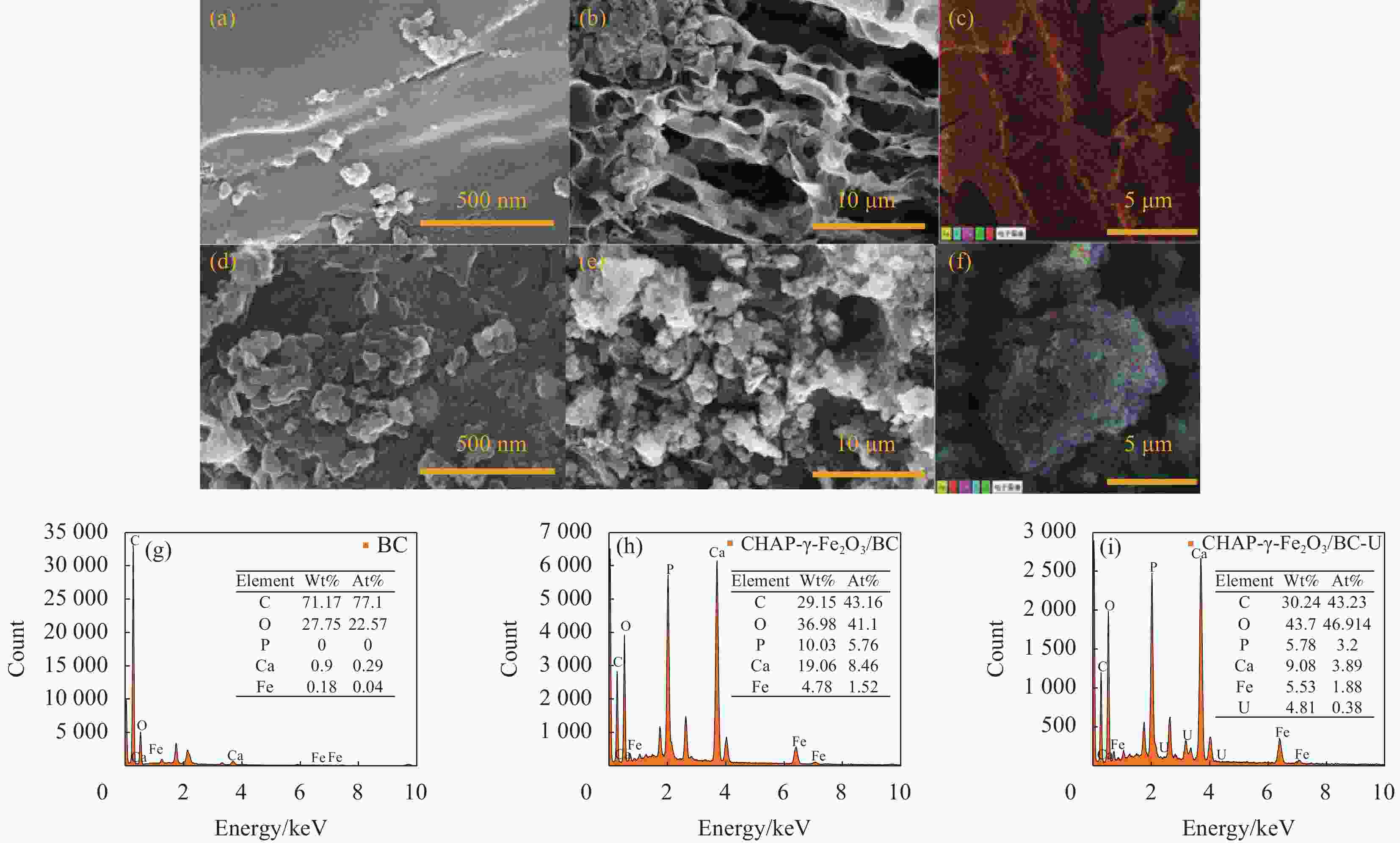
图 2 BC和CHAP-γ-Fe2O3/BC的外表面(a)、(d),横切面(b)、(e)的SEM图;BC和CHAP-γ-Fe2O3/BC的EDS-Mapping图(c)、(f);BC和CHAP-γ-Fe2O3/BC吸附U(VI)前后的EDS图谱(g)、(h)和(i)
Figure 2. SEM maps of BC and CHAP-γ-Fe2O3/BC on the outer surfaces (a), (d), and cross sections (b), (e); EDS-Mapping of BC and CHAP-γ-Fe2O3/BC (c), (f); EDS maps of BC and CHAP-γ-Fe2O3/BC before and after adsorption of U(VI) (g), (h), and (i)
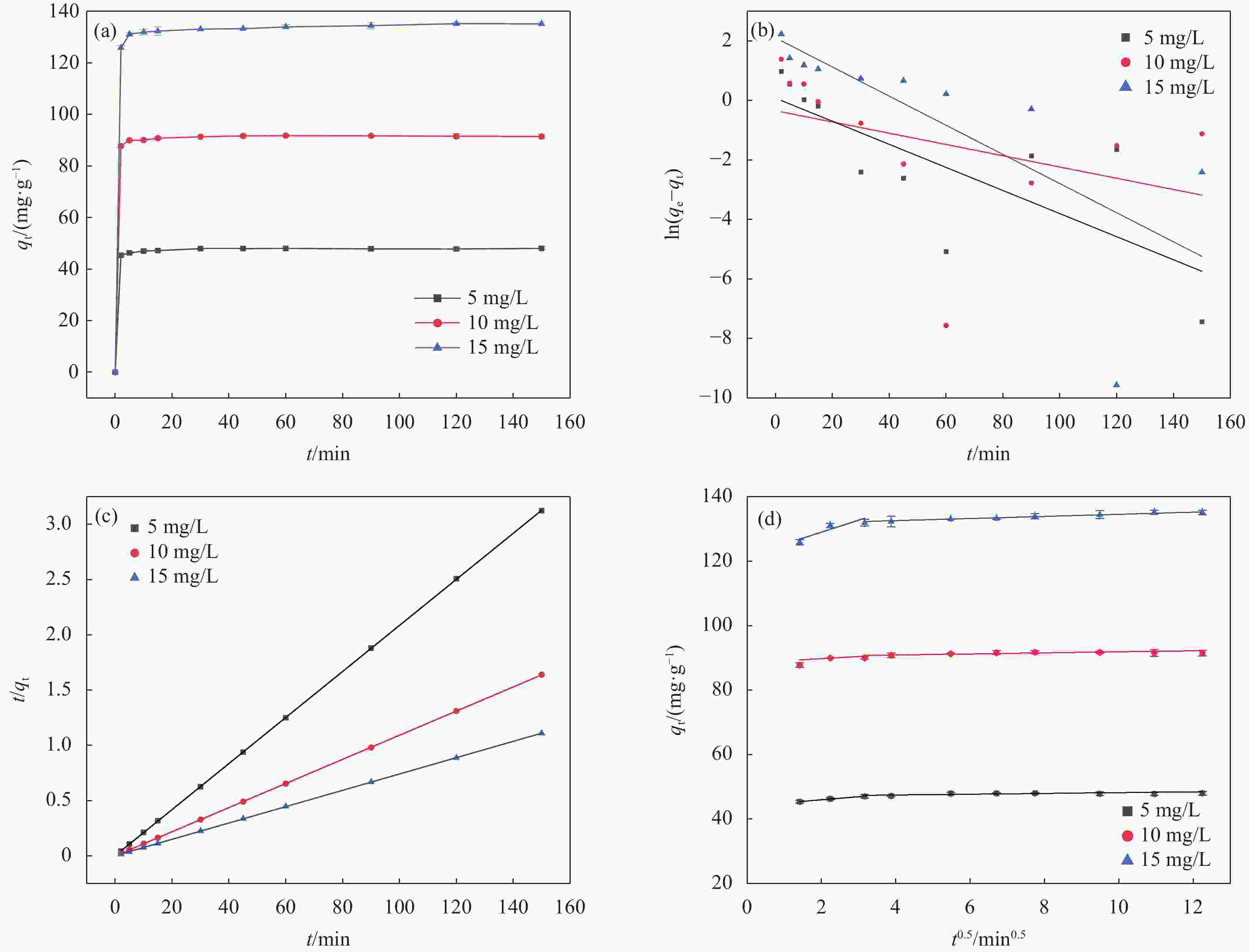
表 1 CHAP-γ-Fe2O3/BC吸附U(VI)的动力学参数
Table 1. Kinetic parameters of U(VI) adsorption by CHAP-γ-Fe2O3/BC
C0/(mg·L−1) 5 10 15 qe,exp/(mg·g−1) 48.02 90.027 125.9 Pseudo-first-order model K1/min−1 0.049 0.189 0.039 qe,cal/(mg·g−1) 8.218 0.711 1.082 R 2 0.529 0.417 0.491 Pseudo-second-order model K2/min−1 0.0077 0.0229 0.158 qe,cal/(mg·g−1) 48.15 89.928 125.3 R 2 0.999 1 0.999 Intraparticle diffusion model Kd1/(mg·(g·min0.5)−1) 0.947 0.648 3.714 C1 44.08 88.471 121.6 R1 2 0.968 0.269 0.584 Kd2/(mg·(g·min0.5)−1) 0.121 0.163 0.327 C2 46.97 90.245 131.3 R2 2 0.486 0.504 0.939 Notes: qe,exp-calculated adsorption equilibrium; qe,cal-actual adsorption equilibrium; K1 and K2-first-order and second-order rate constants; Kd1, Kd2-particle diffusion constants; C-constant; R2-linear correlation coefficient. 表 2 CHAP-γ-Fe2O3/BC对U(VI)的吸附等温线拟合参数
Table 2. Adsorption isotherm fitting parameters of CHAP-γ-Fe2O3/BC for U(VI)
T/K Langmuir model Freundlich model qmax/(mg·g−1) KL/(L·mg−1) R 2 KF/(L·mg−1) n R 2 293 315.86 0.413 0.988 107.54 2.92 0.947 303 324.415 0.456 0.991 137.30 3.70 0.928 313 350.93 0.412 0.983 117.17 2.78 0.969 Notes: qmax-maximum adsorption capacity; KL and KF-Langmuir and Freundlich adsorption equilibrium constants; n-Freundlich equation constant; R2-linear correlation coefficient. 表 4 不同材料对U(VI)吸附效果的比较
Table 4. Comparison of the adsorption effect of different materials on U (VI)
Material T/K pH qmax/(mg·g−1) Reference Magnetic biochar 298 6 17.24 [30] CA-PO4 298 5.5 150.3 [31] HAP microspheres 298 3 199 [32] P-pFGO-7 298 4 266.7 [33] M-α-FeOOH 298 5 127.73 [24] CHAP-γ-Fe2O3/BC 303 6 324.4 This work Notes:CA-PO4—phosphorylated carbon aerogel; HAP—hydroxyapatite; P-pFGO-7—phytic acid functionalized graphene oxide; M-α-FeOOH—goethite (α-FeOOH) and Fe2+-modified magnetic goethite. 表 3 CHAP-γ-Fe2O3/BC吸附U(VI)的热力学参数
Table 3. Thermodynamic parameters of U(VI) adsorption by CHAP-γ-Fe2O3/BC
T/K ΔG/(kJ·mol−1) ΔH/(kJ·mol−1) ΔS/(J·(mol·K)−1) 393 −12.49 16.393 98.574 303 −13.46 313 −14.46 Notes:ΔG—free energy; ΔH—enthalpy change; ΔS—entropy change. -
[1] AKASH S, SIVAPRAKASH B, RAJA V C V, et al. Remediation techniques for uranium removal from polluted environment - Review on methods, mechanism and toxicology[J]. Environ Pollut, 2022, 302: 119068. doi: 10.1016/j.envpol.2022.119068 [2] CHEN T, YU K, DONG C, et al. Advanced photocatalysts for uranium extraction: Elaborate design and future perspectives[J]. Coordination Chemistry Reviews, 2022, 467: 214615. doi: 10.1016/j.ccr.2022.214615 [3] WU M-B, YE H, LIU S-C, et al. Wooden composite separators with ultrahigh uranium/vanadium selectivity and antibacterial property for capturing uranium from seawater[J]. Composites Communications, 2022, 32: 101159. doi: 10.1016/j.coco.2022.101159 [4] WANG Y-Y, LIU Y-X, LU H-H, et al. Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions[J]. Journal of Solid State Chemistry, 2018, 261: 53-61. doi: 10.1016/j.jssc.2018.02.010 [5] ZHANG Q, LU L, LIANG M, et al. Efficient treatment of lead-containing wastewater using bagasse biochar modified via hydroxylapatite[J]. BioResources, 2022, 17(1): 1205-1231. doi: 10.15376/biores.17.1.1205-1231 [6] 程鹏飞, 徐文总, 程传明, 等. 改性鸡蛋壳协效膨胀型阻燃剂对热塑性聚氨酯弹性体阻燃抑烟性能的影响[J]. 复合材料学报, 2022, 40: .CHENG P F, WU W Z, CHENG C M, et al. Effect of modified chicken eggshell and intumescent flame retarant on flame retardancy and smole suppression of thermoplastic polyurethane elastomer[J]. Acta Materiae Compositae Sinica, 2023, 40: (in Chinese). [7] WEN X, SHAO C-T, CHEN W, et al. Mesoporous carbonated hydroxyapatite/chitosan porous materials for removal of Pb(II) ions under flow conditions[J]. RSC Advances, 2016, 6(115): 113940-113950. doi: 10.1039/C6RA20448A [8] JUNG K-W, LEE S Y, CHOI J-W, et al. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media[J]. Chemical Engineering Journal, 2019, 369: 529-541. doi: 10.1016/j.cej.2019.03.102 [9] ZENG R, TANG W, DING C, et al. Preparation of anionic-cationic co-substituted hydroxyapatite for heavy metal removal: Performance and mechanisms[J]. Journal of Solid State Chemistry, 2019, 280: 120960. doi: 10.1016/j.jssc.2019.120960 [10] LIAO J, HE X, ZHANG Y, et al. The construction of magnetic hydroxyapatite-functionalized pig manure-derived biochar for the efficient uranium separation[J]. Chemical Engineering Journal, 2023, 457: 141367. doi: 10.1016/j.cej.2023.141367 [11] 刘金香, 熊芬, 谢水波, 等. 纳米Fe3O4/黑曲霉磁性微球对 U(VI)的吸附性能及机制[J]. 复合材料学报, 2017, 34(12): 2826-2833.LIU Jinxiang, XIONG Fen, XIE shuibo, et al. Adsorption characteristic and mechanism of Uranium(VI) by nano Fe3O4/ Aspergillus niger magnetic microspheres[J]. Acta Materiae Compositae Sinica, 2017, 34(12): 2826-2833(in Chinese). [12] 张连科, 王洋, 王维大, 等. 磁性羟基磷灰石 /生物炭复合材料的制备及对 Pb2+ 的吸附性能[J]. 环境科学学报, 2018, 38(11): 4360-4370.ZHANG L K, WANG Y, WANG W D, et al. 2018. Preparation of magnetic hydroxyapatite /biochar composite and its adsorption behavior of Pb2+ and recycling performance[J]. Acta Scientiae Circumstantiae, 2018, 38(11): 4360-4370(in Chinese). [13] SUN Y, XIA R, ZHANG J, et al. Preparation of ferric oxide for efficient electrocatalytic oxidation of methylene blue[J]. Inorganic Chemistry Communications, 2022, 140: 109364. doi: 10.1016/j.inoche.2022.109364 [14] DAI Z, ZHEN Y, SUN Y, et al. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal[J]. Chemical Engineering Journal, 2021, 415: 129002. doi: 10.1016/j.cej.2021.129002 [15] SANG K, MEI D, WANG Y, et al. Amidoxime-functionalized zeolitic imidazolate frameworks with antimicrobial property for the removal of U (VI) from wastewater[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108344. doi: 10.1016/j.jece.2022.108344 [16] AHMED W, NUNEZ-DELGADO A, MEHMOOD S, et al. Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: Adsorption behavior and mechanism[J]. Environ Res, 2021, 201: 111518. doi: 10.1016/j.envres.2021.111518 [17] OOI C-H, LING Y P, PUNG S-Y, et al. Mesoporous hydroxyapatite derived from surfactant-templating system for p-Cresol adsorption: Physicochemical properties, formation process and adsorption performance[J]. Powder Technology, 2019, 342: 725-734. doi: 10.1016/j.powtec.2018.10.043 [18] MA K, CUI H, ZHOU A, et al. Mesoporous hydroxyapatite: Synthesis in molecular self-assembly and adsorption properties[J]. Microporous and Mesoporous Materials, 2021, 323: 111164. doi: 10.1016/j.micromeso.2021.111164 [19] ZHU S, IRSHAD M K, IBRAHIM M, et al. The distinctive role of nano-hydroxyapatite modified biochar for alleviation of cadmium and arsenic toxicity in aqueous system[J]. Journal of Water Process Engineering, 2022, 49: 103054. doi: 10.1016/j.jwpe.2022.103054 [20] ZHOU C, SONG X, WANG Y, et al. The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil[J]. Chemosphere, 2022, 286(Pt3): 131810. [21] ROUHANI M, ASHRAFI S D, TAGHAVI K, et al. Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions[J]. Journal of Molecular Liquids, 2022, 356: 119040. doi: 10.1016/j.molliq.2022.119040 [22] CHAUDHARY M, SINGH L, REKHA P, et al. Adsorption of uranium from aqueous solution as well as seawater conditions by nitrogen-enriched nanoporous polytriazine[J]. Chemical Engineering Journal, 2019, 378: 122236. doi: 10.1016/j.cej.2019.122236 [23] SHARMA M, CHAUDHARY K, KUMARI M, et al. Highly efficient, economic, and recyclable glutathione decorated magnetically separable nanocomposite for uranium(VI) adsorption from aqueous solution[J]. Materials Today Chemistry, 2020, 18: 100379. doi: 10.1016/j.mtchem.2020.100379 [24] JIANG T-J, ZHANG X-W, XIE C, et al. Effective capture of aqueous uranium using a novel magnetic goethite: Properties and mechanism[J]. Journal of Solid State Chemistry, 2021, 300: 122236. doi: 10.1016/j.jssc.2021.122236 [25] LIU L, LIU X, WANG D, et al. Removal and reduction of Cr(Ⅵ) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge[J]. Journal of Cleaner Production, 2020, 257: 120562. doi: 10.1016/j.jclepro.2020.120562 [26] KAUR M, TEWATIA P, RATTAN G, et al. Diamidoximated cellulosic bioadsorbents from hemp stalks for elimination of uranium (VI) and textile waste in aqueous systems[J]. Journal of Hazardous Materials, 2021, 417: 126060. doi: 10.1016/j.jhazmat.2021.126060 [27] LIU F, HUANG W, WANG S, et al. Investigation of adsorption properties and mechanism of uranium(VI) and europium(III) on magnetic amidoxime-functionalized MCM-41[J]. Applied Surface Science, 2022, 594: 153376. doi: 10.1016/j.apsusc.2022.153376 [28] CHEN Z, WANG J, PU Z, et al. Synthesis of magnetic Fe3O4 /CFA composites for the efficient removal of U(VI) from wastewater[J]. Chemical Engineering Journal, 2017, 320: 448-457. doi: 10.1016/j.cej.2017.03.074 [29] FAN Q H, HAO L M, WANG C L, et al. The adsorption behavior of (VI) on granite[J]. Environmental Science:Processes & Impacts, 2014, 16(3): 534-541. [30] AHMED W, MEHMOOD S, NUNEZ-DELGADO A, et al. Fabrication, characterization and U(VI) sorption properties of a novel biochar derived from Tribulus terrestris via two different approaches[J]. Sci Total Environ, 2021, 780: 146617. doi: 10.1016/j.scitotenv.2021.146617 [31] ZHANG Z, DONG Z, WANG X, et al. Synthesis of ultralight phosphorylated carbon aerogel for efficient removal of U(VI): Batch and fixed-bed column studies[J]. Chemical Engineering Journal, 2019, 370: 1376-1387. doi: 10.1016/j.cej.2019.04.012 [32] WU Y, CHEN D, KONG L, et al. Rapid and effective removal of uranium (VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres[J]. J Hazard Mater, 2019, 371: 397-405. doi: 10.1016/j.jhazmat.2019.02.110 [33] LEI H, ZHOU D, TANG J, et al. Epoxy graphene oxide from a simple photo-Fenton reaction and its hybrid with phytic acid for enhancing U(VI) capture[J]. Sci Total Environ, 2020, 738: 140316. doi: 10.1016/j.scitotenv.2020.140316 [34] WEI-RONG CUI F-F L, RUI-HAN XU, CHENG-RONG, ZHANG X-R C, RUN-HAN YAN, RU-PING LIANG, AND, QIU J-D. Regenerable Covalent Organic Frameworks for Photo-enhanced Uranium Adsorption from Seawater[J]. Angewandte Chemie 2020, 59: 17684-17690. [35] YANG S, LI Q, CHEN L, et al. Synergistic removal and reduction of U(VI) and Cr(VI) by Fe3S4 micro-crystal[J]. Chemical Engineering Journal, 2020, 385: 123909. doi: 10.1016/j.cej.2019.123909 [36] GUO Y, GONG Z, LI C, et al. Efficient removal of uranium (VI) by 3D hierarchical Mg/Fe-LDH supported nanoscale hydroxyapatite: A synthetic experimental and mechanism studies[J]. Chemical Engineering Journal, 2020, 392: 123682. doi: 10.1016/j.cej.2019.123682 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 47
- HTML全文浏览量: 49
- 被引次数: 0




 下载:
下载: