Performance of Cu-BTC-derived CuOx/C for methanol oxidative carbonylation to dimethyl carbonate
-
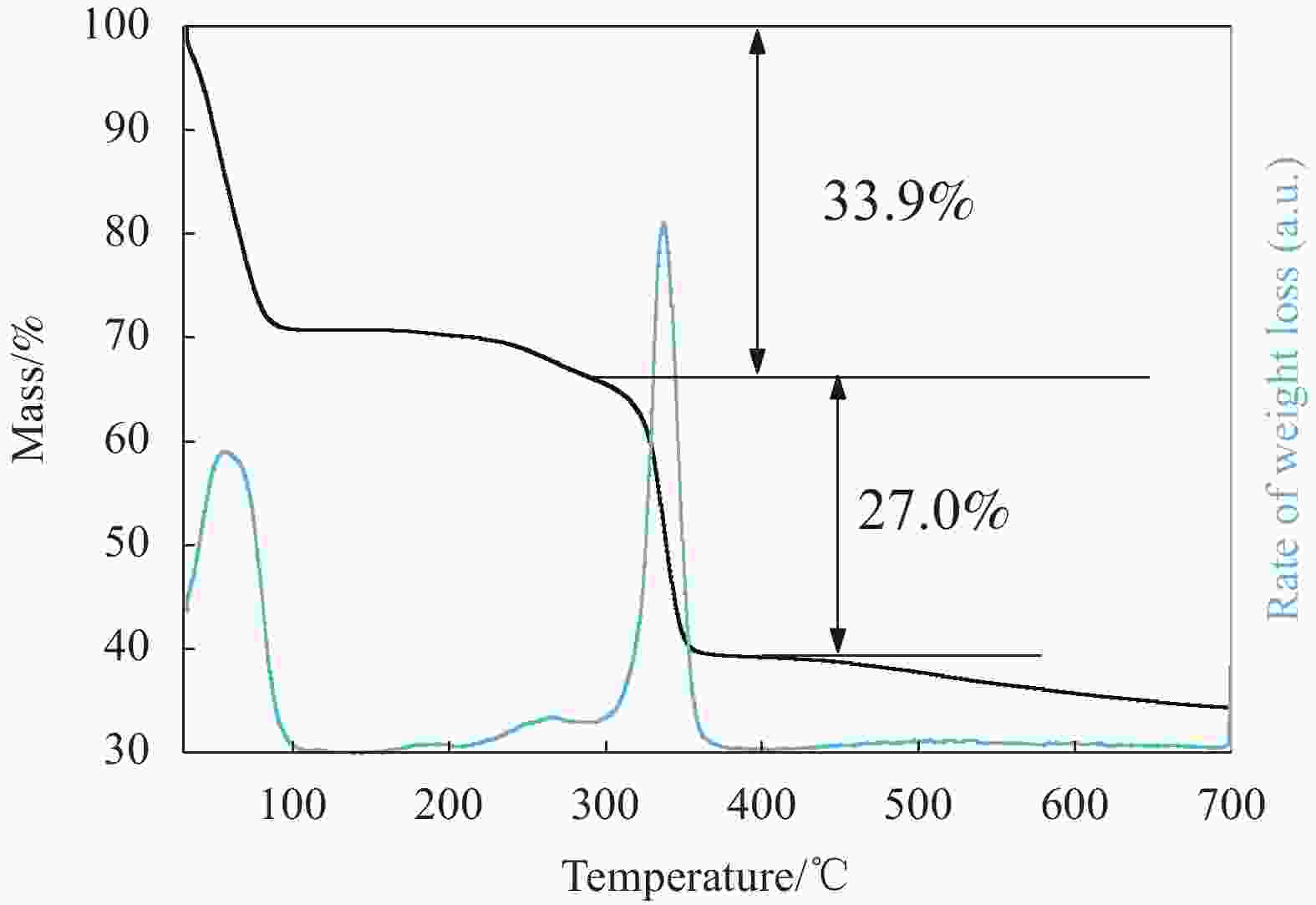
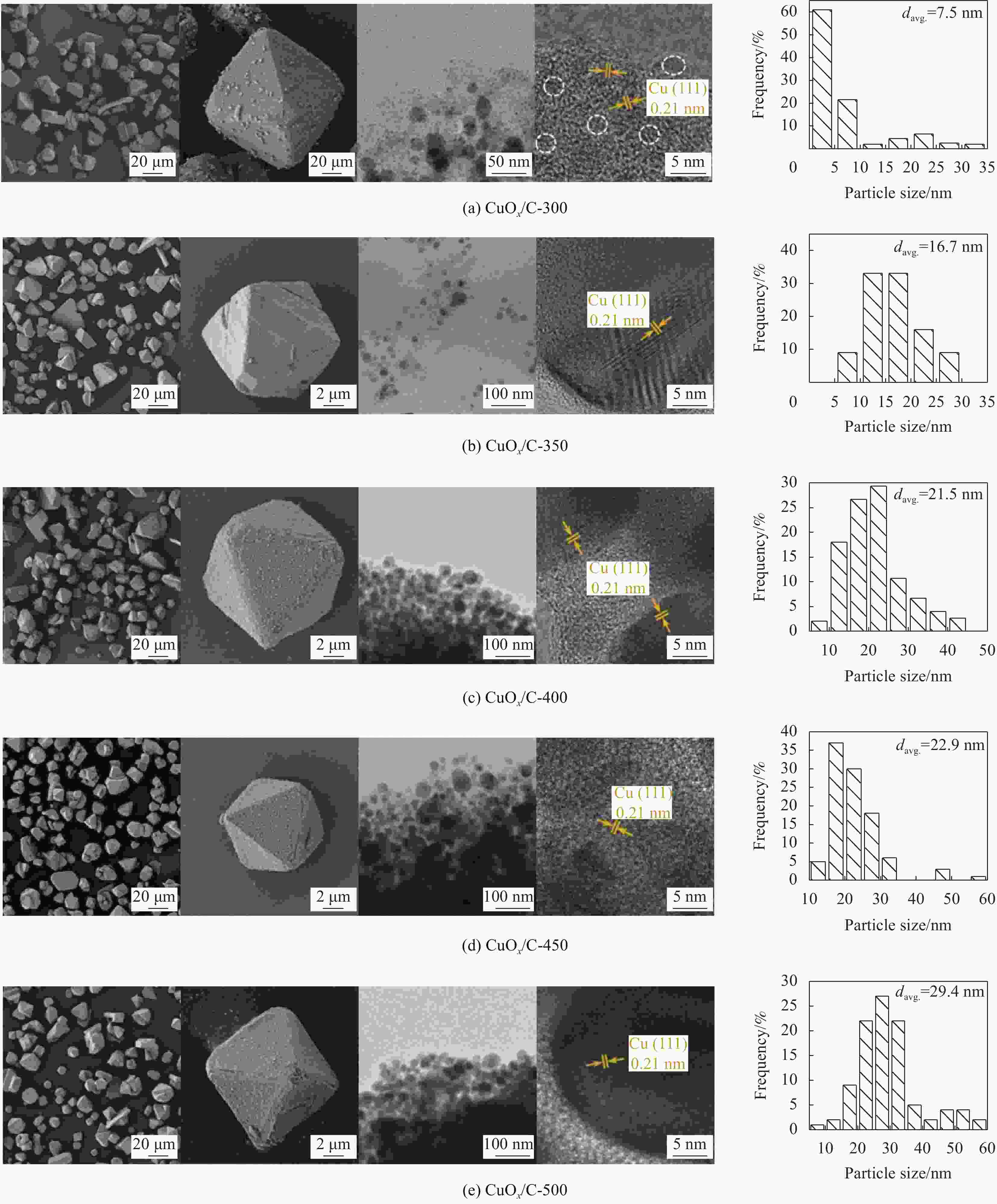
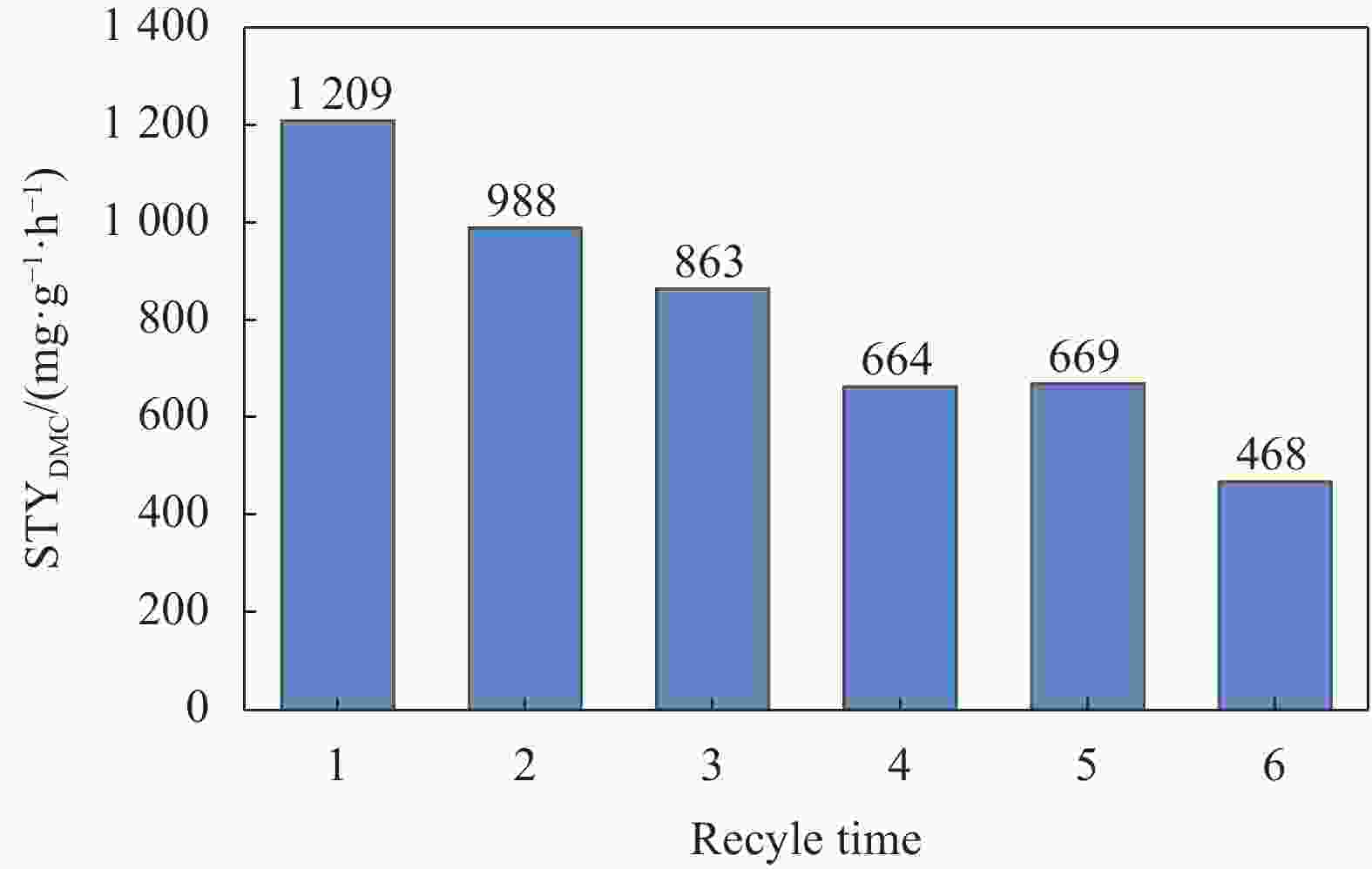
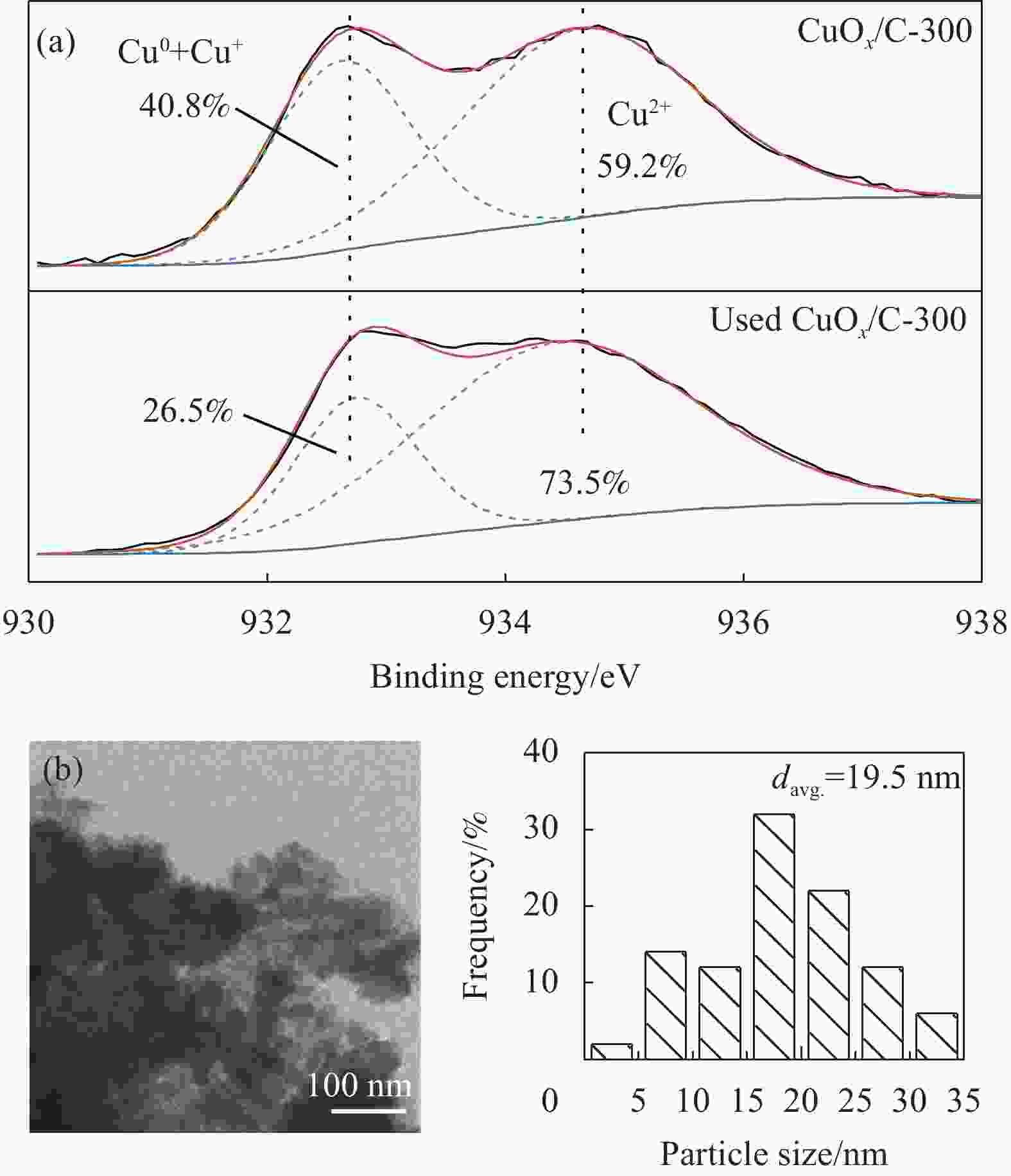
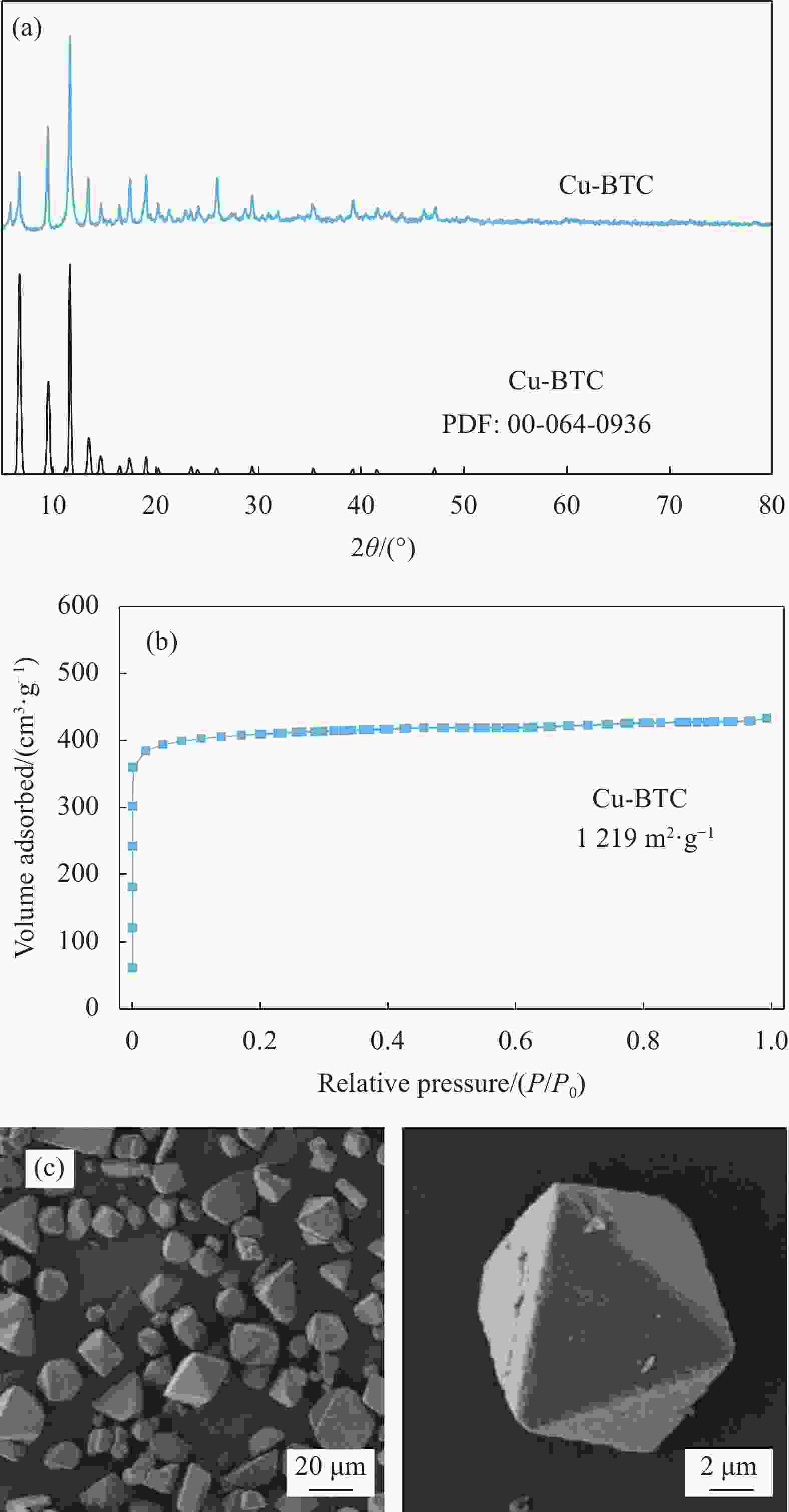
摘要: 碳基材料负载Cu是一类高效的甲醇氧化羰基化合成碳酸二甲酯催化剂,但存在Cu纳米颗粒易团聚和氧化等问题。采用溶剂热法制备了Cu-BTC,并以其为前驱体,在N2气氛下热解,制备了碳负载Cu及其氧化物(CuOx/C)催化剂。考察了热解温度对Cu纳米颗粒粒径、Cu的价态以及甲醇氧化羰基化合成碳酸二甲酯性能的影响。表征结果显示,升高热解温度有利于Cu2+还原为(Cu0+Cu+),但会导致Cu纳米颗粒团聚。催化剂活性随着热解温度升高而降低,当热解温度为300℃时,制备的CuOx/C-300具有最优的催化活性,碳酸二甲酯的时空收率为1209 mg·g−1·h−1,因为其Cu纳米颗粒的粒径最小(7.5 nm)。经过6次循环实验后,碳酸二甲酯的时空收率降至468 mg·g−1·h−1,催化剂失活的主要原因是(Cu0+Cu+)的氧化和Cu纳米颗粒团聚。Abstract: Carbon-based material supported Cu is an efficient catalyst for methanol oxidative carbonylation to dimethyl carbonate, but Cu nanoparticles are prone to agglomeration and oxidation. Cu-BTC were prepared by hydrothermal method. And then CuOx/C catalysts was prepared by pyrolysis Cu-BTC under N2 atmosphere. The effect of pyrolysis temperature on Cu nanoparticle size, Cu valence state and performance for methanol oxidative carbonylation to dimethyl carbonate were investigated. The characterization results show that increasing the pyrolysis temperature is beneficial to the reduction of Cu2+ to (Cu0+Cu+), but would lead to the agglomeration of Cu nanoparticles. Catalytic activity decreases with the increasing of pyrolysis temperature. CuOx/C-300, prepared by pyrolysis of Cu-BTC at 300 ℃, shows the optimized catalytic activity and the space-time yield of dimethyl carbonate is 1209 mg·g−1·h−1. This is attributed to the smallest particle size of Cu nanoparticles (7.5 nm). In addition, the space-time yield of dimethyl carbonate decreased to 468 mg·g−1·h−1 after 6 cycle experiments. The main reasons for catalyst inactivation are the oxidation of (Cu0+Cu+) and the agglomeration of Cu nanoparticles.
-
Key words:
- catalyst /
- carbon-based materials /
- Cu nanoparticles /
- dimethyl carbonate /
- MOFs /
- pyrolysis /
- oxidative carbonylation
-
表 1 CuOx/C催化剂中Cu和表面O含量及组成
Table 1. Cu mass fraction and surface O fraction and content of CuOx/C catalysts
Catalyst Cu/(wt.%)① O/(at.%)② Area of O1 s /% C=O O=C—O C—OH CuOx/C-300 63.1 26.9 90.9 3.6 5.5 CuOx/C-350 58.2 14.3 71.4 3.6 25.0 CuOx/C-400 61.6 13.6 44.3 6.4 49.3 CuOx/C-450 61.8 11.6 34.9 5.9 59.2 CuOx/C-500 56.6 10.5 39.5 4.0 56.5 Notes: ①Determined based on ICP-OES results.
②Determined based on fitting results of XPS survey spectra.表 2 催化剂性能表
Table 2. catalytic performance of catalysts
Catalyst CMeOH①/% SDMC②/% STYDMC③/(mg·g-1·h-1) Cu-BTC 0 0 0 Cu-BTC-250 0 0 0 CuOx/C-300 2.19 100 1209 CuOx/C-350 2.01 100 1111 CuOx/C-400 1.71 100 948 CuOx/C-450 1.32 100 724 CuOx/C-500 1.14 100 634 Notes: ①Coverted methanol/total methanol, mol/mol. ②Methanol converted to DMC/converted methanol, mol/mol. ③The amount of DMC based on per unit of catalyst and per unit of time. -
[1] TAMBOLI A H, CHAUGULE A A, KIM H. Catalytic developments in the direct dimethyl carbonate synthesis from carbon dioxide and methanol[J]. Chemical Engineering Journal, 2017, 323: 530-544. doi: 10.1016/j.cej.2017.04.112 [2] SELVA M, PEROSA A, RODRÍGUEZ-PADRÓN D, et al. Applications of dimethyl carbonate for the chemical upgrading of biosourced platform chemicals[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6471-6479. [3] ZHANG M, XU Y H, WILLIAMS B L, et al. Catalytic materials for direct synthesis of dimethyl carbonate (DMC) from CO2[J]. Journal of Cleaner Production, 2021, 279: 123344. doi: 10.1016/j.jclepro.2020.123344 [4] 丁晓墅, 李乃华, 王淑芳等. 甲醇为原料联合制备碳酸二甲酯、甲缩醛和二甲醚反应体系热力学计算及节能分析[J]. 化工学报, 2015, 66(07): 2377-2386. doi: 10.11949/j.issn.0438-1157.20141673DING X S, LI N H, WANG S F, et al. Thermodynamic calculation and energy saving analysis of combined reaction system for production of dimethyl carbonate, methylal and dimethyl ether from methanol[J]. CIESC Journal, 2015, 66(07): 2377-2386(in Chinese). doi: 10.11949/j.issn.0438-1157.20141673 [5] KUMAR P, SRIVASTAVA V C, ŠTANGAR U L, et al. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts[J]. Catalysis Reviews, 2019, 63(3): 363-421. [6] HUANG S Y, YAN B, WANG S P, et al. Recent advances in dialkyl carbonates synthesis and applications[J]. Chemical Society Reviews, 2015, 44(10): 3079-3116. doi: 10.1039/C4CS00374H [7] ROMANO U, RIVETTI F, MUZIO N D. Process for producing dimethyl carbonate: US4318862A [P]. 1982-03-09. [8] HAN M S, LEE B G, SUH I, et al. Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol over Cu-based catalysts[J]. Journal of Molecular Catalysis A:Chemical, 2001, 170: 225-234 doi: 10.1016/S1381-1169(01)00073-5 [9] 宋宇淙, 丁晓墅, 闫亚辉等. 氧化石墨烯复合金属催化剂催化碳酸二甲酯合成反应性能[J]. 化工学报, 2019, 70(4): 1401-1408.SONG Y C, DING X S, YAN Y H, et al. Catalytic performance of graphene oxide composite metal catalyst in dimethyl carbonate synthesis[J]. CIESC Journal, 2019, 70(4): 1401-1408(in Chinese). [10] MO W L, XIONG H, LI T, et al. The catalytic performance and corrosion inhibition of CuCl/Schiff base system in homogeneous oxidative carbonylation of methanol[J]. Journal of Molecular Catalysis A:Chemical, 2006, 247(1-2): 227-232. doi: 10.1016/j.molcata.2005.11.051 [11] RAAB V, MERZ M, SUNDERMEYER J. Ligand effects in the copper catalyzed aerobic oxidative carbonylation of methanol to dimethyl carbonate (DMC)[J]. Journal of Molecular Catalysis A:Chemical, 2001, 175: 51-63. doi: 10.1016/S1381-1169(01)00220-5 [12] LIU D H, HE J, SUN L B, et al. Cupric bromide-derived complex: An effective homogeneous catalyst for oxidative carbonylation of methanol to dimethyl carbonate[J]. Journal of the Taiwan Institute of Chemical Engineers, 2011, 42(4): 616-621. doi: 10.1016/j.jtice.2010.10.005 [13] 肖艳华, 孙志康, 文武强等. 铜配合物Cu( 5-CH3-1, 10-phenanthroline) Br2催化甲醇氧化羰基化[J]. 精细化工, 2017, 34(04): 424-430.XIAO Y H, SUN Z K, WEN W Q, et al. Oxidative carbonylation of methanol catalyzed by Cu(5-CH3-1, 10-phenanthroline)Br2[J]. Fine Chemicals, 2017, 34(04): 424-430(in Chinese). [14] 邓志勇, 李文杰, 林慧博等. 醇直接氧化羰基化合成碳酸二甲酯催化剂的研究进展[J]. 精细化工, 2022, 39(11): 2196-2202+2214 doi: 10.13550/j.jxhg.20220423DENG Z Y, LI W J, LIN H B, et al. Research progress of catalysts for synthesis of dimethyl carbonate via direct oxidative carbonylation of methanol[J]. Fine Chemicals, 2022, 39(11): 2196-2202+2214 (in Chinese) doi: 10.13550/j.jxhg.20220423 [15] SHI K, HUANG S Y, ZHANG Z Y, et al. Novel fabrication of copper oxides on AC and its enhanced catalytic performance on oxidative carbonylation of methanol[J]. Chinese Chemical Letters, 2017, 28(1): 70-74. doi: 10.1016/j.cclet.2016.06.005 [16] ZHANG G Q, YAN J F, WANG J J, et al. Effect of carbon support on the catalytic performance of Cu-based nanoparticles for oxidative carbonylation of methanol[J]. Applied Surface Science, 2018, 455: 696-704. doi: 10.1016/j.apsusc.2018.05.114 [17] SHI R N, ZHAO J X, QUAN Y H, et al. Fabrication of few-layer graphene-supported copper catalysts using a lithium-promoted thermal exfoliation method for methanol oxidative carbonylation[J]. ACS Applied Materials & Interfaces, 2020, 12(27): 30483-30493. [18] SHI R N, WANG J A, ZHAO J X, et al. Cu nanoparticles encapsulated with hollow carbon spheres for methanol oxidative carbonylation: Tuning of the catalytic properties by particle size control[J]. Applied Surface Science, 2018, 459: 707-715. doi: 10.1016/j.apsusc.2018.08.032 [19] WANG J J, YANG L, FU T J, et al. The confinement effects of ordered mesoporous carbon on copper nanoparticles for methanol oxidative carbonylation[J]. New Journal of Chemistry, 2022, 46(6): 2980-2988. doi: 10.1039/D1NJ05480E [20] ZHANG G Q, LI Z, ZHENG H Y, et al. Influence of surface oxygenated groups on the formation of active Cu species and the catalytic activity of Cu/AC catalyst for the synthesis of dimethyl carbonate[J]. Applied Surface Science, 2016, 390: 68-77. doi: 10.1016/j.apsusc.2016.08.054 [21] ZHANG G Q, ZHAO D, YAN J F, et al. The promotion and stabilization effects of surface nitrogen containing groups of CNT on cu-based nanoparticles in the oxidative carbonylation reaction[J]. Applied Catalysis A:General, 2019, 579: 18-29. doi: 10.1016/j.apcata.2019.04.012 [22] FURUKAWA H, CORDOVA K E, O'KEEFFE M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. doi: 10.1126/science.1230444 [23] KONNERTH H, MATSAGAR B M, CHEN S S, et al. Metal-organic framework (MOF)-derived catalysts for fine chemical production[J]. Coordination Chemistry Reviews, 2020, 416: 213319. doi: 10.1016/j.ccr.2020.213319 [24] LIU X L, VERMA G, CHEN Z S, et al. Metal-organic framework nanocrystal-derived hollow porous materials: Synthetic strategies and emerging applications[J]. Innovation (Camb), 2022, 3(5): 100281. [25] 黄国芳, 程佳, 王娜等. 金属有机框架衍生单原子催化剂的构筑策略及其在制氢反应中的应用[J/OL]. 复合材料学报, 1-14[2023-09-13].HONG G F, CHENG J, WANG N, et al. Construction strategy of metal-organic frameworks derived single-atom catalysts and their application in hydrogen production[J/OL]. Acta Materiae Compositae Sinica, 1-14 [2023-09-13]. (in Chinese) [26] WANG Q, XIAO L P, LV Y H, et al. Metal–organic-framework-derived copper catalysts for the hydrogenolysis of lignin into monomeric phenols[J]. ACS Catalysis, 2022, 12(19): 11899-11909. doi: 10.1021/acscatal.2c02955 [27] JIA Y, LIU Y L, ZHAO P H, et al. Chemical thermodynamic and catalytic mechanism analysis of Cu-BTC-derived CuOx/C catalyst for selective catalytic reduction (SCR)[J]. Molecular Catalysis, 2022, 531: 112710. doi: 10.1016/j.mcat.2022.112710 [28] HUANG K M, CHEN S Y, XIA C J, et al. HKUST-1 derived Cu@CuOx/carbon catalyst for base-free aerobic oxidative coupling of benzophenone imine: high catalytic efficiency and excellent regeneration performance[J]. RSC Advances, 2020, 10(59): 36111-36118. doi: 10.1039/D0RA06367C [29] ZHANG R R, HU L, BAO S X, et al. Surface polarization enhancement: high catalytic performance of Cu/CuOx/C nanocomposites derived from Cu-BTC for CO oxidation[J]. Journal of Materials Chemistry A, 2016, 4(21): 8412-8420. doi: 10.1039/C6TA01199C [30] CHEN K, LING J L, WU C D. In situ generation and stabilization of accessible Cu/Cu2O heterojunctions inside organic frameworks for highly efficient catalysis[J]. Angewandte Chemie International Edition, 2020, 59(5): 1925-1931. doi: 10.1002/anie.201913811 [31] PEI Y L, ZHAO J X, SHI R N, et al. Hierarchical porous carbon-supported copper nanoparticles as an efficient catalyst for the dimethyl carbonate synthesis[J]. Catalysis Letters, 2019, 149(11): 3184-3193. doi: 10.1007/s10562-019-02884-7 [32] 李忠, 文春梅, 王瑞玉等. 醋酸铜热解制备无氯Cu2O/AC催化剂及其催化氧化羰基化[J]. 高等学校化学学报, 2009, 30: 2024-2031. doi: 10.3321/j.issn:0251-0790.2009.10.021LI Z, WEN C M, WANG R W, et al. Chloride-free Cu2O/AC catalyst prepared by pyrolysis of copper acetate and catalytic oxycarbonylation[J]. Chemical Journal of Chinese Universities, 2009, 30: 2024-2031(in Chinese). doi: 10.3321/j.issn:0251-0790.2009.10.021 [33] ZHAO J X, SHI R N, QUAN Y H, et al. Highly efficient synthesis of dimethyl carbonate over copper catalysts supported on resin-derived carbon microspheres[J]. Chemical Engineering Science, 2019, 207: 1060-1071. doi: 10.1016/j.ces.2019.07.039 [34] SHI R N, ZHAO J X, QUAN Y H, et al. Carbon-supported nitrogen-doped graphene-wrapped copper nanoparticles: an effective catalyst for the oxidative carbonylation of methanol[J]. Industrial & Engineering Chemistry Research, 2021, 60(7): 2944-2953. [35] YAN J, WANG H, JIN B, et al. Cu-MOF derived Cu/Cu2O/C nanocomposites for the efficient thermal decomposition of ammonium perchlorate[J]. Journal of Solid State Chemistry, 2021, 297: 122060. doi: 10.1016/j.jssc.2021.122060 [36] KHAN I A, BADSHAH A, NADEEM M A, et al. A copper based metal-organic framework as single source for the synthesis of electrode materials for high-performance supercapacitors and glucose sensing applications[J]. International Journal of Hydrogen Energy, 2014, 39(34): 19609-19620. doi: 10.1016/j.ijhydene.2014.09.106 [37] LIU H L, CHANG Z L, FU J, et al. A CuZn-BTC derived stable Cu/ZnO@SiO2 catalyst for ethanol dehydrogenation[J]. Applied Catalysis B:Environmental, 2023, 324: 122194. doi: 10.1016/j.apcatb.2022.122194 [38] PAN J Y, BAI X T, LI Y Y, et al. HKUST-1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance[J]. Environment Research, 2022, 205: 112425. doi: 10.1016/j.envres.2021.112425 [39] BAO L W, YANG S Q, HU T L, Cu-NPs@C nanosheets derived from a PVP-assisted 2d Cu-MOF with renewable ligand for high-efficient selective hydrogenation of 5-hydroxymethylfurfural[J]. ChemSusChem, 2022, 15(13): e202200392. [40] ZHANG G Q, LI Z, ZHENG H Y, et al. Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol[J]. Applied Catalysis B:Environmental, 2015, 179: 95-105. doi: 10.1016/j.apcatb.2015.05.001 [41] ZHANG G Q, LI Z, ZHENG H Y, et al. Influence of surface oxygenated groups on the formation of active Cu species and the catalytic activity of Cu/AC catalyst for the synthesis of dimethyl carbonate[J]. Applied Surface Science, 2016, 390: 68-77. doi: 10.1016/j.apsusc.2016.08.054 [42] WANG J, SHI R N, HAO P P, et al. Influence of oxygen-containing groups of activated carbon aerogels on copper/activated carbon aerogels catalyst and synthesis of dimethyl carbonate[J]. Journal of Materials Science, 2018, 53: 1833-18050 doi: 10.1007/s10853-017-1639-8 -





 下载:
下载: