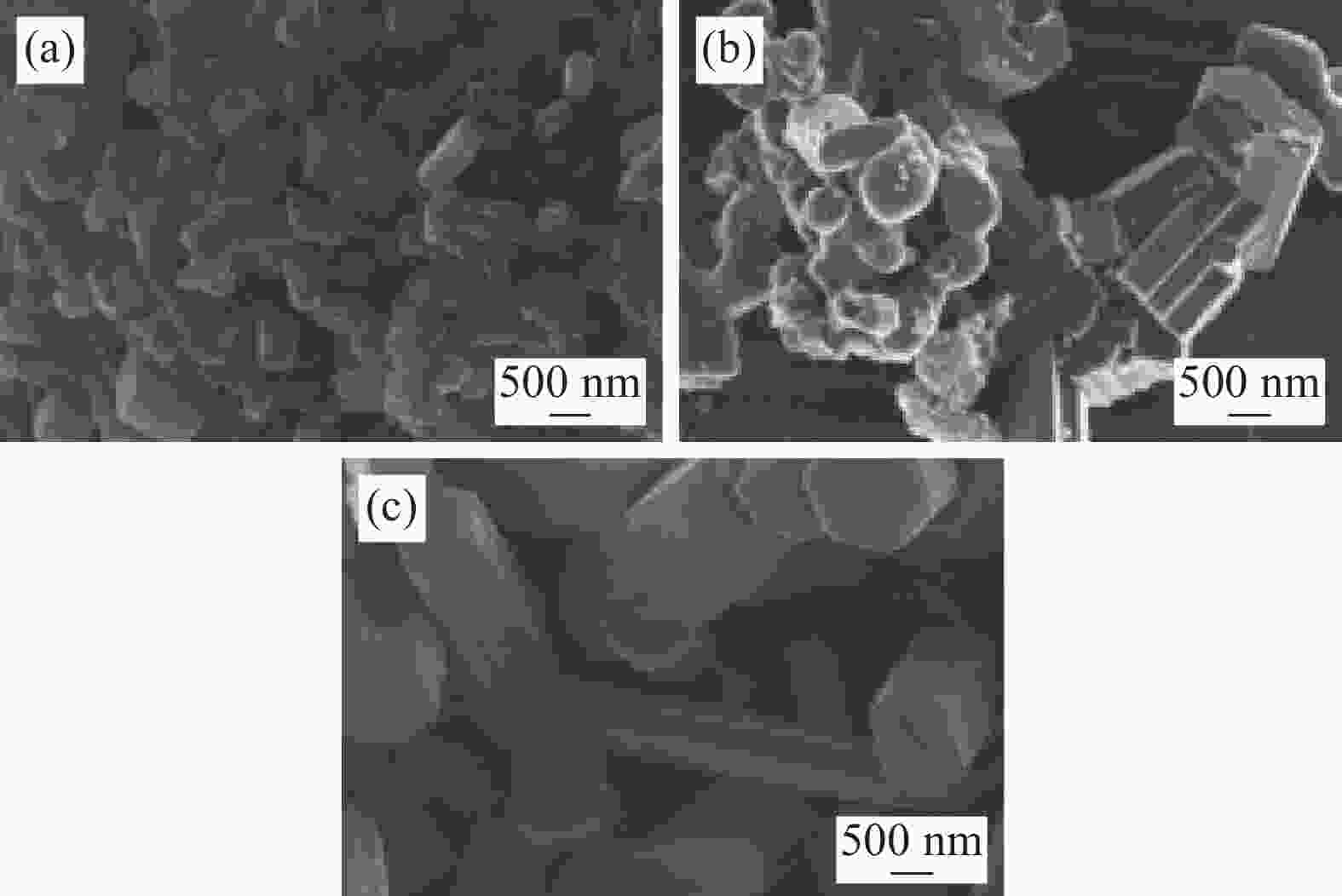
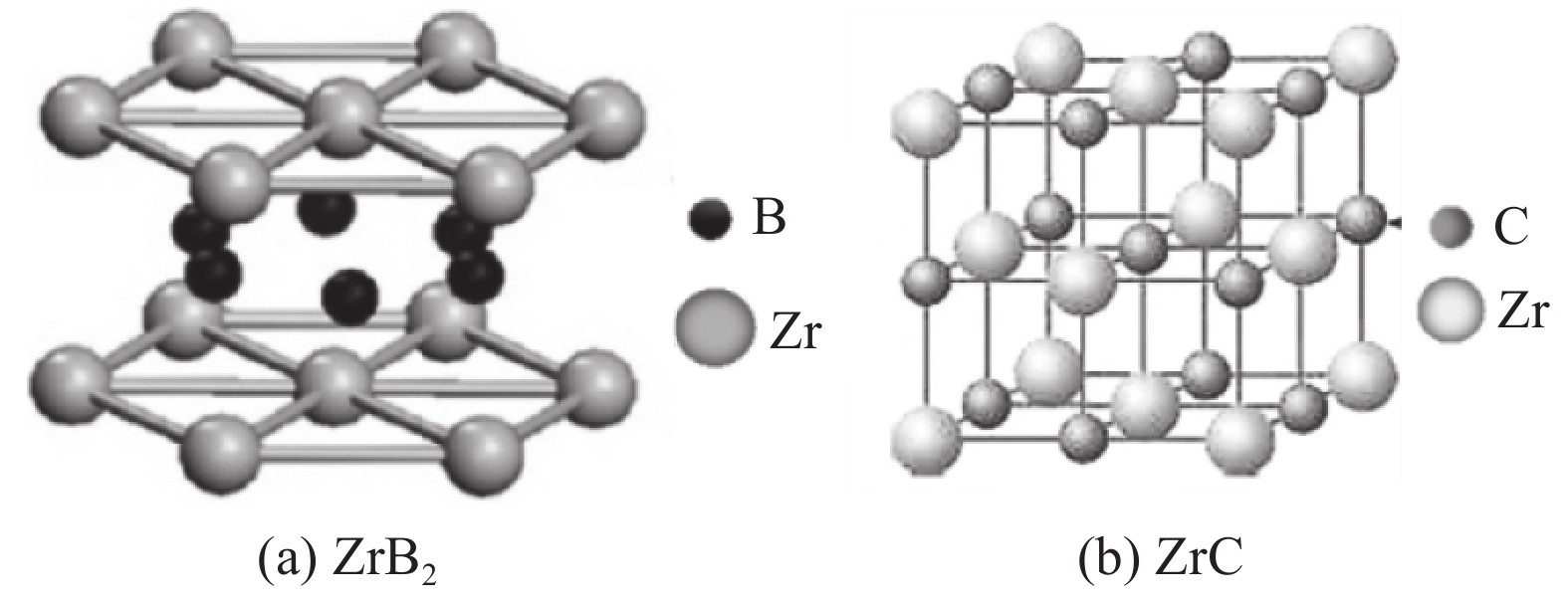
Preparation status of ZrB2, ZrC single-phase powders and ZrB2-ZrC composite powder
-
摘要: 随着科学技术的飞速发展及日益增长的技术需求,不仅能够承受高温同时可以在高温下依然保持高强度、高抗氧化性等优异性能的超高温陶瓷材料成为主要研究趋势。ZrB2和ZrC由于具有高熔点、良好的导电导热性、低密度、较低的热膨胀系数等,同时在高温下具有高强度和良好的抗氧化性等优点,成为非常有潜力的超高温结构陶瓷材料。目前ZrB2、ZrC单相粉末已经很难满足航空航天领域中极端条件的要求,因此ZrB2-ZrC复合粉末的制备研究受到广泛关注。本文对ZrB2、ZrC单相粉末及ZrB2-ZrC复合粉末的合成原理及制备方法进行了综述,分析了目前ZrB2、ZrC单相粉末及ZrB2-ZrC复合粉末制备研究中存在的局限,对其未来研究方向进行了展望。
-
关键词:
- ZrB2粉末 /
- ZrC粉末 /
- ZrB2-ZrC复合粉末 /
- 合成原理 /
- 制备方法
Abstract: With the rapid development of science and technology and the increasing technical demand, ultra-high temperature ceramic materials can not only withstand high temperatures but also maintain high strength and high oxidation resistance at high temperatures, making them become the main research trend. ZrB2 and ZrC have become very potential ultra-high temperature structural ceramic materials because of their high melting point, good electrical and thermal conductivity, low density, low thermal expansion coefficient, and high strength and good oxidation resistance at high temperatures. However, ZrB2 and ZrC single-phase powders are difficult to meet the requirements of extreme conditions in aerospace field, so preparing ZrB2-ZrC composite powders has been widely concerned. The synthesis mechanism and preparation methods of ZrB2 and ZrC single-phase powders and ZrB2-ZrC composite powder are reviewed. The limitations of current preparation and application of ZrB2 and ZrC single-phase powders and ZrB2-ZrC composite powder are analyzed, the future research direction is prospected.-
Key words:
- ZrB2 powder /
- ZrC powder /
- ZrB2-ZrC composite powder /
- synthesis mechanism /
- preparation methods
-
表 1 ZrB2粉末的合成原理
Table 1. Principle of synthesis of ZrB2 powder
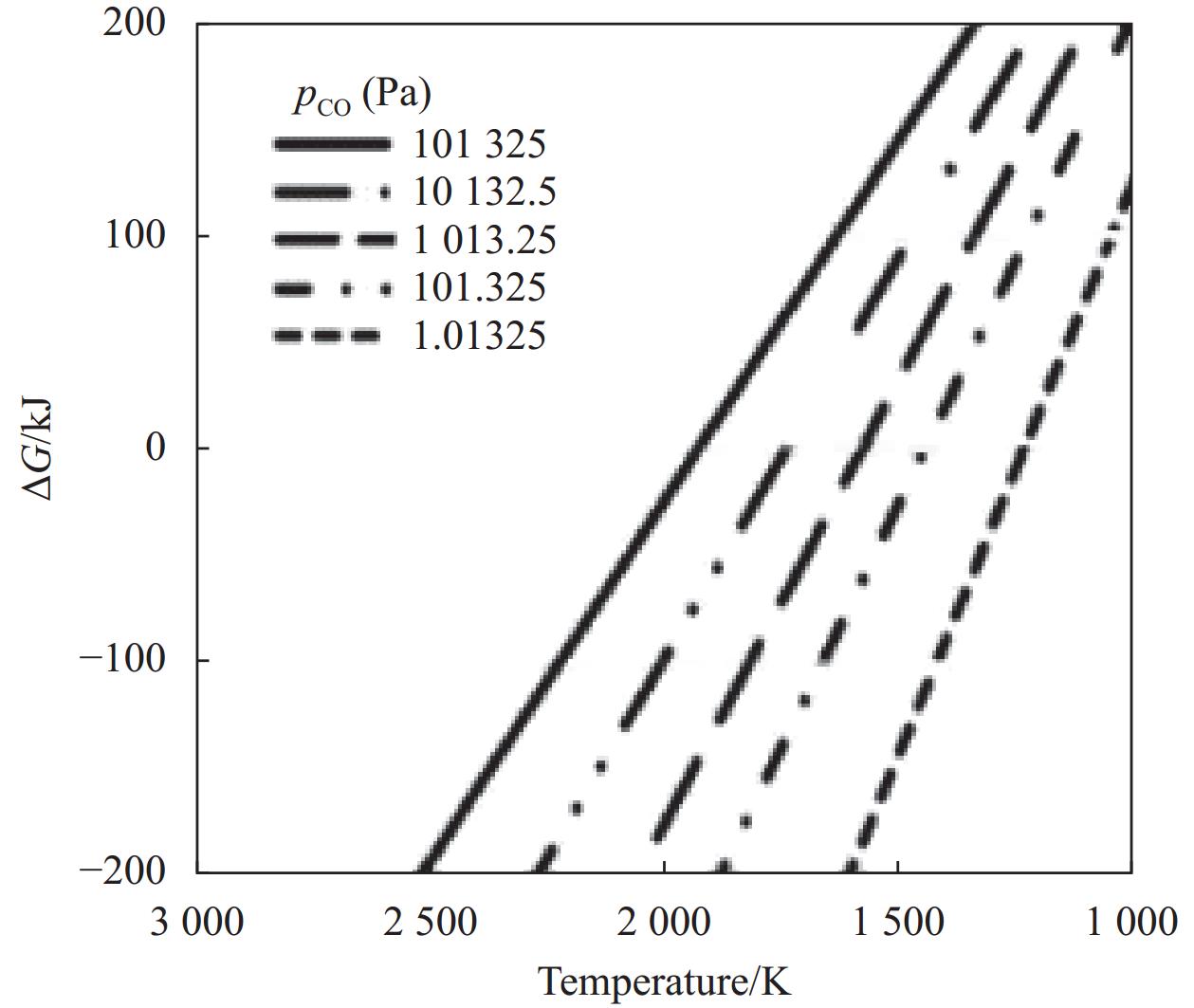
Method Raw materials Reaction equation Carbothermal reduction ZrO2, B2O3/H3BO3, C $ \text{Zr}\text{O}_{\text{2}}\text{ + }\text{B}_{\text{2}}\text{O}_{\text{3}}\text{ + 5C}\to\text{Zr}\text{B}_{\text{2}}\text{ + 5CO} $
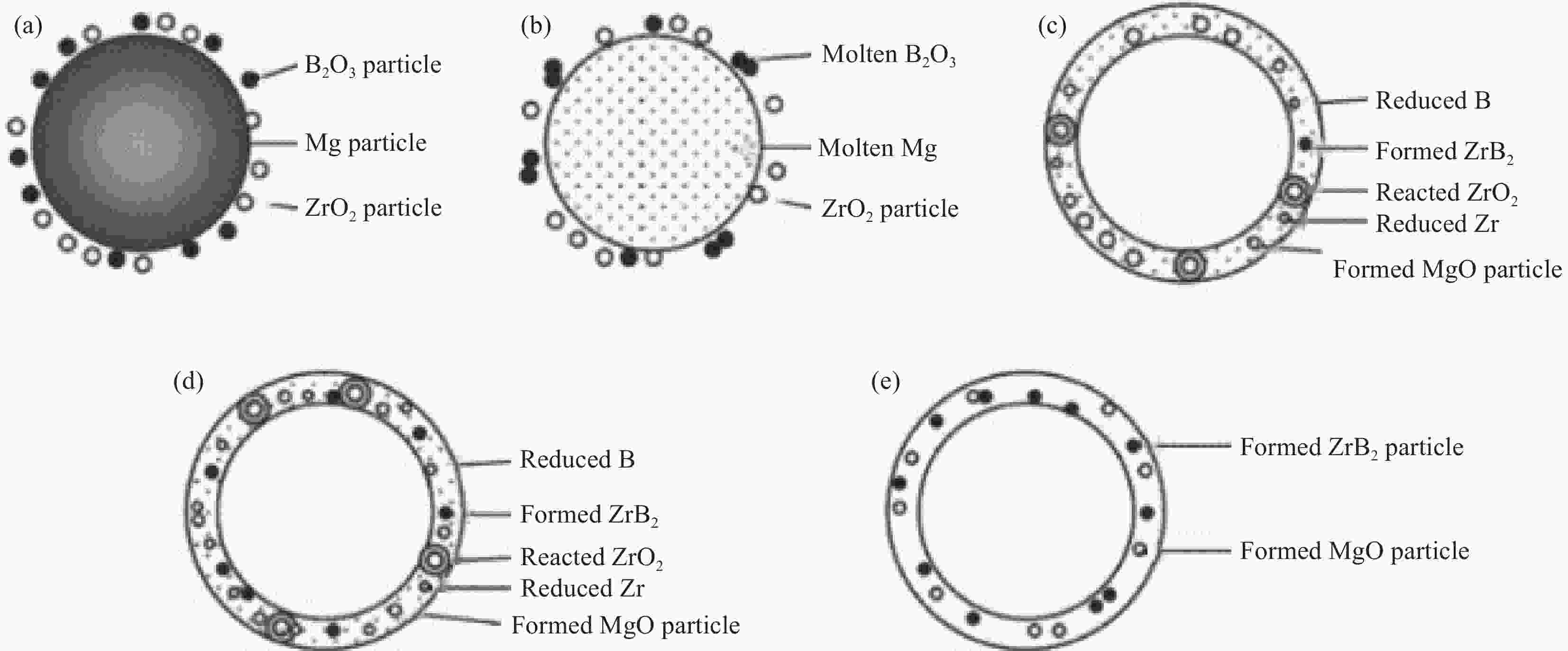
$ \text{Zr}\text{O}_{\text{2}}\text{ + 2}\text{H}_{\text{3}}\text{B}\text{O}_{\text{3}}\text{ + 5C = Zr}\text{B}_{\text{2}}\text{ + 5CO + 3}\text{H}_{\text{2}}\text{O} $Borothermal reduction ZrO2, B $ \text{Zr}\text{O}_{\text{2}}\text{ + 4B = Zr}\text{B}_{\text{2}}\text{ + }\text{B}_{\text{2}}\text{O}_{\text{2}} $ Boron/carbothermal reduction ZrO2, B4C, graphite/carbon black $ \text{2Zr}\text{O}_{\text{2}}\text{ + }\text{B}_{\text{4}}\text{C + 3C}\to\text{2Zr}\text{B}_{\text{2}}\text{ + 4CO} $ Magnesiothermic reduction ZrO2, B2O3, Mg $ \text{2Zr}\text{O}_{\text{2}}\text{ + }\text{B}_{\text{2}}\text{O}_{\text{3}}\text{ + 5Mg}\to\text{Zr}\text{B}_{\text{2}}\text{ + 5MgO} $ 表 2 ZrB2和ZrC粉末制备工艺及其优缺点
Table 2. Preparation of ZrB2 and ZrC powders and their advantages and disadvantages
Preparation Introduce Advantages Disadvantages Self-propagation high-temperature synthesis The mixture of raw materials uses the high temperature and heat generated by the
chemical reaction to make the reaction
proceed spontaneously and obtain the
desired productEnergy saving, rapid response, high efficiency, high purity of the generated powder, less pollution[37] The reaction speed is too fast, resulting in unsatisfactory mixing uniformity of raw materials, the reactant conversion is incomplete, more impurities Sol-gel methed The raw materials are first mixed and heated according to the ratio to form a stable sol, and then the sol is dried into a gel and ground into
a powder to prepare the precursor powder, and the product is obtained after high temperature heat treatment in a tube furnaceSmall particle size and good dispersion, low reaction temperature High cost, cumbersome process, long production cycle, organic materials are not good for human health Liquid phase precursor method The precursor solution is prepared from raw materials in proportion, and then dried and
heat-treated at high temperature in a tube furnace to obtain the required productThe raw materials are fully mixed, simple equipment, short process cycle Pollution of organic raw materials during heat treatment Plasma process The raw material is injected at the top of the plasma torch, rapidly heated in the plasma
area, cooled at high speed after flying out of
the plasma flame, and finally condensed into a very fine spherical powderThe product has high purity and uniform dispersion High equipment requirements, high preparation cost Molten salt method Molten salt with low melting point is used to provide liquid phase environment for the reactants, so that the reactants are mixed
evenly, and the diffusion and migration rate of each reaction component is accelerated to produce productsLow synthesis temperature, short reaction time, the shape and size of the product can be controlled by controlling the synthesis temperature and the type of molten salt The process of removing molten salt impurities is complicated, not suitable for mass production -
[1] FAHRENHOLTZ W G, HILMAS G E. Ultra-high temperature ceramics: Materials for extreme environments[J]. Scripta Materialia, 2017, 129: 94-99. [2] 张幸红, 胡平, 韩杰才, 等. 超高温陶瓷复合材料的研究进展[J]. 科学通报, 2015, 60(3): 257-266. doi: 10.1360/N972014-00456ZHANG Xinghong, HU Ping, HAN Jiecai, et al. Research progress on ultra-high temperature ceramic composites[J]. Chinese Science Bulletin, 2015, 60(3): 257-266(in Chinese). doi: 10.1360/N972014-00456 [3] 宋瑞颖, 刘宁, 张红芹, 等. ZrC陶瓷的性能、制备及应用[J]. 硬质合金, 2009, 26(2): 134-140.SONG Ruiying, LIU Ning, ZHANG Hongqin, et al. Properties, preparation and application of ZrC ceramics[J]. Cemented Carbide, 2009, 26(2): 134-140(in Chinese). [4] 骆吉源, 肖国庆, 丁冬海, 等. 二硼化锆粉体合成研究现状与展望[J]. 材料导报, 2021, 35(21): 21159-21168.LUO Jiyuan, XIAO Guoqing, DING Donghai, et al. Research status and prospect of zirconium diboride powder synthesis[J]. Materials Report, 2021, 35(21): 21159-21168(in Chinese). [5] 沈浩. 粒度形貌可控的二硼化锆粉体制备及生长机制研究[D]. 合肥: 合肥工业大学, 2022.SHEN Hao. Preparation and growth mechanism of zirconium diboride powder with controllable particle size and morphology[D]. Hefei: Hefei University of Technology, 2022(in Chinese). [6] 赵彦伟, 刘宏瑞, 李军平, 等. ZrC粉体制备的研究进展[J]. 宇航材料工艺, 2012, 42(2): 15-18. doi: 10.3969/j.issn.1007-2330.2012.02.004ZHAO Yanwei, LIU Hongrui, LI Junping, et al. Progress on preparation of ZrC powders[J]. Aerospace Materials & Technology, 2012, 42(2): 15-18(in Chinese). doi: 10.3969/j.issn.1007-2330.2012.02.004 [7] ASL M S, NAYEBI B, AHMADI Z, et al. Effects of carbon additives on the properties of ZrB2–based composites: A review[J]. Ceramics International, 2018, 44(7): 7334-7348. doi: 10.1016/j.ceramint.2018.01.214 [8] 马宝霞, 郭二军, 王丽萍. ZrC超高温陶瓷复合材料的研究进展[J]. 材料导报, 2013, 27(3): 49-54.MA Baoxia, GUO Erjun, WANG Liping. Research progress in ZrC ultra-high temperature ceramic composites[J]. Materials Reports, 2013, 27(3): 49-54(in Chinese). [9] LIU J, SIMPSON M D, YAN J, et al. Tracking time-varying cerebral autoregulation in response to changes in respiratory PaCO2[J]. Physiological Measurement, 2010, 31(10): 1291-1307. doi: 10.1088/0967-3334/31/10/001 [10] NEUMAN E W, HILMAS G E, FAHRENHOLTZ W G. Processing, microstructure, and mechanical properties of large-grained zirconium diboride ceramics[J]. Materials Science and Engineering: A, 2016, 670: 196-204. doi: 10.1016/j.msea.2016.06.017 [11] SONBER J K, SURI A K. Synthesis and consolidation of zirconium diboride: Review[J]. Advances in Applied Ceramics, 2013, 110(6): 321-334. [12] INOUE R, ARAI Y, KUBOTA Y, et al. Oxidation of ZrB2 and its composites: A review[J]. Journal of Materials Science, 2018, 53(21): 14885-14906. doi: 10.1007/s10853-018-2601-0 [13] 邹洪伟, 叶金文, 刘颖, 等. 原料粉末碳、氧含量对无粘结相硬质合金性能的影响[J]. 功能材料, 2010, 41(1): 90-93.ZOU Hongwei, YE Jinwen, LIU Ying, et al. Effect of carbon and oxygen content in raw material powder on properties of cemented carbide with unbonded phase[J]. Functional Materials, 2010, 41(1): 90-93(in Chinese). [14] 刘昌奎, 张佳庆, 陈贺贺, 等. 粉末尺寸和氧含量对FGH96合金中PPB形成的影响[J]. 兵器材料科学与工程, 2018, 41(3): 33-38.LIU Changkui, ZHANG Jiaqing, CHEN Hehe, et al. Effect of powder size and oxygen content on PPB formation in FGH96 alloy[J]. Weapons Materials Science and Engineering, 2018, 41(3): 33-38(in Chinese). [15] 贺涛, 胡继林. ZrC粉末制备技术的研究进展[J]. 山东陶瓷, 2021, 44(5): 11-14.HE Tao, HU Jilin. Progress in preparation of ZrC powder[J]. Shandong Ceramics, 2021, 44(5): 11-14(in Chinese). [16] 方舟, 王皓, 傅正义. Zr-B2O3-Mg体系自蔓延高温合成ZrB2陶瓷粉末[J]. 硅酸盐学报, 2004(6): 755-758.FANG Zhou, WANG Hao, FU Zhengyi. ZrB2 ceramic powder in Zr-B2O3-Mg system prepared by self-propagating high-temperature synthesis[J]. Journal of the Chinese Ceramic Society, 2004(6): 755-758(in Chinese) [17] ZHAO Y, LI J P, WANG T Y, et al. Synthesis of ZrB2 nanoparticles by using xylitol[J]. Journal of Inorganic Materials, 2016, 31(6): 597-601. [18] 周晓波, 苏勋家, 侯根良, 等. 液相先驱体制备ZrC粉末研究[J]. 材料导报, 2009, 23(S1): 142-144.ZHOU Xiaobo, SU Xunjia, HOU Genliang, et al. Study on preparation of ZrC powder by liquid precursors[J]. Materials Reports, 2009, 23(S1): 142-144(in Chinese). [19] ZHANG M, ZOU B, XU J, et al. Reaction behavior, microstructure and application in coating of in situ ZrC-ZrB2 ceramic composites powders from a Co-Zr-B4C system[J]. Materials & Design, 2015, 81: 65-72. [20] BARIS M, SIMSEK T, SIMSEK T, et al. High purity synthesis of ZrB2 by a combined ball milling and carbothermal method: Structural and magnetic properties[J]. Advanced Powder Technology, 2018, 29(10): 2440-2446. doi: 10.1016/j.apt.2018.06.024 [21] CHEN Z B, ZHAO X T, LI M L, et al. Synthesis of rod-like ZrB2 crystals by boro/carbothermal reduction[J]. Ceramics International, 2019, 45(11): 13726-13731. doi: 10.1016/j.ceramint.2019.04.068 [22] LIU H T, QIU H Y, GUO W M, et al. Synthesis of rod-like ZrB2 powders[J]. Advances in Applied Ceramics, 2014, 114(8): 418-422. [23] JUNG E Y, KIM J H, JUNG S H, et al. Synthesis of ZrB2 powders by carbothermal and borothermal reduction[J]. Journal of Alloys and Compounds, 2012, 538: 164-168. doi: 10.1016/j.jallcom.2012.05.076 [24] MA L, YU J, GUO X, et al. Effects of HBO2 on phase and morphology of ZrB2 powders synthesized by carbothermal reduction[J]. Ceramics International, 2017, 43(15): 12975-12978. doi: 10.1016/j.ceramint.2017.05.355 [25] 王恩元, 谢建林, 李学钊. 碳热还原法合成ZrB2粉体及其游离碳的去除[J]. 耐火材料, 2015, 39(6): 435-437. doi: 10.3969/j.issn.1001-1935.2015.06.008WANG Enyuan, XIE Jianlin, LI Xuezhao. Synthesis of ZrB2 powders by carbon thermal reduction process and free carbon removal[J]. Refractories, 2015, 39(6): 435-437(in Chinese). doi: 10.3969/j.issn.1001-1935.2015.06.008 [26] SEO M, KANG S, KIM Y, et al. Preparation of highly dispersed ultra-fine ZrC by combination of carbothermal reduction of ball-milled ZrO2 and C mixture and bead milling[J]. International Journal of Refractory Metals and Hard Materials, 2013, 41: 345-350. doi: 10.1016/j.ijrmhm.2013.05.007 [27] 曾广, 杨鑫, 苏哲安, 等. 碳源及反应温度对碳热还原法制备纳米ZrC形貌与物相的影响[J]. 粉末冶金材料科学与工程, 2018, 23(1): 17-24.ZENG Guang, YANG Xin, SUN Zhe'an, et al. Effects of carbon source and synthesized temperature on the morphology and phase composition of nano-sized ZrC powders prepared by carbothermal reduction method[J]. Materials Science and Engineering of Powder Metallurgy, 2018, 23(1): 17-24(in Chinese). [28] ARIANPOUR F, KAZEMI F, REZAIE H R. Thermodynamic study of zirconium carbide synthesis via a low-temperature pyrovacuum method[J]. Journal of the Australian Ceramic Society, 2019, 56(3): 969-977. [29] RAN S, VAN DER BIEST O, VLEUGELS J. ZrB2 powders synthesis by borothermal reduction[J]. Journal of the American Ceramic Society, 2010, 93(6): 1586-1590. [30] GUO W M, WU L X, SUN S K, et al. Particle refinement of ZrB2 by the combination of borothermal reduction and solid solution[J]. Journal of the American Ceramic Society, 2017, 100(2): 524-528. doi: 10.1111/jace.14693 [31] GUO W M, TAN D W, ZHANG Z L, et al. Synthesis of fine ZrB2 powders by new borothermal reduction of coarse ZrO2 powders[J]. Ceramics International, 2016, 42(13): 15087-15090. doi: 10.1016/j.ceramint.2016.06.051 [32] 刘海, 杨静, 唐明国, 等. 碳热还原法制备高纯ZrB2粉体[J]. 金属热处理, 2019, 44(2): 7-11.LIU Hai, YANG Jing, TANG Mingguo, et al. High purity ZrB2 powders prepared by boro/carbothermal reduction[J]. Heat Treatment of Metals, 2019, 44(2): 7-11(in Chinese). [33] QIU H Y, GUO W M, ZOU J, et al. ZrB2 powders prepared by boro/carbothermal reduction of ZrO2: The effects of carbon source and reaction atmosphere[J]. Powder Technology, 2012, 217: 462-466. doi: 10.1016/j.powtec.2011.11.002 [34] MURTHY S S N, PATEL M, REDDY J J, et al. Influence of B4C particle size on the synthesis of ZrB2 by boro/carbothermal reduction method[J]. Transactions of the Indian Institute of Metals, 2017, 71(1): 57-65. [35] ZHENG Y T, LI H B, XU Z H, et al. Reaction mechanism of self-propagating magnesiothermic reduction of ZrB2 powders[J]. Rare Metals, 2013, 32(4): 408-413. doi: 10.1007/s12598-013-0069-2 [36] SHISHKIN R A, KUDYAKOVA V S, ZHIRENKINA N V. Physicochemical transformations during low-temperature synthesis of zirconium carbide[J]. Refractories and Industrial Ceramics, 2019, 60(1): 105-108. doi: 10.1007/s11148-019-00317-x [37] 张薇, 肖国庆, 丁冬海. 硼砂对自蔓延高温合成ZrB2粉体的影响[J]. 材料导报, 2017, 31(24): 125-128, 44. doi: 10.11896/j.issn.1005-023X.2017.024.025ZHANG Wei, XIAO Guoqing, DING Donghai. Effect of Na2B4O7 on ZrB2 powder prepared by self-propagating high-temperature synthesis[J]. Materials Reports, 2017, 31(24): 125-128, 44(in Chinese). doi: 10.11896/j.issn.1005-023X.2017.024.025 [38] JALALY M, BAFGHI M S, TAMIZIFAR M, et al. An investigation on the formation mechanism of nano ZrB2 powder by a magnesiothermic reaction[J]. Journal of Alloys and Compounds, 2014, 588: 36-41. doi: 10.1016/j.jallcom.2013.11.050 [39] 李静, 傅正义, 王为民, 等. 自蔓延高温技术制备ZrC粉体[J]. 硅酸盐学报, 2010, 38(5): 979-985.LI Jing, FU Zhengyi, WANG Weimin, et al. Preparation of ZrC by self-propagating high-temperature synthesis[J]. Journal of the Chinese Ceramic Society, 2010, 38(5): 979-985(in Chinese). [40] 宋谋胜, 冉茂武. 自蔓延反应制备ZrC粉末的形成机制研究[J]. 粉末冶金技术, 2011, 29(3): 177-182.SONG Mousheng, RAN Maowu. Investigation of formation mechanism of ZrC powder fabricated by self-propagating reaction[J]. Powder Metallurgy Technology, 2011, 29(3): 177-182(in Chinese). [41] DA A, LONG F, WANG J, et al. Preparation of nano-sized zirconium carbide powders through a novel active dilution self-propagating high temperature synthesis method[J]. Journal of Wuhan University of Technology-Materials Science Edition, 2015, 30(4): 729-734. doi: 10.1007/s11595-015-1220-8 [42] 李锐星, 张云, 赵斌, 等. 碳热还原协同溶胶-凝胶法合成纳米ZrB2粉末[J]. 中国材料进展, 2012, 31(7): 59-63.LI Ruixing, ZHANG Yun, ZHAO Bin, et al. Carbothermal reduction acted synergistically with sol-gel method to synthesize ZrB2 nanoparticles[J]. Materials China, 2012, 31(7): 59-63(in Chinese). [43] YANG B, LI J, ZHAO B, et al. Synthesis of hexagonal-prism-like ZrB2 by a sol-gel route[J]. Powder Technology, 2014, 256: 522-528. doi: 10.1016/j.powtec.2014.01.067 [44] 喻冲, 杨静, 张良, 等. 溶胶-凝胶法制备核级ZrB2粉体[J]. 粉末冶金技术, 2018, 36(5): 377-381.YU Chong, YANG Jing, ZHANG Liang, et al. Preparation of nuclear-grade ZrB2 powders by sol-gel method[J]. Powder Metallurgy Technology, 2018, 36(5): 377-381(in Chinese). [45] 沈浩, 闫健, 胡小晔, 等. 凝胶-溶胶协同碳热还原法合成二硼化锆粉体及生长机制探究[J]. 金属功能材料, 2022, 29(1): 8-12, 19.SHEN Hao, YAN Jian, HU Xiaoye, et al. Synthesis and growth mechanism of zirconium diboride powder by gel-sol cooperative carbothermal reduction method[J]. Metal Functional Materials, 2022, 29(1): 8-12, 19(in Chinese). [46] 陈琪. 二硼化锆合成及涂层制备研究[D]. 北京: 华北电力大学(北京), 2022.CHEN Qi. Synthesis of zirconium diboride and preparation of its coating [D]. Beijing: North China Electric Power University (Beijing), 2022(in Chinese). [47] CAO Y, LI F, ZHANG H, et al. Low-temperature preparation of ZrC powders using a combined sol-gel and microwave carbothermal reduction method[J]. Journal of the Ceramic Society of Japan, 2016, 124(11): 1171-1174. doi: 10.2109/jcersj2.16065 [48] 廉晓庆, 隆万坤, 徐菊花, 等. 液相先驱体转化结合溶胶凝胶法合成ZrC-SiC纳米复合粉体[J]. 硅酸盐通报, 2018, 37(1): 195-199.LIAN Xiaoqing, LONG Wankun, XU Juhua, et al. ZrC-SiC nanocomposite powders were synthesized by sol-gel method combined with liquid precursor transformation[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(1): 195-199(in Chinese). [49] 孙楚函, 王洪磊, 周新贵. 前驱体转化法制备超高温陶瓷粉体研究进展[J]. 硅酸盐通报, 2023, 42(8): 2865-2880.SUN Chuhan, WANG Honglei, ZHOU Xingui. Research progress in preparation of ultrahigh temperature ceramic powder by precursor transformation method [J]. Bulletin of the Chinese Ceramic Society, 2023, 42(8): 2865-2880(in Chinese). [50] 李运涛, 陶雪钰, 邱文丰, 等. 液相前驱体转化法制备ZrB2粉末[J]. 北京化工大学学报(自然科学版), 2010, 37(4): 78-82.LI Yuntao, TAO Xueyu, QIU Wenfeng, et al. Preparation of powdered zirconium diboride by a solution precursor convertion method[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2010, 37(4): 78-82(in Chinese). [51] ZHANG X, DONG Z, HUANG Q, et al. Preparation of zirconium diboride powders by co-pyrolysis of a zirconium-containing organic precursor and polyborazine using a solution based method[J]. Ceramics International, 2014, 40(9): 15207-15214. doi: 10.1016/j.ceramint.2014.07.002 [52] 梅冰, 苏勋家, 侯根良, 等. 液相先驱体转化法制备ZrC粉末及合成机理[J]. 固体火箭技术, 2008(3): 275-278, 287.MEI Bing, SUN Xunjia, HOU Genliang, et al. Preparation of ZrC powder by means of liquid precursor conversion method and its synthetic mechanism [J]. Journal of Solid Rocket Technology, 2008(3): 275-278, 287(in Chinese). [53] 李庆刚, 程新, 王志, 等. 液相前驱体转化法制备ZrC及其表征[J]. 济南大学学报(自然科学版), 2013, 27(3): 221-224.LI Qinggang, CHENG Xin, WANG Zhi, et al. Preparation and characterization of ZrC powders by liquid precursor conversion method [J]. Journal of University of Jinan (Science and Technology), 2013, 27(3): 221-224(in Chinese). [54] CHU A, QIN M, RAFI UD D, et al. Carbothermal synthesis of ZrC powders using a combustion synthesis precursor[J]. International Journal of Refractory Metals and Hard Materials, 2013, 36: 204-210. doi: 10.1016/j.ijrmhm.2012.08.016 [55] 白柳杨, 张海宝, 袁方利, 等. 高频热等离子体合成超细ZrB2和ZrC粉体材料[J]. 宇航材料工艺, 2012, 42(2): 88-90. doi: 10.3969/j.issn.1007-2330.2012.02.022BAI Liuyang, ZHANG Haibao, YUAN Fangli, et al. Production of ultra-fine ZrB2 and ZrC powders via RF plasma[J]. Aerospace Materials & Technology, 2012, 42(2): 88-90(in Chinese). doi: 10.3969/j.issn.1007-2330.2012.02.022 [56] CINERT J, CTIBOR P, BROZEK V, et al . Preparation of ZrB₂ by boro/carbothermal reduction in SPS device[J]. Ceramics-Silikaty, 2019, 63(1): 93-99. [57] YU L, FENG L, LEE H I, et al. Synthesis and densification of ultra-fine ZrC powders-effects of C/Zr ratio[J]. International Journal of Refractory Metals and Hard Materials, 2019, 81: 149-154. doi: 10.1016/j.ijrmhm.2019.03.001 [58] FENG L, LEE S, LEE H. Nano-sized zirconium carbide powder: Synthesis and densification using a spark plasma sintering apparatus[J]. International Journal of Refractory Metals and Hard Materials, 2017, 64: 98-105. doi: 10.1016/j.ijrmhm.2017.01.006 [59] 谭操, 段红娟, 王军凯, 等. 熔盐镁热还原法合成ZrB2超细粉体[J]. 材料导报, 2017, 31(8): 109-112. doi: 10.11896/j.issn.1005-023X.2017.08.022TAN Cao, DUAN Hongjuan, WANG Junkai, et al. Preparation of ZrB2 ultrafine powders via morten-salt-mediated magnesiothermic reduction[J]. Materials Reports, 2017, 31(8): 109-112(in Chinese). doi: 10.11896/j.issn.1005-023X.2017.08.022 [60] WANG Y, WU Y D, WU K H, et al. Effect of NaCl on synthesis of ZrB2 by a borothermal reduction reaction of ZrO2[J]. International Journal of Minerals, Metallurgy, and Materials, 2019, 26(7): 831-838. doi: 10.1007/s12613-019-1794-9 [61] ZHANG S, KHANGKHAMANO M, ZHANG H, et al. Novel synthesis of ZrB2 powder via molten-salt-mediated magnesiothermic reduction[J]. Journal of the American Ceramic Society, 2014, 97(6): 1686-1688. doi: 10.1111/jace.12945 [62] 白晨, 王周福, 邓承继, 等. 熔盐中镁热还原合成碳化锆粉体[J]. 人工晶体学报, 2018, 47(3): 505-509.BAI Chen, WANG Zhoufu, DENG Chengji, et al. Magnecium reduction synthesis of ZrC powders in molten salt[J]. Journal of Synthetic Crystals, 2018, 47(3): 505-509(in Chinese). [63] 许珂, 占发琦, 张华, 等. 混合盐辅助燃烧合成纳米ZrC粉体及影响机制[J]. 功能材料, 2021, 52(10): 10177-10186, 10220. doi: 10.3969/j.issn.1001-9731.2021.10.026XU Ke, ZHAN Faqi, ZHANG Hua, et al. Synthesis of nanometer ZrC powder by mixed salt-assisted combustion and its mechanism[J]. Functional Materials, 2021, 52(10): 10177-10186, 10220(in Chinese). doi: 10.3969/j.issn.1001-9731.2021.10.026 [64] 闫永杰, 张辉, 黄政仁, 等. 无机盐溶胶-凝胶法制备超细ZrB2-ZrC复合粉体[J]. 无机材料学报, 2008(4): 815-818.YAN Yongjie, ZHANG Hui, HUANG Zhengren, et al. Synthesis of ultral-fine ZrB2-ZrC composite powders by inorganic sol-gel method[J]. Journal of Inorganic Materials, 2008(4): 815-818(in Chinese). [65] TIAN Y, SUN W. Synthesis of ZrB2-ZrC hybrid powders via boro-carbothermal reduction of ZrO2 by B4C and carbon black[J]. Ceramics International, 2022, 48(18): 26499-26507. doi: 10.1016/j.ceramint.2022.05.346 [66] SAOWANEE S, SUTHAM N, CHANADEE T. Synthesis and characterization of ZrB2-ZrC composite powders from zircon sand by self-propagating high-temperature synthesis method[J]. Materials Science Forum, 2018, 934: 66-70. doi: 10.4028/www.scientific.net/MSF.934.66 [67] TSUCHIDA T, YAMAMOTO S. Spark plasma sintering of ZrB2-ZrC powder mixtures synthesized by MA-SHS in air[J]. Journal of Materials Science, 2007, 42(3): 772-778. doi: 10.1007/s10853-006-0719-y [68] BAI L, YUAN F, FANG Z, et al. RF thermal plasma synthesis of ultrafine ZrB2-ZrC composite powders[J]. Nanomaterials (Basel), 2020, 10(12): 2497. [69] WU S J, WANG Y G, GUI D M. Synthesis of ZrB2-ZrC composite powders by pirac method[J]. The American Ceramic Society, 2014, 248: 541-545. [70] WANG Y, ZHANG G, CHOU K. Preparation and oxidation characteristics of ZrB2-ZrC composite powders with different proportions[J]. International Journal of Minerals, Metallurgy and Materials, 2021, 29(3): 521-528. -






 下载:
下载: