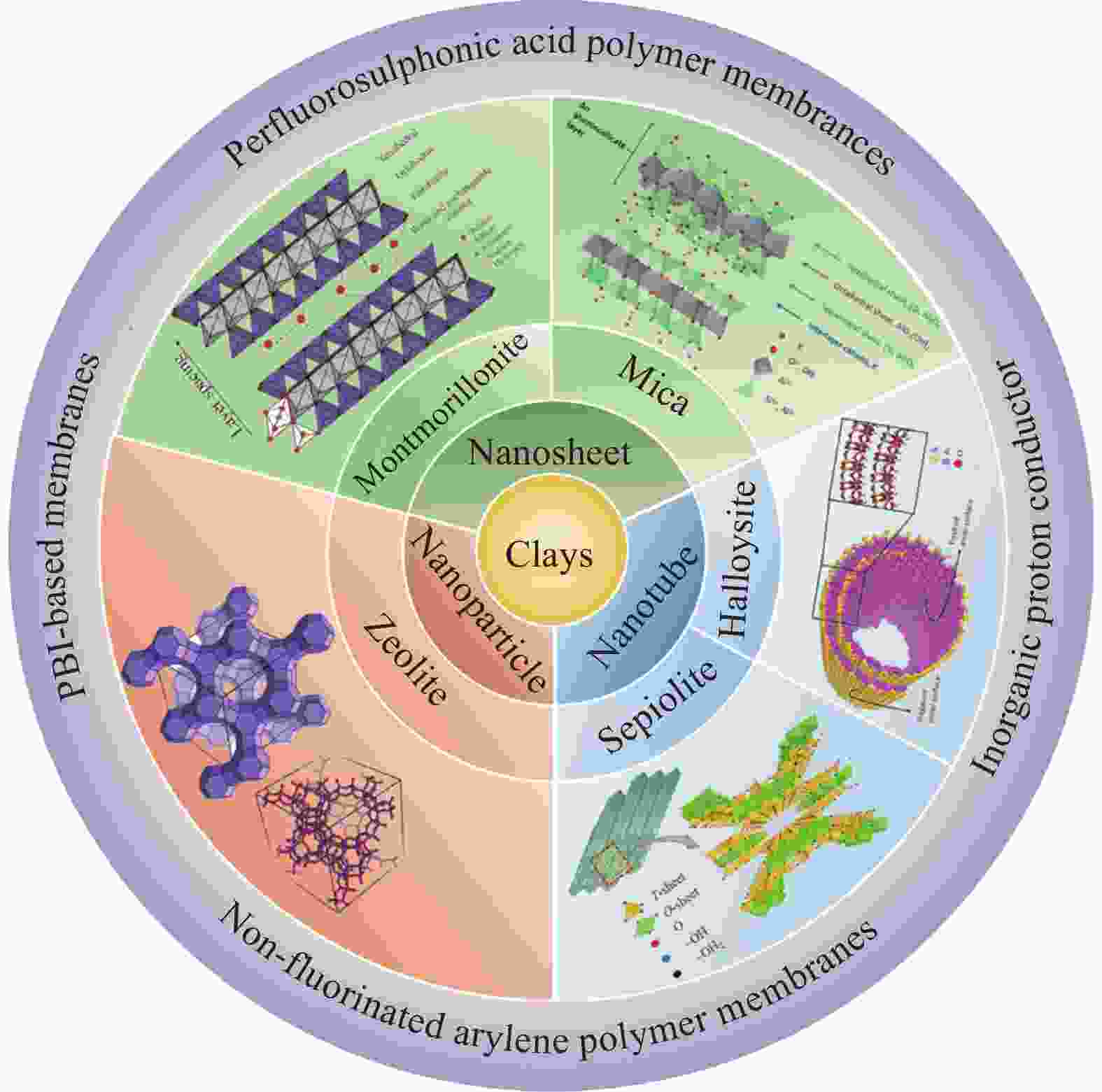
Application progress of natural clays in proton exchange membrane
-
摘要: 质子交换膜(PEM)作为聚合物电解质燃料电池关键部件直接影响着电池性能,拓宽其运行温度和湿度范围有利于简化燃料电池水、热管理设计,从而促进电池小型化和降低成本。近些年来,开发天然粘土/聚合物复合膜已成为提升传统PEM性能和拓宽其应用温、湿度范围的重要途径之一。天然粘土矿物多为含水层状硅酸盐化合物,特殊的孔、层结构和纳米尺度赋予其较大的比表面积和表面效应,其表面和层间富含的羟基在提高复合膜机械强度的同时固定了传质介质,从而在复合膜中构建新的质子传导通道用以提高膜的性能。从纳米微观多个维度综述了不同类别粘土矿物的结构与性能,以及其在质子交换膜中的研究进展,对天然粘土矿物复合质子交换膜的研究进行了总结与展望。Abstract: As a key component of polymer electrolyte fuel cells, the proton exchange membrane (PEM) directly impacts cell performance. Expanding its operational temperature and humidity range is advantageous for simplifying fuel cell water and thermal management designs, thereby promoting miniaturization and cost reduction. In recent years, the development of polymer composite membranes based on natural clay has emerged as a crucial avenue for enhancing traditional PEM performance and broadening its applicability across varying environmental conditions. Natural clay minerals predominantly consist of hydrated layered silicate compounds, characterized by unique pore and layer structures at the nanoscale, endowing them with substantial specific surface area and surface effects. The abundance of hydroxyl groups on their surfaces and interlayers not only enhances the mechanical strength of composite membranes but also immobilizes mass transport media. Consequently, these materials facili-tate the creation of novel proton-conductive pathways within the composite membrane, thereby elevating membrane performance. We comprehensively review, from a nanoscale perspective, various categories of clay minerals, elucidating their structural and performance attributes. Furthermore, we provide a comprehensive summary and future prospects of research advancements in natural clay mineral-composite proton exchange membranes.
-
Key words:
- natural clays /
- proton exchange membrane /
- composite membrane /
- nanoscale /
- fuel cells
-
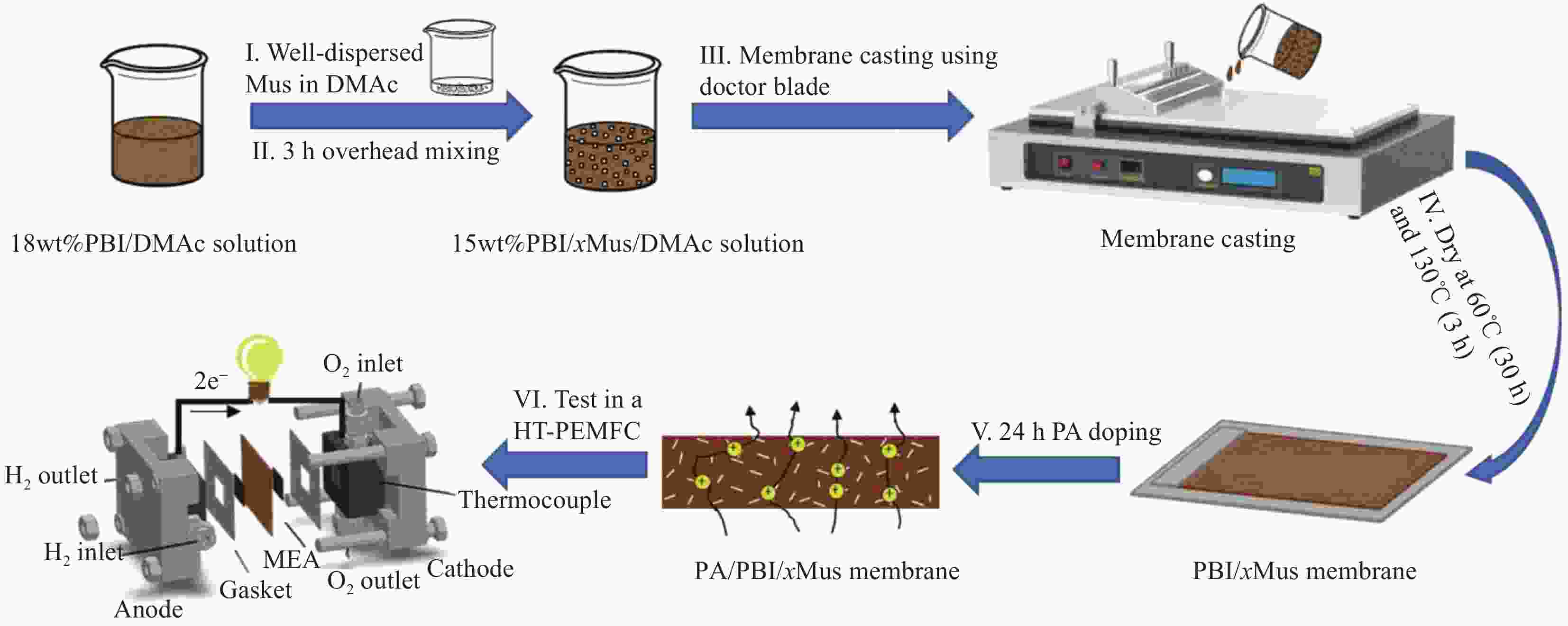
图 4 (a) 聚乙烯亚胺@海泡石纳米棒(PEI@SNR)纳米颗粒、聚(2, 5-苯并咪唑) (ABPBI)/PEI@SNR膜的制备图;(b) 磷酸(PA)掺杂的ABPBI/PEI@SNR膜的质子传导途径[25]
Figure 4. (a) Schematic representation of the preparation of polyethyleneimine (PEI)@silicon nanorods (SNR) nanoparticles, poly(2, 5-benzimidazole) (ABPBI)/PEI@SNR membranes; (b) Proton conducting pathways in phosphoric acid (PA)-doped ABPBI/ PEI@SNR membrane[25]
图 5 离子液体(IL)@埃洛石(HNTs)和ABPBI/IL@HNTs复合膜的制备过程示意图[28]
Figure 5. Preparation process schematic of ionic liquid (IL)@halloysite (HNTs) and ABPBI/IL@HNTs composite membrane[28]
AHNTs—HNTs were subjected to microwave-assisted heating in hydrochloric acid to obtain AHNTs; DABA—3, 4-diaminobenzoic acid; MEA—Membrane electrode assembly
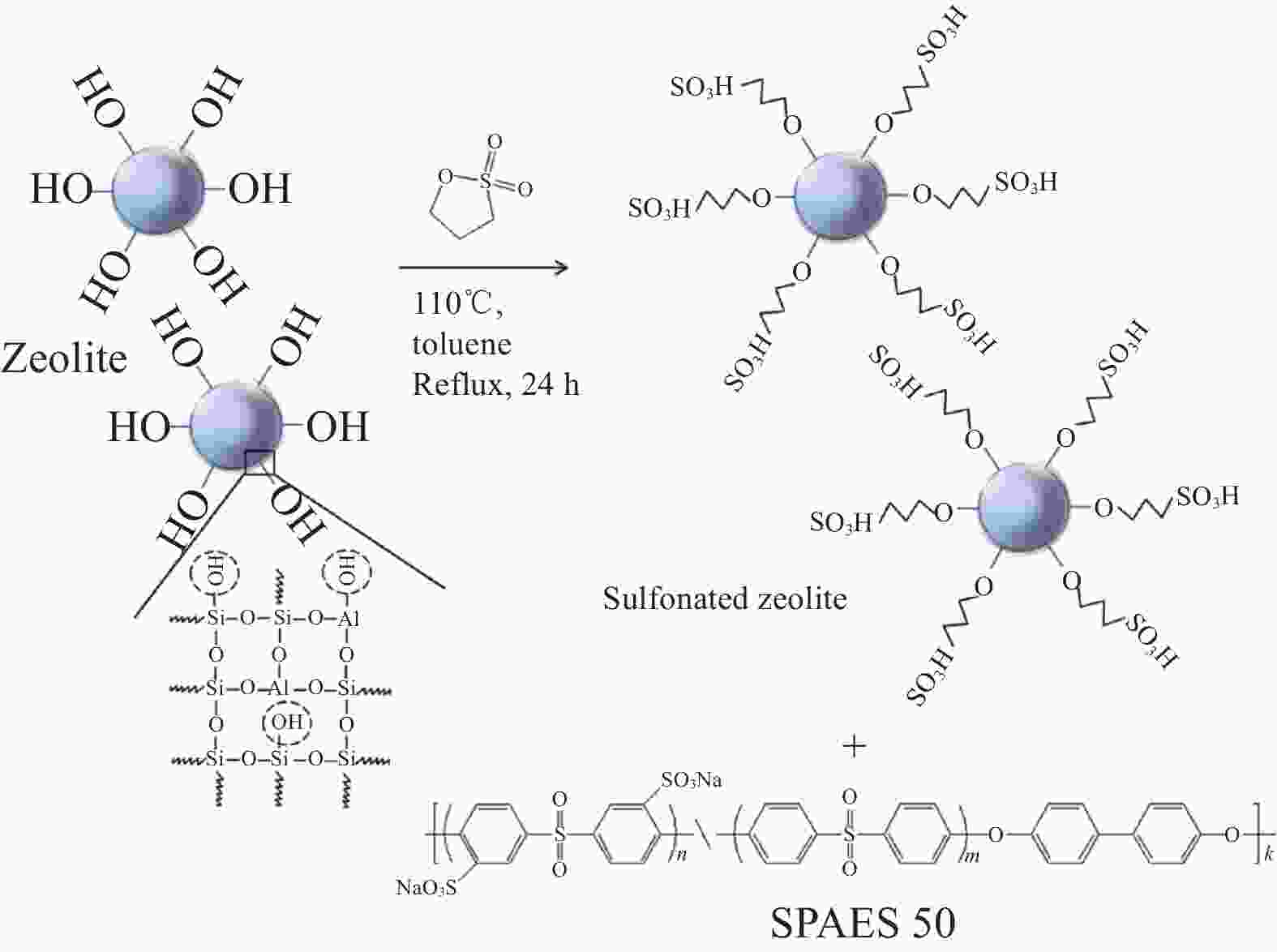
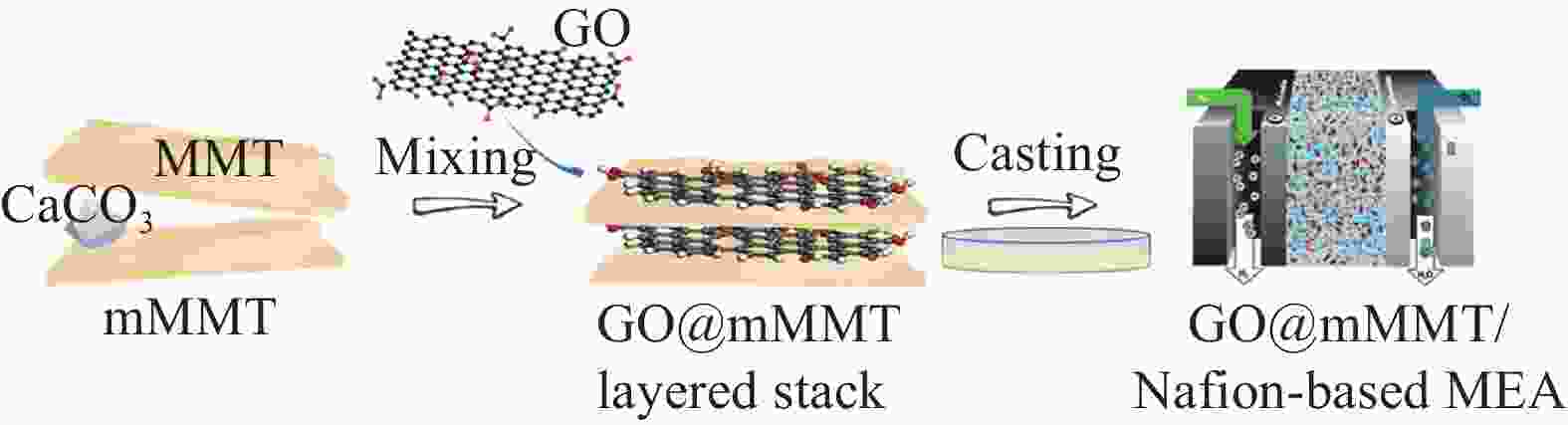
表 1 各类应用于质子交换膜燃料电池(PEMFCs)的天然粘土/聚合物复合膜性能对比
Table 1. Comparison of performance of polymer/natural clay composite membranes applied to proton exchange membrane fuel cells (PEMFCs)
Composite
membraneClay Tensile strength/
MPaProton conductivity/
(S·cm−1)Peak power density/
(W·cm−2)Temperature
T/℃Ref. Nafion/Zeolite Zeolite — 0.129 (80℃) 0.51 (85℃/100%RH) 70-120 [83] Nafion/HZSM/IL Zeolite — 0.2 (80℃/60%RH) 0.6 (80℃) 100 [84] Aquvion/pHNT-SF HNT — 0.28 (90℃/90%RH) — — [60] Nafion/HNTs-SO3H HNT — 0.073 (80℃/90%RH) — — [85] MgAl-Sep Nafion Sep — 0.132 (100℃/100%RH) 0.55 (80℃/100%RH) — [59] GO@mMMT/Nafion MMT 19.2 0.0364 (80℃/98%RH) 0.546 (50℃/100%RH) 40-90 [34] PBI+IL/NaY Zeolite — 0.054 (200℃) 0.269 (150℃) 20-200 [50] ABPBI/IL@HNTs HNT 149.28 0.045 (180℃/0%RH)
0.071 (90℃/98%RH)0.38 (160℃/0%RH) 20-180 [28] ABPBI/S-Sep Sep 99 0.051 (180℃/0%RH) 0.23 (180℃/0%RH) 40-180 [59] ABPBI/IL@SNR Sep 204.24 (undoped) 0.048 (180℃/0%RH) 0.28 (180℃/0%RH) 40-180 [26] ABPBI/PEI@SNR Sep 190 (undoped)
54 (PA-doped)0.04 (180℃/0%RH) 0.27 (180℃/0%RH) 40-180 [25] ABPBI-MMT/SPVA MMT 79.39 (undoped)
60.35 (PA-doped)0.157 (140℃/100%RH) 1.1 (140℃/100%RH) 20-140 [86] PBI/Mus Mica 135 (undoped)
7.5 (PA-doped)0.042 (150℃/0%RH) 0.586 (150℃/0%RH) 140-170 [31] SP/SZ Zeolite 18.72 0.03 (120℃/50%RH) 0.791 (80℃/100%RH) 120 [33] SPEES-SA/SMZ Zeolite 62 0.124 (80℃) 0.45 (80℃/30%RH) 80 [37] SPEEK/DHNTs/HPW HNT 41.3 0.117 (25℃/20%RH) — — [63] SPEEK/DSNT-A@B HNT 47.4 0.084 (80℃/100%RH) — — [87] LDH/Sepiolite/SPEEK Sep 47.5 0.093 (110℃/100%RH) — — [55] Notes: Sep—Sepiolite; MMT—Montmorillonite; HZSM—Na-ZSM zeolite in acid form; pHNT-SF—Pretreated-perfluorosulfonated halloysite; HNTs-SO3H—Sulphonic acid functionalized halloysite; S-Sep—Sulfonated sepiolite; SPVA—Sulfonated polyvinyl alcohol; SP—Sulfonated poly(arylene ether sulfone); SZ—Sulfonated zeolite; SPEES-SA—Sulfanilic acid functionalized poly(1,4-phenylene ether ether sulfone); SMZ—Sulfonic acid functionalized zeolites of Na-mordenite zeolite; SPEEK—Sulfonated poly(ether ether ketone); DHNTs—Polydopamine coated halloysite nanotubes; HPW—Phosphotungstic acid; DSNT-A@B—Acid-base double-shell nanotubes with carboxylate inner shell and an imidazole outer shell; LDH—Layered double hydroxide; RH—Relative humidity. 表 2 各类应用于直接甲醇燃料电池(DMFCs)的天然粘土/聚合物复合膜性能对比
Table 2. Comparison of performance of polymer/natural clay composite membranes applied to direct methanol fuel cells (DMFCs)
Composite membrane Clay Tensile strength/
MPaProton conductivity/
(S·cm−1)Peak power density/
(W·cm−2)Temperature
T/℃Ref. Nafion/AFB Zeolite 25 0.088
(room temperature)5 mol/L MeOH:
0.12 (70℃)— [88] MOR/NF Zeolite 16.6 0.0501 (30℃) 4 mol/L MeOH:
0.01064 (70℃)— [47] Nafion®/BMMT MMT — 0.08 (25℃/95%RH) — — [89] PBI/m-MMT MMT 70.4 (undoped)
47.3 (PA-doped)— — — [90] SSA-sPEEK-HSO3-zeolite-13X Zeolite 11.9 0.12 (70℃) 0.130 (70℃) 25-70 [91] SMMT/SPEEK MMT 51.2 0.105
(100℃/100%RH)1.5 mol/L MeOH:
0.021 (60℃)— [92] Notes: AFB—Acid functionalized zeolite Beta; MOR/NF—Mordenite/Nafion; BMMT—Bio-functionalized montmorillonite; m-MMT—Modified MMT; SSA-sPEEK—Sulfosuccinic acid-sulfonated polyether ether ketone; SMMT—Sulfonated montmorillonite. -
[1] HAIDER R, WEN Y, MA Z F, et al. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies[J]. Chemical Society Reviews, 2021, 50(2): 1138-1187. doi: 10.1039/D0CS00296H [2] STEFFEN W, RICHARDSON K, ROCKSTRÖM J, et al. Plane-tary boundaries: Guiding human development on a chang-ing planet[J]. Science, 2015, 347(6223): 1259855. doi: 10.1126/science.1259855 [3] KITTNER N, LILL F, KAMMEN D M. Energy storage deployment and innovation for the clean energy transition[J]. Nature Energy, 2017, 2(9): 17125. doi: 10.1038/nenergy.2017.125 [4] PENG P, ANASTASOPOULOU A, BROOKS K, et al. Cost and potential of metal–organic frameworks for hydrogen back-up power supply[J]. Nature Energy, 2022, 7(5): 448-458. doi: 10.1038/s41560-022-01013-w [5] SAEEDMANESH A, MAC KINNON M A, BROUWER J. Hydrogen is essential for sustainability[J]. Current Opinion in Electrochemistry, 2018, 12: 166-181. doi: 10.1016/j.coelec.2018.11.009 [6] SCOFIELD M E, LIU H, WONG S S. A concise guide to sustainable PEMFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes[J]. Chemical Society Reviews, 2015, 44(16): 5836-5860. doi: 10.1039/C5CS00302D [7] PAN M, PAN C, LI C, et al. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability[J]. Renewable & Sustainable Energy Reviews, 2021, 141: 110771. [8] DEBE M K. Electrocatalyst approaches and challenges for automotive fuel cells[J]. Nature, 2012, 486(7401): 43-51. doi: 10.1038/nature11115 [9] XIAO T, WANG R, CHANG Z, et al. Electrolyte membranes for intermediate temperature proton exchange membrane fuel cell[J]. Progress in Natural Science: Materials International, 2020, 30(6): 743-750. doi: 10.1016/j.pnsc.2020.08.014 [10] WANG F, HE J. Speeding protons with metal vacancies[J]. Science, 2020, 370(6516): 525-526. doi: 10.1126/science.abe6166 [11] LEE K S, SPENDELOW J S, CHOE Y K, et al. An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs[J]. Nature Energy, 2016, 1(9): 16120. doi: 10.1038/nenergy.2016.120 [12] TANG H, GENG K, WU L, et al. Fuel cells with an operational range of −20℃ to 200℃ enabled by phosphoric acid-doped intrinsically ultramicroporous membranes[J]. Nature Energy, 2022, 7(2): 153-162. doi: 10.1038/s41560-021-00956-w [13] ASENSIO J A, SÁNCHEZ E M, GÓMEZ-ROMERO P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest[J]. Chemical Society Reviews, 2010, 39(8): 3210-3239. doi: 10.1039/b922650h [14] GUO Z, PEREZ-PAGE M, CHEN J, et al. Recent advances in phosphoric acid-based membranes for high-temperature proton exchange membrane fuel cells[J]. Journal of Energy Chemistry, 2021, 63: 393-429. doi: 10.1016/j.jechem.2021.06.024 [15] LIU Y, CHEN J, FU X, et al. Constructing proton transport channels in low phosphoric-acid doped polybenzi-midazole membrane by introducing metal–organic frameworks containing phosphoric-acid groups[J]. Journal of Power Sources, 2021, 507: 230316. doi: 10.1016/j.jpowsour.2021.230316 [16] JIAO K, XUAN J, DU Q, et al. Designing the next generation of proton-exchange membrane fuel cells[J]. Nature, 2021, 595(7867): 361-369. doi: 10.1038/s41586-021-03482-7 [17] PENG J, FU X, LUO J, et al. Constructing novel cross-linked polybenzimidazole network for high-performance high-temperature proton exchange membrane[J]. Journal of Membrane Science, 2022, 643: 120037. doi: 10.1016/j.memsci.2021.120037 [18] YIN B, WU Y, LIU C, et al. An effective strategy for the preparation of a wide-temperature-range proton exchange membrane based on polybenzimidazoles and polyacry-lamide hydrogels[J]. Journal of Materials Chemistry A, 2021, 9(6): 3605-3615. doi: 10.1039/D0TA08872B [19] ELLINGSEN L A W, HUNG C R, MAJEAU BETTEZ G, et al. Nanotechnology for environmentally sustainable electromobility[J]. Nature Nanotechnology, 2016, 11(12): 1039-1051. doi: 10.1038/nnano.2016.237 [20] BRETTI C, CATALDO S, GIANGUZZA A, et al. Thermodynamics of proton binding of halloysite nanotubes[J]. Journal of Physical Chemistry C, 2016, 120(14): 7849-7859. doi: 10.1021/acs.jpcc.6b01127 [21] SAHA K, DEKA J, GOGOI R K, et al. Applications of lamellar membranes reconstructed from clay mineral-based nanosheets: A review[J]. ACS Applied Nano Materials, 2022, 5(11): 15972-15999. doi: 10.1021/acsanm.1c03207 [22] ZHOU Y, LACHANCE A M, SMITH A T, et al. Strategic design of clay-based multifunctional materials: From natural minerals to nanostructured membranes[J]. Advanced Functional Materials, 2019, 29(16): 1807611. doi: 10.1002/adfm.201807611 [23] MEUNIER A. Clays[M]. Heidelberg: Springer-Verlag Berlin Heidelberg, 2005: 472. [24] LYU P, LIU C, RAO Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications[J]. Renewable and Sustainable Energy Reviews, 2017, 68: 707-726. doi: 10.1016/j.rser.2016.10.014 [25] LIU Q, WANG X, ZHANG X, et al. Polyethyleneimine-filled sepiolite nanorods-embedded poly(2, 5-benzimidazole) composite membranes for wide-temperature PEMFCs[J]. Journal of Cleaner Production, 2022, 359: 131977. doi: 10.1016/j.jclepro.2022.131977 [26] ZHANG X, FU X, YANG S, et al. Design of sepiolite-supported ionogel-embedded composite membranes without proton carrier wastage for wide-temperature-range operation of proton exchange membrane fuel cells[J]. Journal of Materials Chemistry A, 2019, 7(25): 15288-15301. doi: 10.1039/C9TA03666K [27] YU L, MU D, LIU L, et al. Bifunctional effects of halloysite nanotubes in vanadium flow battery membrane[J]. Jour-nal of Membrane Science, 2018, 564: 237-246. doi: 10.1016/j.memsci.2018.07.033 [28] LIU Q, XIONG C, SHI H, et al. Halloysite ionogels enabling poly(2, 5-benzimidazole)-based proton-exchange membranes for wide-temperature-range applications[J]. Jour-nal of Membrane Science, 2023, 668: 121192. doi: 10.1016/j.memsci.2022.121192 [29] PIDKO E A, HENSEN E J M. Chapter 3—Computational chemistry of zeolite catalysis[M]//SELS B F, KUSTOV L M. Zeolites and Zeolite-like Materials. Amsterdam: Elsevier, 2016: 111-135. [30] GKOUMA E, GIANNI E, AVGOUSTAKIS K, et al. Applications of halloysite in tissue engineering[J]. Applied Clay Science, 2021, 214: 106291. doi: 10.1016/j.clay.2021.106291 [31] GUO Z, CHEN J, BYUN J J, et al. Insights into the performance and degradation of polybenzimidazole/muscovite composite membranes in high-temperature proton exchange membrane fuel cells[J]. Journal of Membrane Science, 2022, 641: 119868. doi: 10.1016/j.memsci.2021.119868 [32] DJEBBI M A, BOUBAKRI S, BOUAZIZ Z, et al. Extended-release of chlorpromazine intercalated into montmorillonite clays[J]. Microporous and Mesoporous Materials, 2018, 267: 43-52. doi: 10.1016/j.micromeso.2018.03.017 [33] YU D M, YOON Y J, KIM T H, et al. Sulfonated poly(arylene ether sulfone)/sulfonated zeolite composite membrane for high temperature proton exchange membrane fuel cells[J]. Solid State Ionics, 2013, 233: 55-61. doi: 10.1016/j.ssi.2012.12.006 [34] MENG Z, ZOU Y, LI N, et al. Graphene oxide-intercalated microbial montmorillonite to moderate the dependence of nafion-based pemfcs in high-humidity environments[J]. ACS Applied Energy Materials, 2023, 6(3): 1771-1780. doi: 10.1021/acsaem.2c03666 [35] KU B C, KIM D W, STEEVES D, et al. Molecularly ordered structure and permeability properties of amphiphilic polyacetylene-multilayer nanocomposites[J]. Composites Science and Technology, 2008, 68(15): 3215-3219. [36] LI X, ROBERTS E P L, HOLMES S M, et al. Functionalized zeolite A–nafion composite membranes for direct me-thanol fuel cells[J]. Solid State Ionics, 2007, 178(19): 1248-1255. [37] MUNAVALLI B B, KARIDURAGANAVAR M Y. Development of novel sulfonic acid functionalized zeolites incorporated composite proton exchange membranes for fuel cell application[J]. Electrochim Acta, 2019, 296: 294-307. doi: 10.1016/j.electacta.2018.11.056 [38] ATHENS G L, EIN-ELI Y, CHMELKA B F. Acid-functiona-lized mesostructured aluminosilica for hydrophilic proton conduction membranes[J]. Advanced Materials, 2007, 19(18): 2580-2587. doi: 10.1002/adma.200602781 [39] HAN W, KWAN S M, YEUNG K L. Zeolite proton conducting membrane for micro fuel cell applications[J]. Topics in Catalysis, 2010, 53(19-20): 1394-1400. doi: 10.1007/s11244-010-9599-0 [40] FADAEE TAKMIL N, JALEH B, FEIZI MOHAZZAB B, et al. Hydrogen production by electrochemical reaction using waste zeolite boosted with titania and Au nanoparticles[J]. Inorganic Chemistry Communications, 2021, 133: 108891. doi: 10.1016/j.inoche.2021.108891 [41] REEVE P J, FALLOWFIELD H J. Natural and surfactant modified zeolites: A review of their applications for water remediation with a focus on surfactant desorption and toxi-city towards microorganisms[J]. Journal of Environmen-tal Management, 2018, 205: 253-261. [42] WANG S, PENG Y. Natural zeolites as effective adsorbents in water and wastewater treatment[J]. Chemical Engineering Journal, 2010, 156(1): 11-24. doi: 10.1016/j.cej.2009.10.029 [43] INGLEZAKIS V J, ZORPAS A A. Handbook of natural zeolites[M]. Netherlands: Bentham Science Publishers, 2012: 705. [44] HUNGER B, KLEPEL O, KIRSCHHOCK C, et al. Interaction of water with alkali-metal cation-exchanged X type zeolites: A temperature-programmed desorption (TPD) and X-ray diffraction study[J]. Langmuir, 1999, 15(18): 5937-5941. doi: 10.1021/la981284s [45] MECHERI B, FELICE V, ZHANG Z, et al. DSC and DVS investigation of water mobility in nafion/zeolite composite membranes for fuel cell applications[J]. The Journal of Physical Chemistry C, 2012, 116: 20820-20829. doi: 10.1021/jp301762h [46] ZHANG Z, DÉSILETS F, FELICE V, et al. On the proton conductivity of Nafion-Faujasite composite membranes for low temperature direct methanol fuel cells[J]. Journal of Power Sources, 2011, 196(22): 9176-9187. doi: 10.1016/j.jpowsour.2011.07.009 [47] PRAPAINAINAR P, DU Z, KONGKACHUICHAY P, et al. Mordenite/Nafion and analcime/Nafion composite membranes prepared by spray method for improved direct methanol fuel cell performance[J]. Applied Surface Science, 2017, 421: 24-41. doi: 10.1016/j.apsusc.2017.02.004 [48] YOONOO C, DAWSON C P, ROBERTS E P L, et al. Nafion®/mordenite composite membranes for improved direct methanol fuel cell performance[J]. Journal of Membrane Science, 2011, 369(1): 367-374. [49] ARICÒ A S, BAGLIO V, DI BLASI A, et al. Surface properties of inorganic fillers for application in composite membranes-direct methanol fuel cells[J]. Journal of Power Sources, 2004, 128(2): 113-118. doi: 10.1016/j.jpowsour.2003.09.063 [50] EGUIZÁBAL A, LEMUS J, PINA M P. On the incorporation of protic ionic liquids imbibed in large pore zeolites to polybenzimidazole membranes for high temperature proton exchange membrane fuel cells[J]. Journal of Power Sources, 2013, 222: 483-492. doi: 10.1016/j.jpowsour.2012.07.094 [51] BEAUGER C, LAINÉ G, BURR A, et al. Improvement of Nafion®-sepiolite composite membranes for PEMFC with sulfo-fluorinated sepiolite[J]. Journal of Membrane Science, 2015, 495: 392-403. doi: 10.1016/j.memsci.2015.08.014 [52] THMAINI N, CHARRADI K, AHMED Z, et al. Nanoarchitectonics of fibrous clays as fillers of improved proton-conducting membranes for fuel-cell applications[J]. Applied Clay Science, 2023, 242: 107019. doi: 10.1016/j.clay.2023.107019 [53] ARANDA P, RUIZ-HITZKY E. Immobilization of nanoparticles on fibrous clay surfaces: Towards promising nanoplatforms for advanced functional applications[J]. The Chemical Record, 2018, 18(7-8): 1125-1137. doi: 10.1002/tcr.201700113 [54] CHARRADI K, AHMED Z, THMAINI N, et al. Incorporating of layered double hydroxide/sepiolite to improve the performance of sulfonated poly(ether ether ketone) compo-site membranes for proton exchange membrane fuel cells[J]. Journal of Applied Polymer Science, 2021, 138(19): 50364. doi: 10.1002/app.50364 [55] ZHOU C H, LI G L, ZHUANG X Y, et al. Roles of texture and acidity of acid-activated sepiolite catalysts in gas-phase catalytic dehydration of glycerol to acrolein[J]. Molecular Catalysis, 2017, 434: 219-231. doi: 10.1016/j.mcat.2016.12.022 [56] BEAUGER C, LAINÉ G, BURR A, et al. Nafion®-sepiolite composite membranes for improved proton exchange membrane fuel cell performance[J]. Journal of Membrane Science, 2013, 430: 167-179. doi: 10.1016/j.memsci.2012.11.037 [57] TARTAGLIONE G, TABUANI D, CAMINO G. Thermal and morphological characterisation of organically modified sepiolite[J]. Microporous and Mesoporous Materials, 2008, 107(1): 161-168. [58] ZHANG X, LIU Q, XIA L, et al. Poly(2, 5-benzimidazole)/sulfonated sepiolite composite membranes with low phosphoric acid doping levels for PEMFC applications in a wide temperature range[J]. Journal of Membrane Science, 2019, 574: 282-298. doi: 10.1016/j.memsci.2018.12.085 [59] CHARRADI K, AHMED Z, CID R E, et al. Amelioration of PEMFC performance at high temperature by incorporation of nanofiller (sepiolite/layered double hydroxide) in Nafion membrane[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10666-10676. doi: 10.1016/j.ijhydene.2019.02.183 [60] WOO S H, TAGUET A, OTAZAGHINE B, et al. Composite short-side-chain PFSA electrolyte membranes containing selectively modified halloysite nanotubes (HNTs)[J]. Journal of Materials Science, 2021, 56(23): 13108-13127. doi: 10.1007/s10853-021-06109-4 [61] FENG Y, LUO X, WU F, et al. Systematic studies on blood coagulation mechanisms of halloysite nanotubes-coated PET dressing as superior topical hemostatic agent[J]. Chemical Engineering Journal, 2022, 428: 132049. doi: 10.1016/j.cej.2021.132049 [62] DANYLIUK N, TOMASZEWSKA J, TATARCHUK T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications[J]. Journal of Molecular Liquids, 2020, 309: 113077. doi: 10.1016/j.molliq.2020.113077 [63] HE S, DAI W, YANG W, et al. Nanocomposite proton exchange membranes based on phosphotungstic acid immobilized by polydopamine-coated halloysite nanotubes[J]. Polymer Testing, 2019, 73: 242-249. doi: 10.1016/j.polymertesting.2018.11.038 [64] MOGG L, HAO G P, ZHANG S, et al. Atomically thin micas as proton-conducting membranes[J]. Nature Nanotechnology, 2019, 14(10): 962-966. doi: 10.1038/s41565-019-0536-5 [65] SHAO J J, RAIDONGIA K, KOLTONOW A R, et al. Self-assembled two-dimensional nanofluidic proton channels with high thermal stability[J]. Nature Communications, 2015, 6(1): 7602. doi: 10.1038/ncomms8602 [66] DING Z, LI J, ZHANG B, et al. Rapid and high-concentration exfoliation of montmorillonite into high-quality and mono-layered nanosheets[J]. Nanoscale, 2020, 12(32): 17083-17092. doi: 10.1039/D0NR04514D [67] JIA F, SONG S. Exfoliation and characterization of layered silicate minerals: A review[J]. Surface Review and Letters, 2013, 21: 1430001. [68] LIU Y, DU M, LI Z, et al. Molecular dynamics study on swelling and exfoliation properties of montmorillonite nanosheets for application as proton exchange membranes[J]. ACS Applied Nano Materials, 2023, 6(3): 2133-2140. doi: 10.1021/acsanm.2c05167 [69] TAUFIQ MUSA M, SHAARI N, KAMARUDIN S K. Carbon nanotube, graphene oxide and montmorillonite as conductive fillers in polymer electrolyte membrane for fuel cell: An overview[J]. International Journal of Energy Research, 2021, 45(2): 1309-1346. doi: 10.1002/er.5874 [70] KALAISELVIMARY J, SELVAKUMAR K, RAJENDRAN S, et al. Effect of surface-modified montmorillonite incorpo-rated biopolymer membranes for PEM fuel cell applications[J]. Polymer Composites, 2019, 40(S1): E301-E311. [71] LIU M L, HUANG M, TIAN L Y, et al. Two-dimensional nanochannel arrays based on flexible montmorillonite membranes[J]. ACS Applied Materials & Interfaces, 2018, 10(51): 44915-44923. [72] RADMANESH F, RIJNAARTS T, MOHEB A, et al. Enhanced selectivity and performance of heterogeneous cation exchange membranes through addition of sulfonated and protonated montmorillonite[J]. Journal of Colloid and Interface Science, 2019, 533: 658-670. doi: 10.1016/j.jcis.2018.08.100 [73] KOH M J, HWANG H Y, KIM D J, et al. Preparation and characterization of porous PVDF-HFP/clay nanocompo-site membranes[J]. Journal of Materials Science & Technology, 2010, 26(7): 633-638. [74] DEKA M, KUMAR A. Electrical and electrochemical stu-dies of poly(vinylidene fluoride)-clay nanocomposite gel polymer electrolytes for Li-ion batteries[J]. Journal of Power Sources, 2011, 196(3): 1358-1364. doi: 10.1016/j.jpowsour.2010.09.035 [75] PAUL D R, ROBESON L M. Polymer nanotechnology: Nanocomposites[J]. Polymer, 2008, 49(15): 3187-3204. doi: 10.1016/j.polymer.2008.04.017 [76] HASANI-SADRABADI M M, GHAFFARIAN S R, RENAUD P. Nafion/benzotriazole functionalized montmorillonite nanocomposites: Novel high-performance proton exchange membranes[J]. RSC Advances, 2013, 3(42): 19357-19365. doi: 10.1039/c3ra42142b [77] WANG F, WANG D, ZHU H. Montmorillonite-polybenzimidazole inorganic-organic composite membrane with electric field-aligned proton transport channel for high tempe-rature proton exchange membranes[J]. Polymer-Plastics Technology and Engineering, 2018, 57: 1-8. doi: 10.1080/03602559.2017.1298802 [78] ARIAS J J R, CARLOS DUTRA J, GOMES A D S. Hybrid membranes of sulfonated poly ether ether ketone, ionic liquid and organically modified montmorillonite for proton exchange membranes with enhanced ionic conductivity and ionic liquid lixiviation protection[J]. Journal of Membrane Science, 2017, 537: 353-361. doi: 10.1016/j.memsci.2017.05.044 [79] SURANSH J, TIWARI A K, MUNGRAY A K. Modification of clayware ceramic membrane for enhancing the perfor-mance of microbial fuel cell[J]. Environmental Progress & Sustainable Energy, 2020, 39(6): e13427. [80] SASIKALA S, MEENAKSHI S, BHAT S D, et al. Functionalized bentonite clay-sPEEK based composite membranes for direct methanol fuel cells[J]. Electrochimica Acta, 2014, 135: 232-241. doi: 10.1016/j.electacta.2014.04.180 [81] WANG Y, HAN G, TIAN Z, et al. Nafion®/SiO2/m-BOT composite membranes for improved direct methanol fuel cell performance[J]. RSC Advances, 2014, 4(88): 47129-47135. doi: 10.1039/C4RA06365A [82] TANAKA H, TANIGUCHI M. Atomically flat nickel film grown on synthetic mica[J]. Japanese Journal of Applied Physics, 2016, 55(7): 078003. doi: 10.7567/JJAP.55.078003 [83] DEVRIM Y, ALBOSTAN A. Enhancement of PEM fuel cell performance at higher temperatures and lower humidities by high performance membrane electrode assembly based on Nafion/zeolite membrane[J]. International Journal of Hydrogen Energy, 2015, 40(44): 15328-15335. doi: 10.1016/j.ijhydene.2015.02.078 [84] ZANCHET L, DA TRINDADE L G, TROMBETTA F, et al. Improving Nafion/zeolite nanocomposite with a CF3SO3− based ionic liquid for PEMFC application[J]. Ionics, 2021, 27(5): 2027-2036. doi: 10.1007/s11581-021-03918-0 [85] RESSAM I, EL KADIB A, LAHCINI M, et al. Enhanced proton transport properties of Nafion via functionalized halloysite nanotubes[J]. International Journal of Hydrogen Energy, 2018, 43(40): 18578-18591. doi: 10.1016/j.ijhydene.2018.05.025 [86] ALTAF F, BATOOL R, GILL R, et al. Synthesis and electrochemical investigations of ABPBI grafted montmorillonite based polymer electrolyte membranes for PEMFC applications[J]. Renewable Energy, 2021, 164: 709-728. doi: 10.1016/j.renene.2020.09.104 [87] SUN X, ZHANG T, LIU X, et al. Multi-functionalized acid-base double-shell nanotubes are incorporated into the proton exchange membrane to cope with low humidity conditions[J]. International Journal of Hydrogen Energy, 2020, 45(55): 30673-30688. doi: 10.1016/j.ijhydene.2020.08.009 [88] CHEN Z, HOLMBERG B, LI W, et al. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell[J]. Chemistry of Materials, 2006, 18(24): 5669-5675. doi: 10.1021/cm060841q [89] HASANI SADRABADI M M, DASHTIMOGHADAM E, MAJEDI F S, et al. Nafion®/bio-functionalized montmoril-lonite nanohybrids as novel polyelectrolyte membranes for direct methanol fuel cells[J]. Journal of Power Sources, 2009, 190(2): 318-321. doi: 10.1016/j.jpowsour.2009.01.043 [90] CHUANG S W, HSU S L C, HSU C L. Synthesis and properties of fluorine-containing polybenzimidazole/montmorillonite nanocomposite membranes for direct methanol fuel cell applications[J]. Journal of Power Sources, 2007, 168(1): 172-177. doi: 10.1016/j.jpowsour.2007.03.021 [91] SASIKALA S, GOPI K H, BHAT S D. Sulfosuccinic acid-sulfonated polyether ether ketone/organo functionalized microporous zeolite-13X membrane electrolyte for direct methanol fuel cells[J]. Microporous and Mesoporous Materials, 2016, 236: 38-47. doi: 10.1016/j.micromeso.2016.08.029 [92] GOSALAWIT R, CHIRACHANCHAI S, SHISHATSKIY S, et al. Sulfonated montmorillonite/sulfonated poly(ether et-her ketone) (SMMT/SPEEK) nanocomposite membrane for direct methanol fuel cells (DMFCs)[J]. Journal of Membrane Science, 2008, 323(2): 337-346. doi: 10.1016/j.memsci.2008.06.038 -





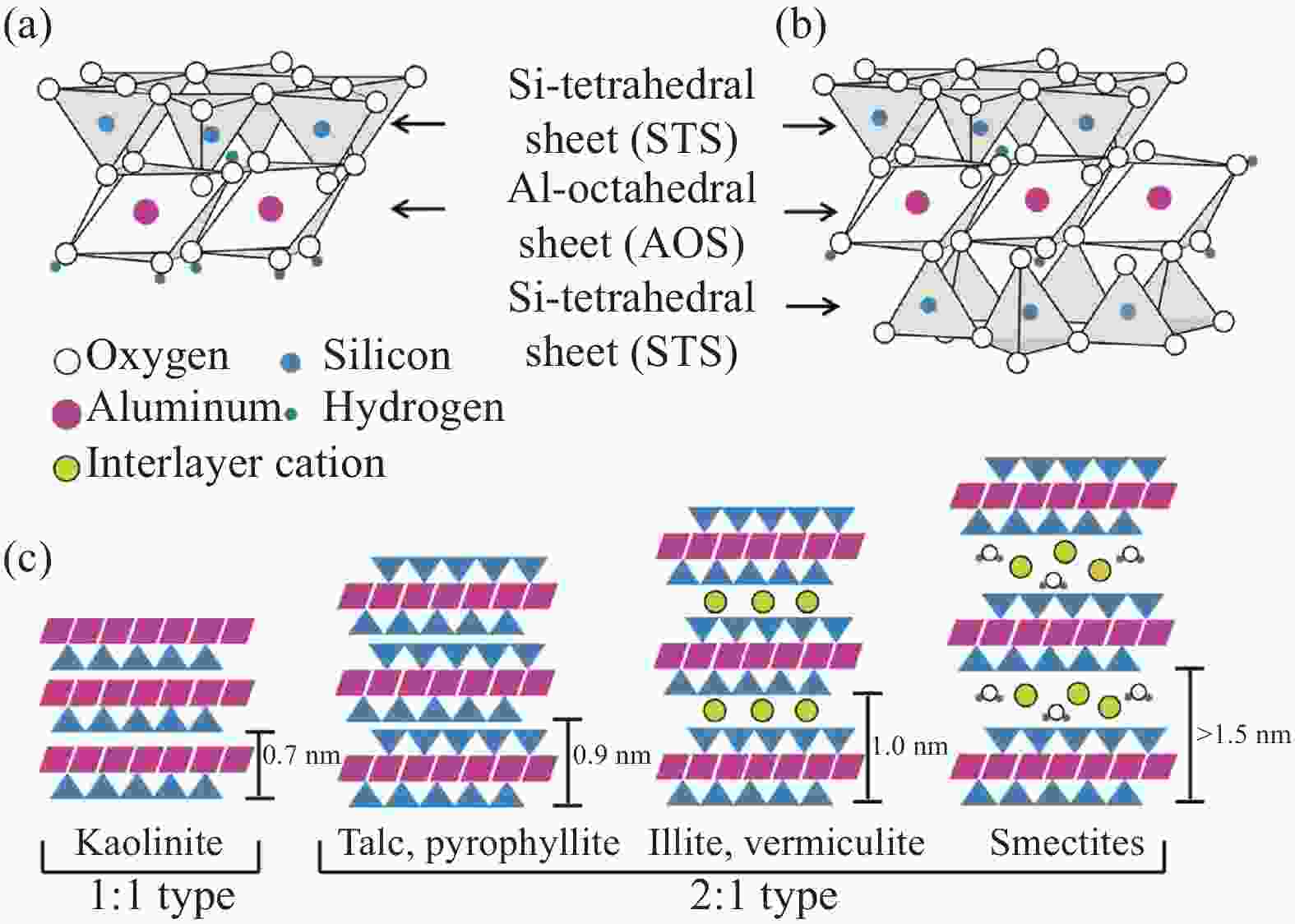
 下载:
下载: