| [1] |
GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by sulfate radical-based advanced oxidation processes (SR-AOPs)[J]. Chemical Engineering Journal, 2021, 406: 127083. doi: 10.1016/j.cej.2020.127083
|
| [2] |
ZHANG W X, LI Z H, LUO R, et al. Design of tandem CuO/CNTs composites for enhanced tetracycline degradation and antibacterial activity[J]. Separation and Purification Technology, 2023, 306: 122548. doi: 10.1016/j.seppur.2022.122548
|
| [3] |
PIMENTEL D, BERGER B, FILIBERTO D, et al. Water resources: Agricultural and environmental issues[J]. BioScience, 2004, 54(10): 909-918.
|
| [4] |
DONG G H, CHEN B, LIU B, et al. Advanced oxidation processes in microreactors for water and wastewater treatment: Development, challenges, and opportunities[J]. Water Research, 2022, 211: 118047. doi: 10.1016/j.watres.2022.118047
|
| [5] |
XU F, ZHANG W, WANG X, et al. Multi-level FeCo/N-doped carbon nanosheet for peroxymonosulfate oxidation and sterilization inactivation[J]. Journal of Colloid and Interface Science, 2024, 661: 840-852.
|
| [6] |
LI X Y, JIE B R, LIN H D, et al. Application of sulfate radicals-based advanced oxidation technology in degradation of trace organic contaminants (TrOCs): Recent advances and prospects[J]. Journal of Environmental Management, 2022, 308: 114664. doi: 10.1016/j.jenvman.2022.114664
|
| [7] |
LI Z H, ZHANG W X, LIU X Y, et al. Iron-cobalt magnetic porous carbon beads activated peroxymonosulfate for enhanced degradation and Microbial inactivation[J]. Journal of Colloid and Interface Science, 2023, 652: 1878-1888. doi: 10.1016/j.jcis.2023.09.018
|
| [8] |
ZHANG H Y, TIAN W J, DUAN X G, et al. Catalysis of a single transition metal site for water oxidation: From mononuclear molecules to single atoms[J]. Advanced Materials, 2020, 32(18): 1904037. doi: 10.1002/adma.201904037
|
| [9] |
DUAN X G, TIAN W J, ZHANG H Y, et al. Interfacial-engineered cobalt@carbon hybrids for synergistically boosted evolution of sulfate radicals toward green oxidation[J]. Applied Catalysis B: Environmental, 2019, 256: 117795. doi: 10.1016/j.apcatb.2019.117795
|
| [10] |
LI J L, ZHU W H, GAO Y, et al. The catalyst derived from the sulfurized Co-doped metal-organic framework (MOF) for peroxymonosulfate (PMS) activation and its application to pollutant removal[J]. Separation and Purification Technology, 2022, 285: 120362. doi: 10.1016/j.seppur.2021.120362
|
| [11] |
PENG Q, DING Y B, ZHU L H, et al. Fast and complete degradation of norfloxacin by using Fe/Fe3C@NG as a bifunctional catalyst for activating peroxymonosulfate[J]. Separation and Purification Technology, 2018, 202: 307-317. doi: 10.1016/j.seppur.2018.03.049
|
| [12] |
KAJAL N, SINGH V, GUPTA R, et al. Metal organic frameworks for electrochemical sensor applications: A review[J]. Environmental Research, 2022, 204: 112320. doi: 10.1016/j.envres.2021.112320
|
| [13] |
FU Q, YANG F, BAO X H. Interface-confined oxide nanostructures for catalytic oxidation reactions[J]. Accounts of Chemical Research, 2013, 46(8): 1692-1701. doi: 10.1021/ar300249b
|
| [14] |
FU Q, BAO X H. Surface chemistry and catalysis confined under two-dimensional materials[J]. Chemical Society Reviews, 2017, 46(7): 1842-1874. doi: 10.1039/C6CS00424E
|
| [15] |
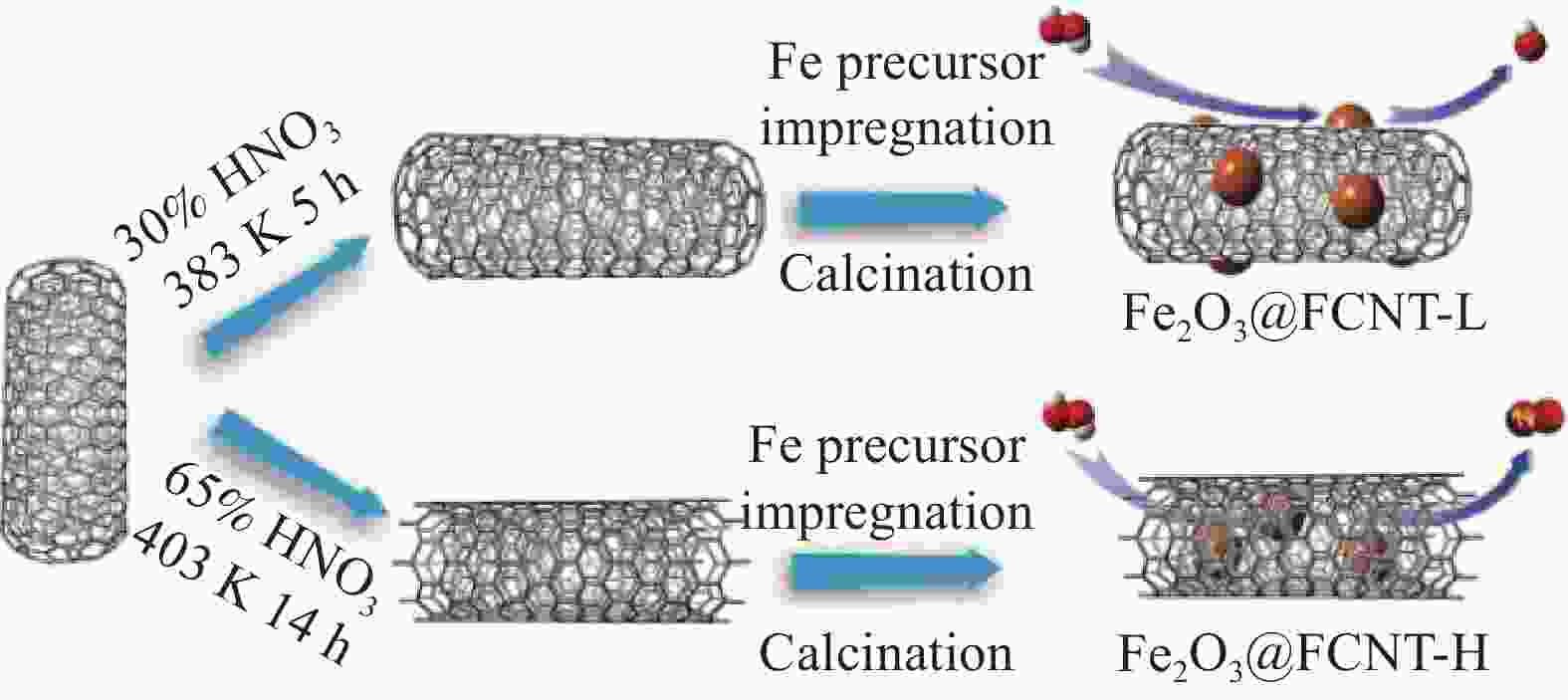
PAN X L, BAO X H. The effects of confinement inside carbon nanotubes on catalysis[J]. Accounts of Chemical Research, 2011, 44(8): 553-562. doi: 10.1021/ar100160t
|
| [16] |
ZHANG S W, GAO H H, XU X T, et al. MOF-derived CoN/N-C@SiO2 yolk-shell nanoreactor with dual active sites for highly efficient catalytic advanced oxidation processes[J]. Chemical Engineering Journal, 2020, 381: 122670. doi: 10.1016/j.cej.2019.122670
|
| [17] |
LI X M, WU D H, HUA T, et al. Micro/macrostructure and multicomponent design of catalysts by MOF-derived strategy: Opportunities for the application of nanomaterials-based advanced oxidation processes in wastewater treatment[J]. Science of the Total Environment, 2022, 804: 150096. doi: 10.1016/j.scitotenv.2021.150096
|
| [18] |
LY Q V, CUI L L, ASIF M B, et al. Membrane-based nanoconfined heterogeneous catalysis for water purification: A critical review[J]. Water Research, 2023, 230: 119577.
|
| [19] |
MA H R, WANG G L, XU Z H, et al. Confining peroxymonosulfate activation in carbon nanotube intercalated nitrogen doped reduced graphene oxide membrane for enhanced water treatment: The role of nanoconfinement effect[J]. Journal of Colloid and Interface Science, 2022, 608: 2740-2751. doi: 10.1016/j.jcis.2021.11.007
|
| [20] |
ZHU J L, WANG J, SHAN C, et al. Durable activation of peroxymonosulfate mediated by Co-doped mesoporous FePO4 via charge redistribution for atrazine degradation[J]. Chemical Engineering Journal, 2019, 375: 122009. doi: 10.1016/j.cej.2019.122009
|
| [21] |
LI C L, WU M C, LIU R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-N-C nanofibers with embedding FeCo alloy nanoparticles[J]. Applied Catalysis B: Environmental, 2019, 244: 150-158. doi: 10.1016/j.apcatb.2018.11.039
|
| [22] |
LIU D S, LI M N, LI X C, et al. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation[J]. Chemical Engineering Journal, 2020, 387: 124008. doi: 10.1016/j.cej.2019.124008
|
| [23] |
LI X M, YAN X L, HU X Y, et al. Hollow Cu-Co/N-doped carbon spheres derived from ZIFs as an efficient catalyst for peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 397: 125533. doi: 10.1016/j.cej.2020.125533
|
| [24] |
WANG G L, NIE X W, JI X J, et al. Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: The synergism between Co and N[J]. Environmental Science: Nano, 2019, 6(2): 399-410. doi: 10.1039/C8EN01231H
|
| [25] |
HOU C C, ZOU L L, XU Q. A hydrangea-like superstructure of open carbon cages with hierarchical porosity and highly active metal sites[J]. Advanced Materials, 2019, 31(46): 1904689. doi: 10.1002/adma.201904689
|
| [26] |
TIAN W J, ZHANG H Y, DUAN X G, et al. Porous carbons: Structure-oriented design and versatile applications[J]. Advanced Functional Materials, 2020, 30(17): 1909265. doi: 10.1002/adfm.201909265
|
| [27] |
CHEN X, OH W D, ZHANG P H, et al. Surface construction of nitrogen-doped chitosan-derived carbon nanosheets with hierarchically porous structure for enhanced sulfacetamide degradation via peroxymonosulfate activation: Maneuverable porosity and active sites[J]. Chemical Engineering Journal, 2020, 382: 122908. doi: 10.1016/j.cej.2019.122908
|
| [28] |
LIU C, LIU S Q, LIU L Y, et al. Novel carbon-based Fe-Co oxides derived from prussian blue analogues activating peroxymonosulfate: Refractory drugs degradation without metal leaching[J]. Chemical Engineering Journal, 2020, 379: 122274. doi: 10.1016/j.cej.2019.122274
|
| [29] |
ZHANG Y J, HUANG G X, CHEN J J, et al. Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination[J]. Nature Communications, 2022, 13(1): 3005. doi: 10.1038/s41467-022-30560-9
|
| [30] |
XIONG Z K, JIANG Y N, WU Z L, et al. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review[J]. Chemical Engineering Journal, 2021, 421: 127863. doi: 10.1016/j.cej.2020.127863
|
| [31] |
LI X R, WANG P X, LU Q Y, et al. A hierarchical porous aerohydrogel for enhanced water evaporation[J]. Water Research, 2023, 244: 120447.
|
| [32] |
DUAN X G, AO Z M, ZHANG H Y, et al. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: Core-shell layer dependence[J]. Applied Catalysis B: Environmental, 2018, 222: 176-181. doi: 10.1016/j.apcatb.2017.10.007
|
| [33] |
YI Y Y, ZHAO W, ZENG Z H, et al. ZIF-8@ZIF-67-derived nitrogen-doped porous carbon confined CoP polyhedron targeting superior potassium-ion storage[J]. Small, 2020, 16(7): 1906566. doi: 10.1002/smll.201906566
|
| [34] |
CHEN J H, LIU J W, XIE J Q, et al. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range[J]. Nano Energy, 2019, 56: 225-233. doi: 10.1016/j.nanoen.2018.11.051
|
| [35] |
ZHANG W X, SONG H, CHENG Y, et al. Core-shell prussian blue analogs with compositional heterogeneity and open cages for oxygen evolution reaction[J]. Advanced Science, 2019, 6(7): 1801901. doi: 10.1002/advs.201801901
|
| [36] |
YUAN H D, NAI J W, FANG Y J, et al. Double-shelled C@MoS2 structures preloaded with sulfur: An additive reservoir for stable lithium metal anodes[J]. Angewandte Chemie International Edition, 2020, 59(37): 15839-15843. doi: 10.1002/anie.202001989
|
| [37] |
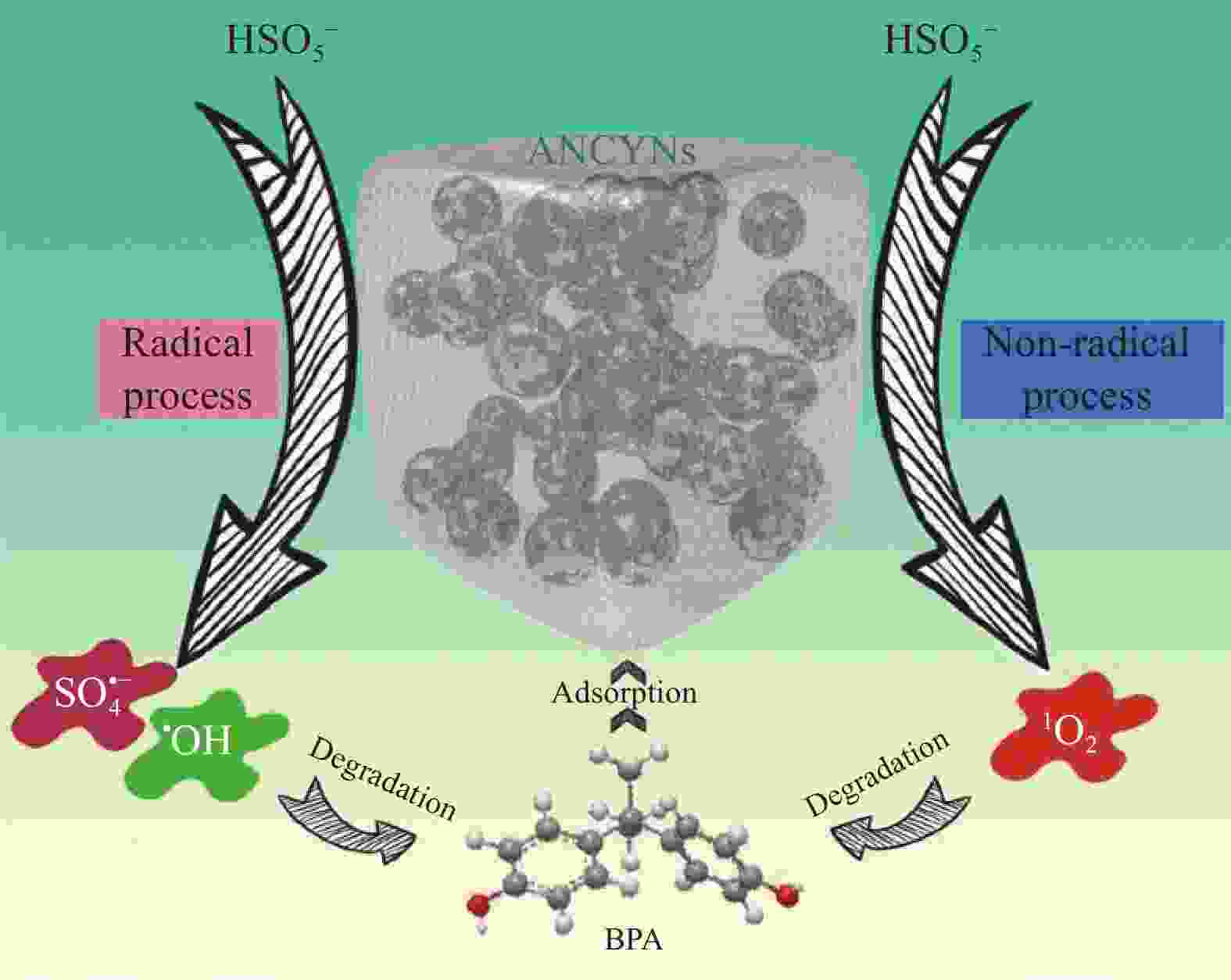
YANG Z C, QIAN J S, YU A Q, et al. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement[J]. Proceedings of the National Academy of Sciences, 2019, 116(14): 6659-6664. doi: 10.1073/pnas.1819382116
|
| [38] |
ZHANG H C, KANG Z X, HAN J J, et al. Photothermal nanoconfinement reactor: Boosting chemical reactivity with locally high temperature in a confined space[J]. Angewandte Chemie International Edition, 2022, 61(26): e202200093. doi: 10.1002/anie.202200093
|
| [39] |
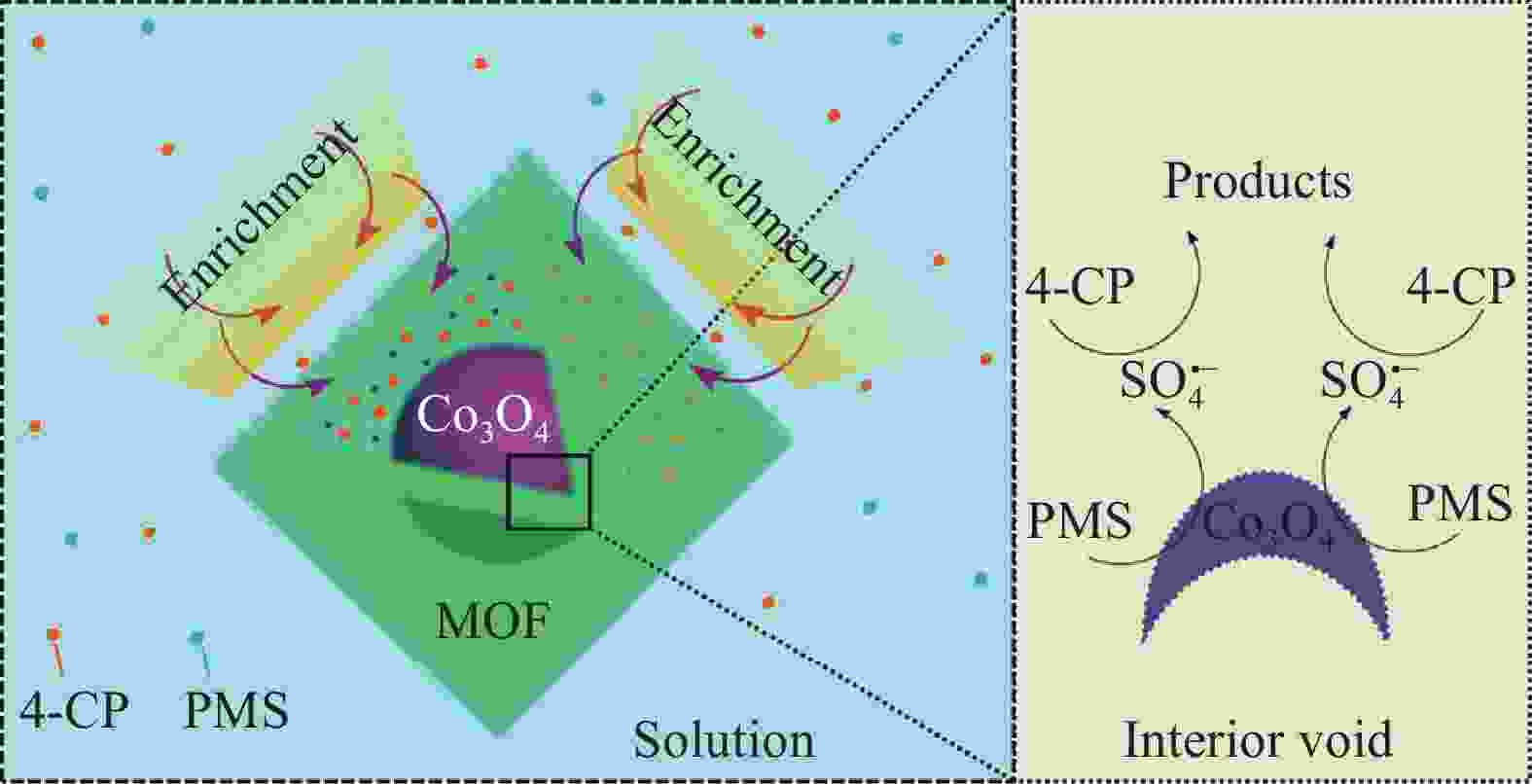
ZENG T, ZHANG X L, WANG S H, et al. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants[J]. Environmental Science & Technology, 2015, 49(4): 2350-2357.
|
| [40] |
WU Z L, XIONG Z K, LIU R, et al. Pivotal roles of N-doped carbon shell and hollow structure in nanoreactor with spatial confined Co species in peroxymonosulfate activation: Obstructing metal leaching and enhancing catalytic stability[J]. Journal of Hazardous Materials, 2022, 427: 128204. doi: 10.1016/j.jhazmat.2021.128204
|
| [41] |
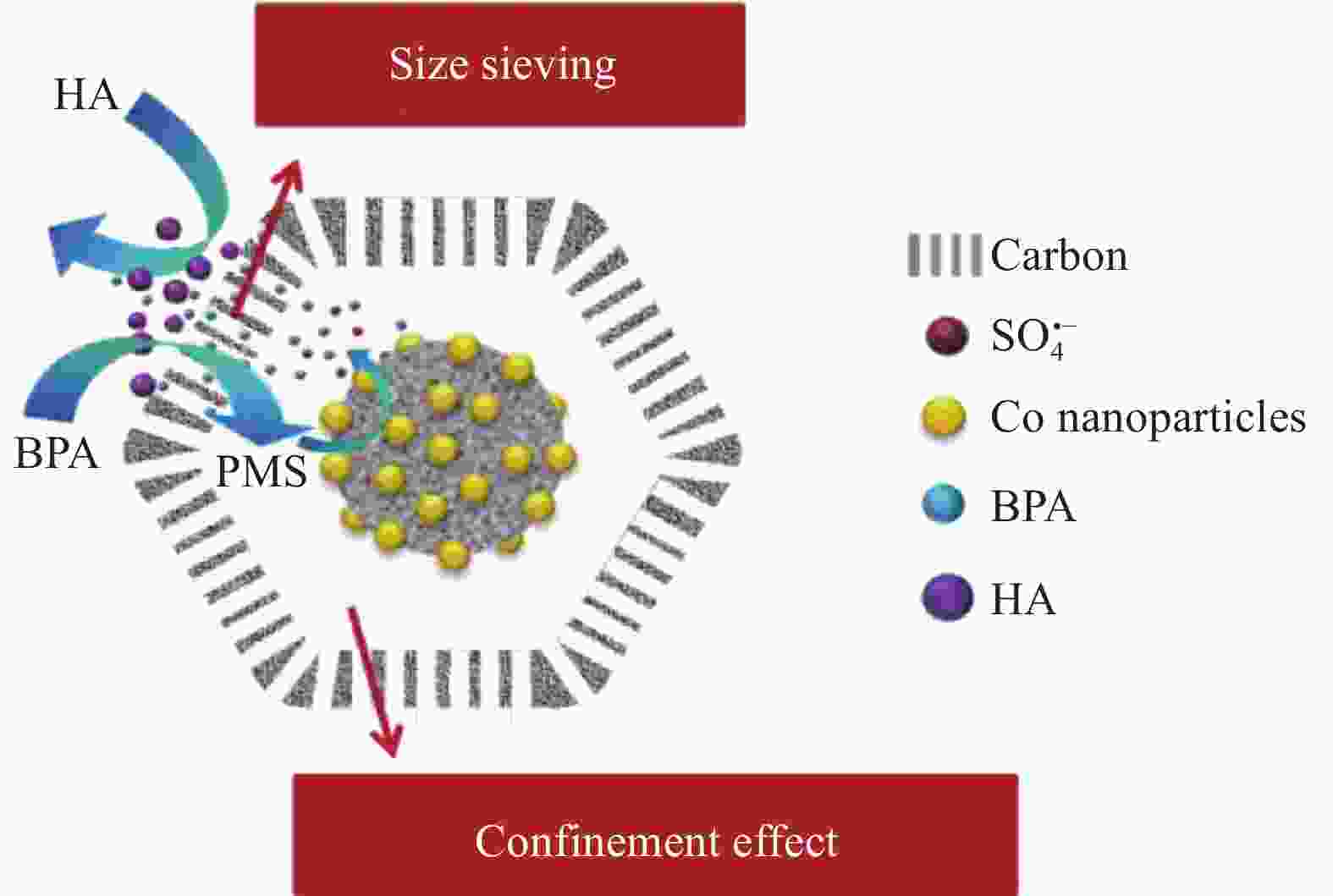
ZHANG M, XIAO C M, YAN X, et al. Efficient removal of organic pollutants by metal-organic framework derived Co/C yolk-shell nanoreactors: Size-exclusion and confinement effect[J]. Environmental Science & Technology, 2020, 54(16): 10289-10300. doi: 10.1021/acs.est.0c00914
|
| [42] |
ZHANG W X, YANG M, ZHANG H, et al. A confinement approach to fabricate hybrid PBAs-derived FeCo@NC yolk-shell nanoreactors for bisphenol A degradation[J]. Chemical Engineering Journal, 2022, 428: 131080. doi: 10.1016/j.cej.2021.131080
|
| [43] |
FANG C, HAO Z X, WANG Y L, et al. Carbon nanotube as a nanoreactor for efficient degradation of 3-aminophenol over CoO x/CNT catalyst[J]. Journal of Cleaner Production, 2023, 405: 136912. doi: 10.1016/j.jclepro.2023.136912
|
| [44] |
LI C Y, YANG L, WANG J, et al. A newly-integrated FeCo-layered double hydroxides photocatalytic system for UV-induced degradation of various heterocyclic amines against complex sample matrix[J]. Separation and Purification Technology, 2023, 304: 122341. doi: 10.1016/j.seppur.2022.122341
|
| [45] |
YANG Y, ZENG Z T, ZHANG C, et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight[J]. Chemical Engineering Journal, 2018, 349: 808-821. doi: 10.1016/j.cej.2018.05.093
|
| [46] |
VINOD K G, TAWFIK A S, JAIN R, et al. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions[J]. Materials Science and Engineering: C, 2012, 32(1): 12-17. doi: 10.1016/j.msec.2011.08.018
|
| [47] |
YANG X X, CAO C D, ERICKSON L, et al. Photo-catalytic degradation of rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation[J]. Applied Catalysis B: Environmental, 2009, 91(3-4): 657-662. doi: 10.1016/j.apcatb.2009.07.006
|
| [48] |
DU C Y, ZHANG Z, YU G L, et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis[J]. Chemosphere, 2021, 272: 129501. doi: 10.1016/j.chemosphere.2020.129501
|
| [49] |
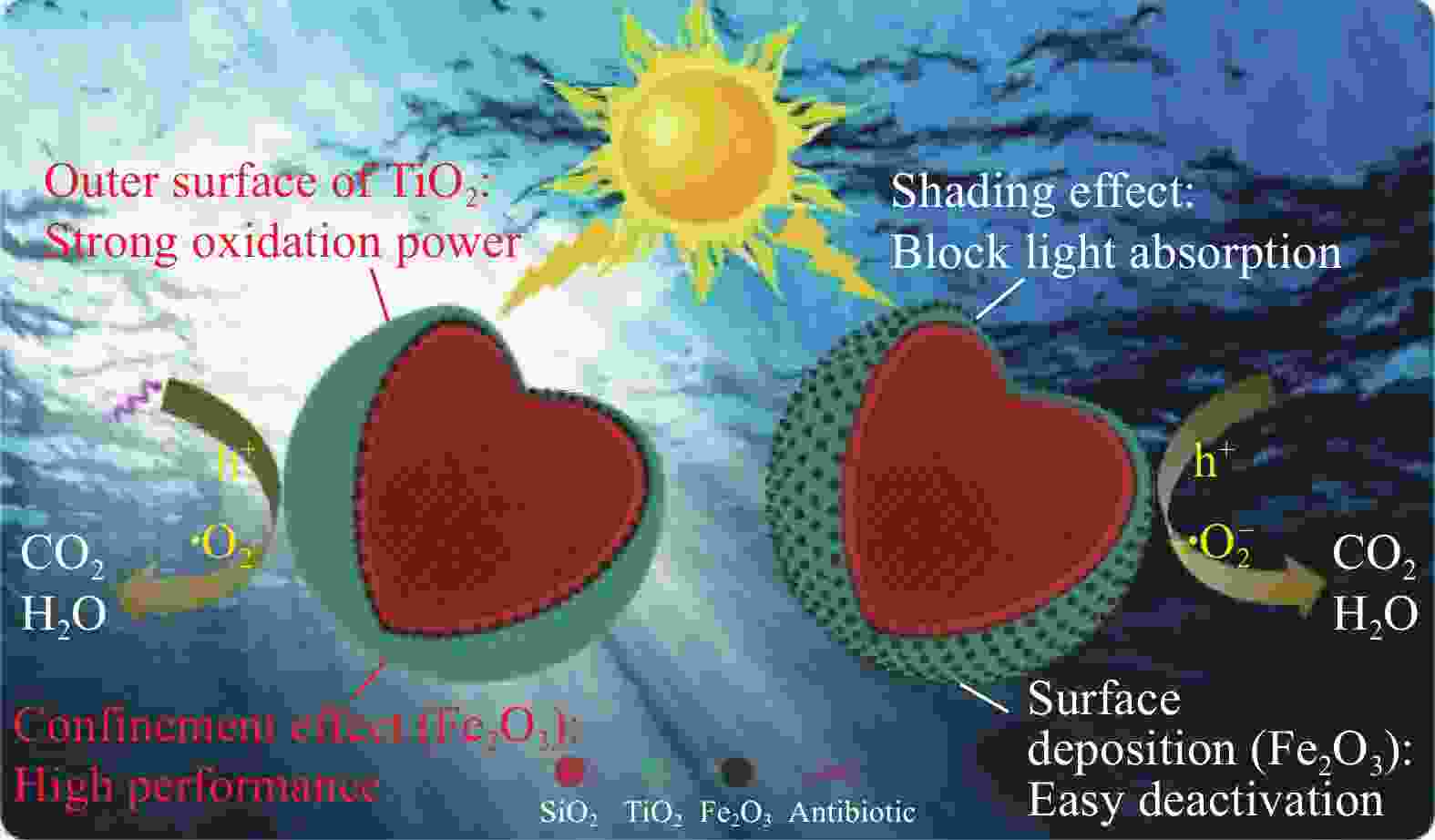
ZHANG S, YI J J, CHEN J R, et al. Spatially confined Fe2O3 in hierarchical SiO2@TiO2 hollow sphere exhibiting superior photocatalytic efficiency for degrading antibiotics[J]. Chemical Engineering Journal, 2020, 380: 122583. doi: 10.1016/j.cej.2019.122583
|
| [50] |
MA Y Y, PENG Q, SUN M, et al. Photocatalytic oxidation degradation of tetracycline over La/Co@TiO2 nanospheres under visible light[J]. Environmental Research, 2022, 215: 114297. doi: 10.1016/j.envres.2022.114297
|
| [51] |
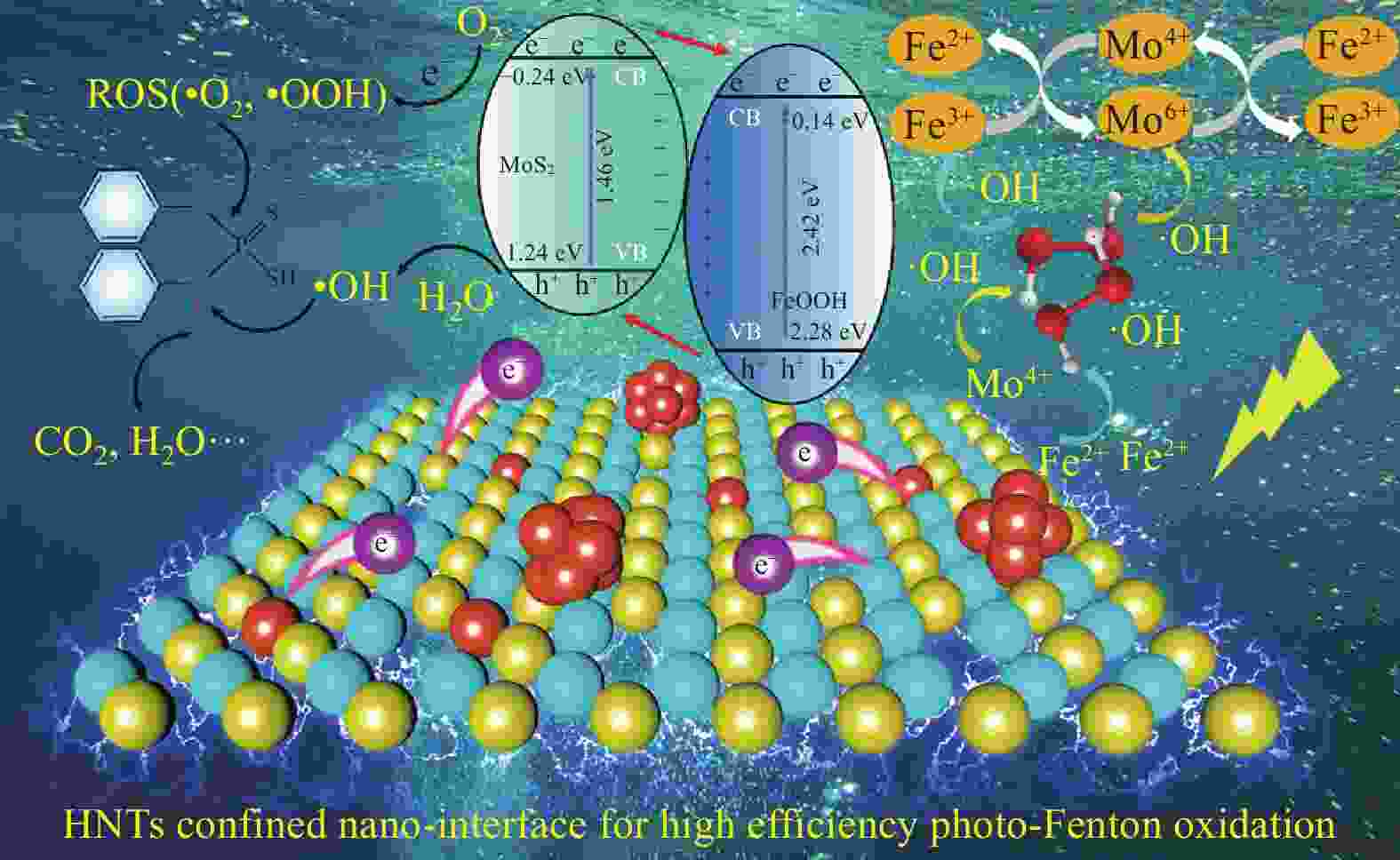
LIU W, DONG Y B, LIU J F, et al. Halloysite nanotube confined interface engineering enhanced catalytic oxidation of photo-Fenton reaction for aniline aerofloat degradation: Defective heterojunction for electron transfer regulation[J]. Chemical Engineering Journal, 2023, 451: 138666. doi: 10.1016/j.cej.2022.138666
|
| [52] |
DANG T J, LU G H, JIANG R R, et al. Bi-etched MIL-125 promotes visible-light-driven photocatalytic performance based on the surface plasmon resonance and spatial confinement effects[J]. Separation and Purification Technology, 2023, 306: 122597. doi: 10.1016/j.seppur.2022.122597
|
| [53] |
YUAN J W, LI H, WANG G, et al. Adsorption, isolated electron/hole transport, and confined catalysis coupling to enhance the photocatalytic degradation performance[J]. Applied Catalysis B: Environmental, 2022, 303: 120892. doi: 10.1016/j.apcatb.2021.120892
|
| [54] |
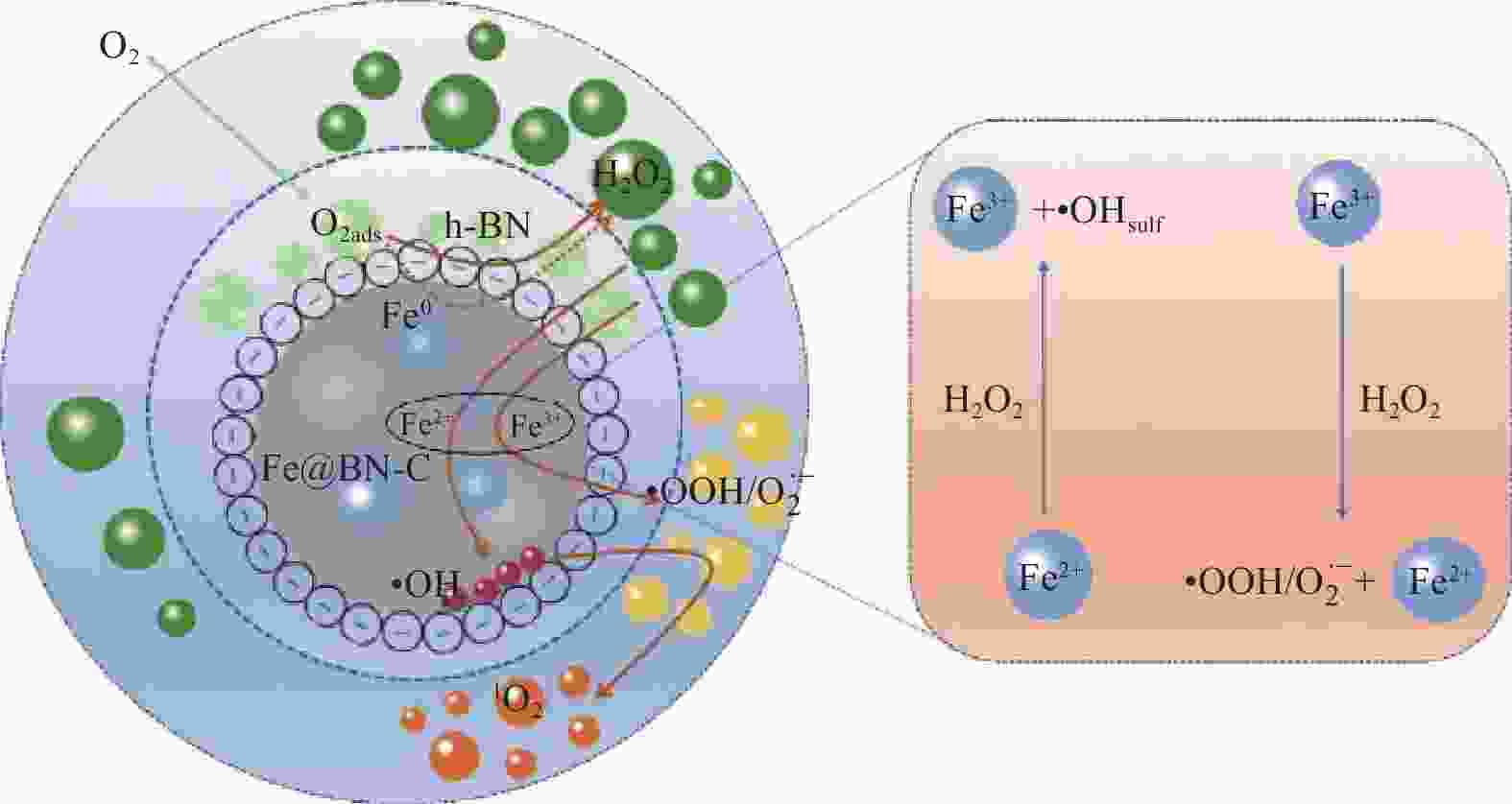
SU P, FU W Y, DU X D, et al. Cost-effective degradation of pollutants by in-situ electrocatalytic process on Fe@BN-C bifunctional cathode: Formation of 1O2 with high selectivity under nanoconfinement[J]. Chemical Engineering Journal, 2023, 452: 139693. doi: 10.1016/j.cej.2022.139693
|
| [55] |
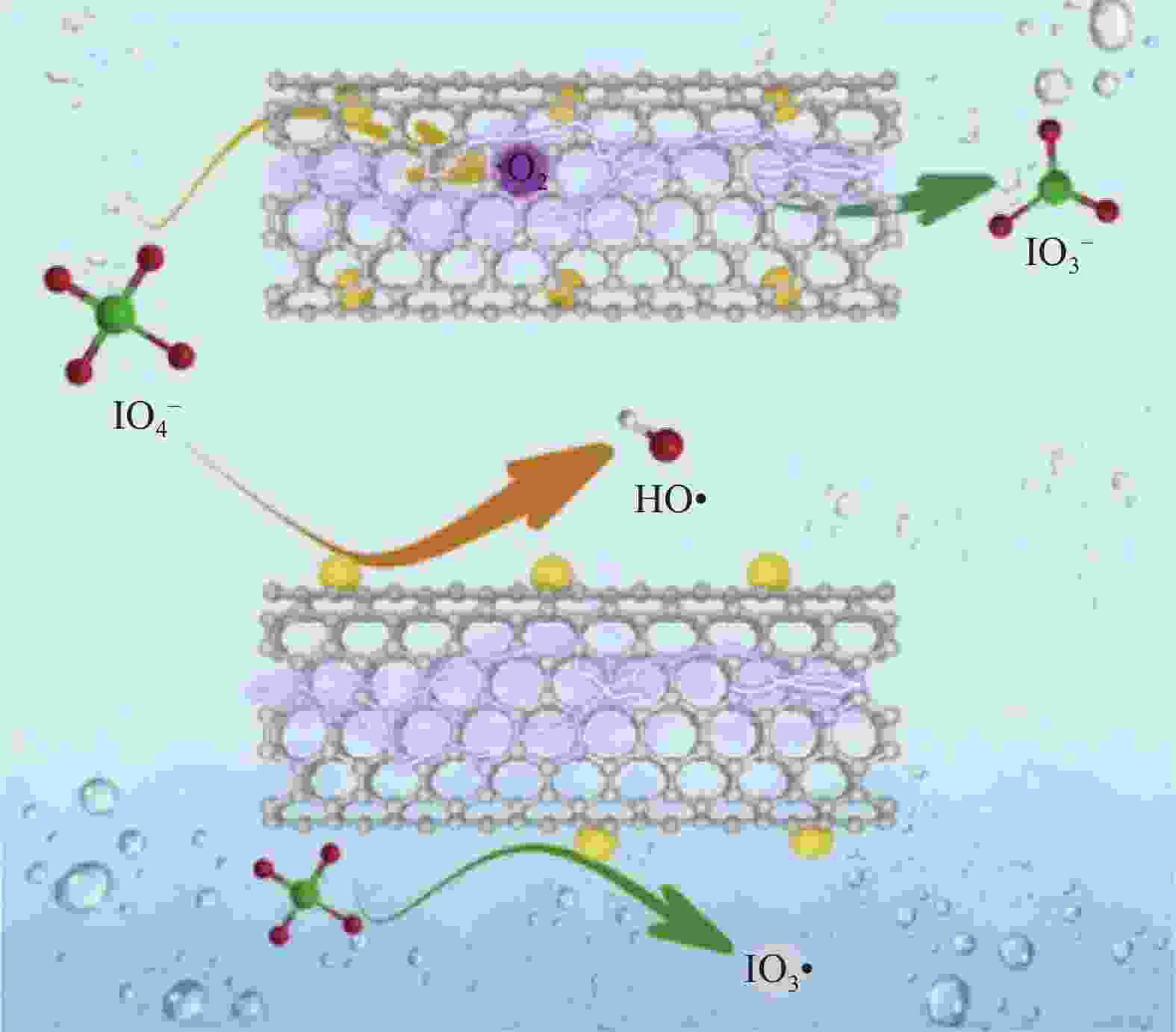
GUO D L, YAO Y, YOU S J, et al. Ultrafast degradation of micropollutants in water via electro-periodate activation catalyzed by nanoconfined Fe2O3[J]. Applied Catalysis B: Environmental, 2022, 309: 121289. doi: 10.1016/j.apcatb.2022.121289
|
| [56] |
GUO D L, JIANG S T, JIN L M, et al. CNT encapsulated MnO x for an enhanced flow-through electro-Fenton process: The involvement of Mn(IV)[J]. Journal of Materials Chemistry A, 2022, 10: 15981-15989. doi: 10.1039/D2TA03445J
|
| [57] |
XU H, CHEN J L, ZHANG Z H, et al. In situ confinement of ultrasmall metal nanoparticles in short mesochannels for durable electrocatalytic nitrate reduction with high efficiency and selectivity[J]. Advanced Materials, 2023, 35(2): 2207522. doi: 10.1002/adma.202207522
|
| [58] |
SUN M, WANG X X, LEA R W, et al. Electrified membranes for water treatment applications[J]. ACS ES&T Engineering, 2021, 1(4): 725-752.
|
| [59] |
MENG C C, DING B F, ZHANG S Z, et al. Angstrom-confined catalytic water purification within Co-TiO(x) laminar membrane nanochannels[J]. Nature Communications, 2022, 13(1): 4010. doi: 10.1038/s41467-022-31807-1
|
| [60] |
ZHANG Z H, ZHANG S Z, QIU L, et al. Ultrahigh-permeance functionalized boron nitride membrane for nanoconfined heterogeneous catalysis[J]. Chemical Catalysis, 2022, 2(3): 550-562. doi: 10.1016/j.checat.2022.01.003
|
| [61] |
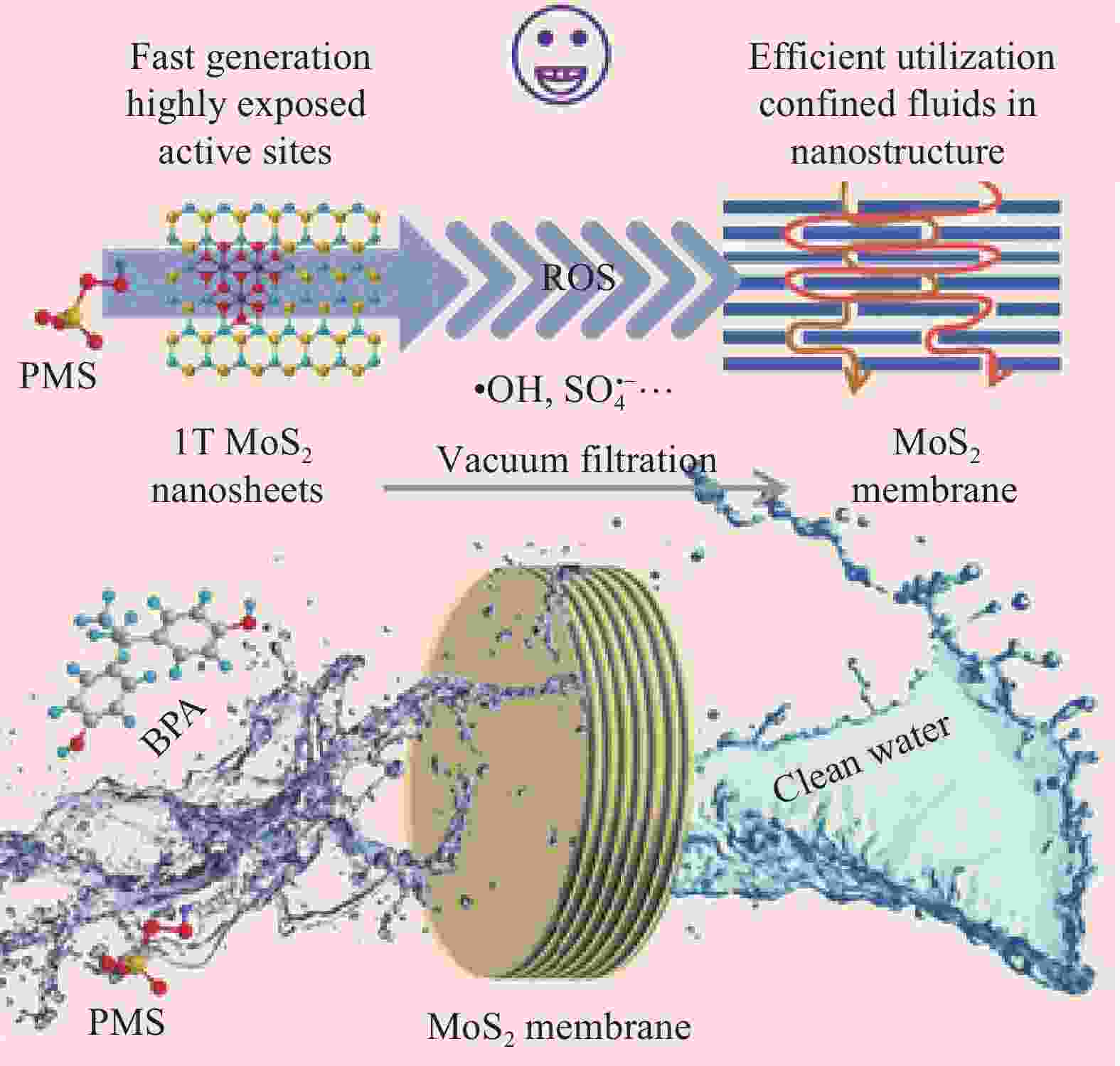
CHEN Y, ZHANG G, LIU H J, et al. Confining free radicals in close vicinity to contaminants enables ultrafast Fenton-like processes in the Interspacing of MoS2 membranes[J]. Angewandte Chemie International Edition, 2019, 58(24): 8134-8138. doi: 10.1002/anie.201903531
|
| [62] |
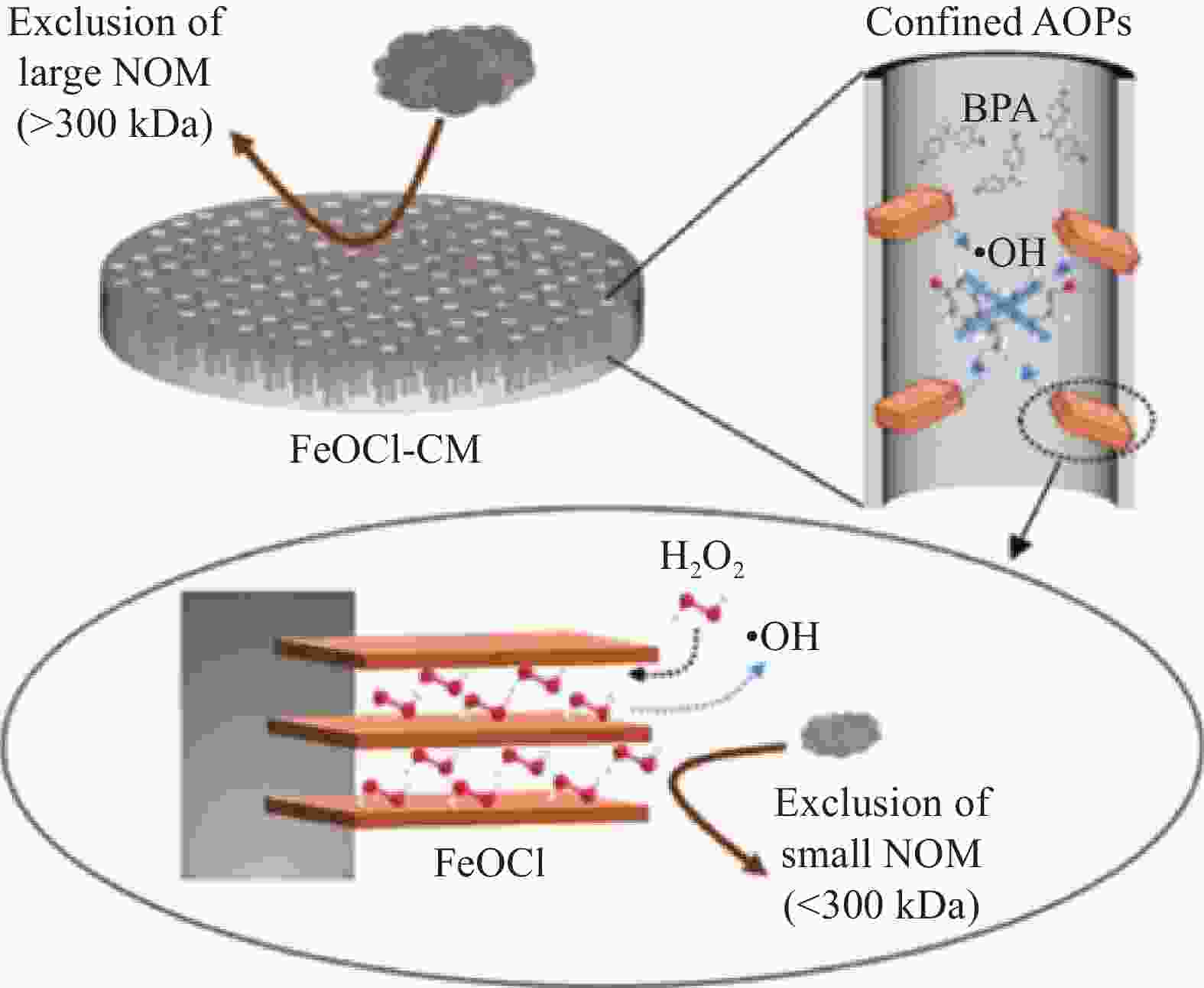
ZHANG S, HEDTKE T, ZHU Q H, et al. Membrane-confined iron oxychloride nanocatalysts for highly efficient heterogeneous Fenton water treatment[J]. Environmental Science & Technology, 2021, 55(13): 9266-9275.
|
| [63] |
HAN Y H, JIANG B, ZHANG C C, et al. Co@N-C nanocatalysts anchored in confined membrane pores for instantaneous pollutants degradation and antifouling via peroxymonosulfate activation[J]. Journal of Water Process Engineering, 2022, 47: 102639. doi: 10.1016/j.jwpe.2022.102639
|
| [64] |
ZHANG S, SUN M, HEDTKE T, et al. Mechanism of heterogeneous Fenton reaction kinetics enhancement under nanoscale spatial confinement[J]. Environmental Science & Technology, 2020, 54(17): 10868-10875.
|
| [65] |
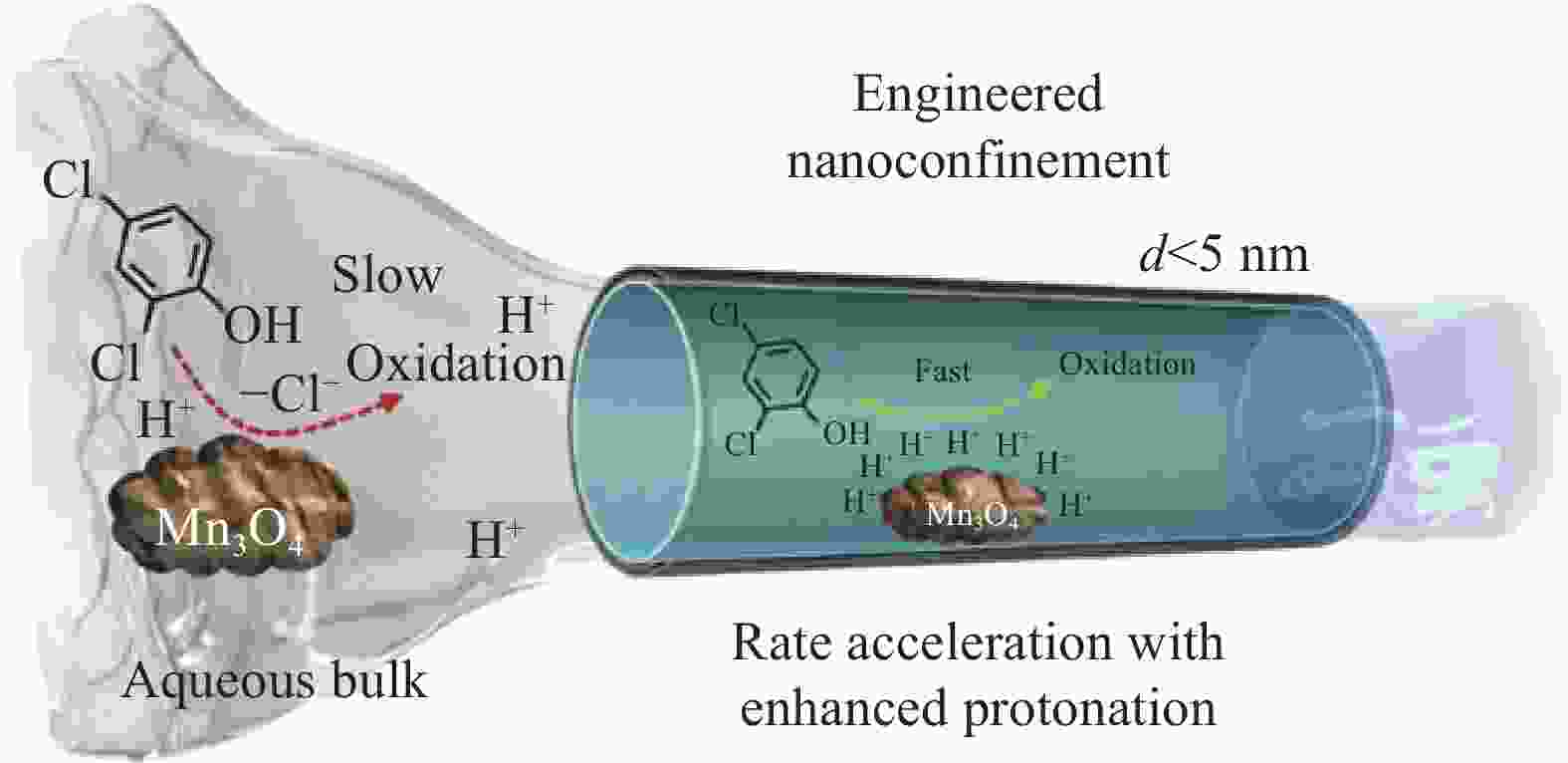
ZHANG S, HEDTKE T, WANG L, et al. Engineered nanoconfinement accelerating spontaneous manganese-catalyzed degradation of organic contaminants[J]. Environmental Science & Technology, 2021, 55(24): 16708-16715.
|






 下载:
下载: