Research progress of organic-inorganic composite electrolytes for solid-statelithium batteries
-
摘要: 相比于传统液态锂电池,固态锂电池兼具高安全性和高比能量,在学术界和工业界引起了广泛关注。发展具备优异力学性能、高离子电导率和宽电化学窗口的有机-无机复合固态电解质是开发高性能固态锂电池的有效途径之一。近年来,基于聚合物电解质与无机材料的复合型固态电解质成为了研究的热点。基于此,本文回顾了有机-无机复合固态电解质的研究进展,综述了改善固态电解质离子电导率的研究策略,梳理了有机-无机复合固态电解质在固态锂金属电池、固态锂-硫电池和固态锂-空气电池等领域的应用,并对固态锂电池用有机-无机复合固态电解质存在的挑战和未来的发展趋势进行了展望。Abstract: Compared to traditional liquid-state lithium batteries, solid-state lithium batteries have distinct advantages such as high safety and high specific energy, and have attracted widespread attention in both academia and industry. Exploring organic-inorganic composite solid electrolytes that combine excellent mechanical properties, high ion conductivity, and large electrochemical windows is a feasible solution to developing high-performance solid-state lithium batteries. In recent years, composite solid-state electrolytes based on polymer electrolytes and inorganic materials have become a hot topic. In this tutorial review, we focus on recent advances in various classes of organic-inorganic composite electrolytes and summarize the state-of-the-art strategies for improving the performance (Especially the ionic conductivity) of solid-state electrolytes. This is followed by detailed discussions on the implementation of composite solid-electrolytes in various energy storage systems, including solid-state lithium-metal batteries, solid-state lithium-sulfur batteries and solid-state lithium-air batteries, and the current challenges and future opportunities of organic-inorganic composite solid-state electrolytes for lithium batteries are also provided.
-
图 1 (a) 石榴石构型Li7La3Zr2O12 (LLZO)[26];(b) 聚偏氟乙烯 (PVDF)/ Li6.75La3Zr1.75Ta0.25O12 (LLZTO)复合固态电解质的结构模型[28];PVDF-LLZO纳米纤维固态电解质形貌((c), (d))和对称电池测试(e)[29];三维珊瑚状LLZO-PVDF复合电解质制备示意图(f)及其SEM图像((g), (h))[30]
Figure 1. (a) Garnet-type Li7La3Zr2O12 (LLZO)[26]; (b) Possible complex structures in the poly(vinylidene fluoride) (PVDF)/Li6.75La3Zr1.75Ta0.25O12 (LLZTO)[28]; SEM images ((c), (d)) of PVDF-LLTO nanofibers solid-state electrolyte and symmetrical battery test (e)[29]; Schematic illustration (f) for the preparation procedures of the coral-like LLZO/PVDF electrolyte and SEM images ((g), (h))[30]
NFs—Nanofibers; RT—Room temperature
图 2 (a) NASICON构型Li1+xAlxGe2−x(PO4)3 (LAGP)[26];(b) 聚环氧乙烷(PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)的循环稳定测试[40];(c) PVDF-LATP的电化学窗口测试[42];(d) 贝壳启发下 LAGP陶瓷-聚合物复合电解质的制备[43];(e) 冰模板法制备LAGP-PEO复合电解质流程图[44]
Figure 2. (a) NASICON-type Li1+xAlxGe2−x(PO4)3 (LAGP)[26]; (b) Cycle stability of polyethylene oxide (PEO)-Li1+xAlxTi2−x(PO4)3 (LATP)[40]; (c) Electrochemical stability windows of PVDF-LATP[42]; (d) Design and fabrication of LAGP ceramic-polymer composite electrolyte[43]; (e) Schematic of preparation process of the ice-templated LAGP-PEO composite electrolyte[44]
图 3 (a)钙钛矿构型LLTO[26];(b) 纳米线/纳米颗粒-聚合物固态电解质中Li+传导路径对比;(c) PAN-LLTO的Arrhenius曲线[33]
Figure 3. (a) Perovskite-type LLTO[26]; (b) Comparison of possible lithium-ion conduction pathway in nanowire-filled and nanoparticle-filled composite electrolytes; (c) Arrhenius plots of PAN-LLTO[33]
σ—Conductivity; T—Temperature
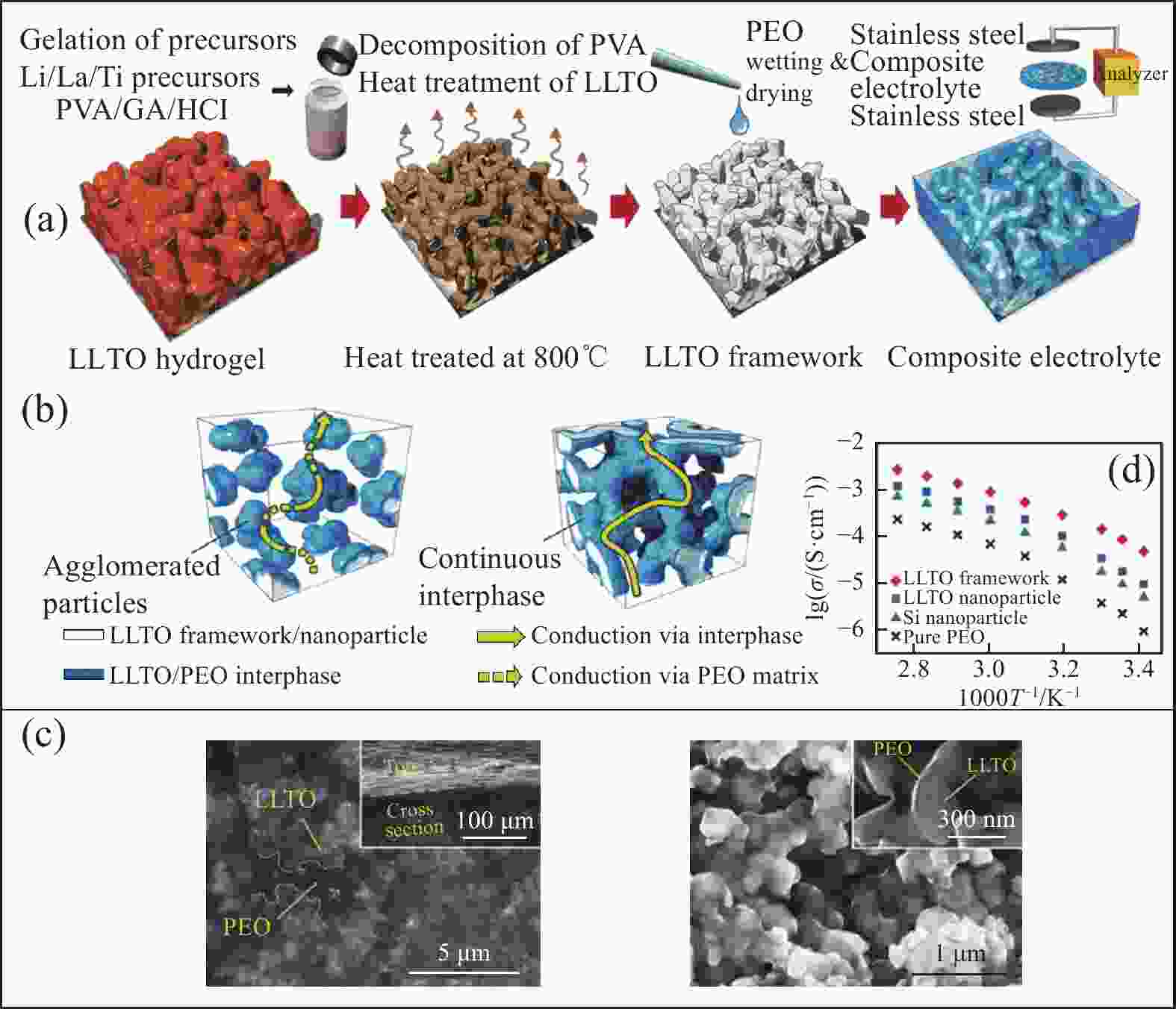
图 4 PEO-LLTO合成示意图(a)、复合电解质中Li+传导机制(b)、扫描电镜图(c)、Arrhenius曲线(d)[54]
Figure 4. Schematic representation of the synthesis of PEO-LLTO composite electrolytes (a), the possible conduction mechanism in composite electrolytes (b), top view (left) and cross-section (right) SEM images (c) of the composite electrolyte, Arrhenius plots (d)[54]
PVA—Polyvinyl alcohol; GA—Glutaraldehyde
图 5 (a) 硫化物构型Li10GeP2S12 (LGPS)[26];Arrhenius曲线(b)和充放电曲线(c)[65];(d) PEO-LGPS原位合成示意图[66];Li6.25PS5.25Cl0.75晶格(e)和Arrhenius曲线(f)[67]
Figure 5. (a) Sulfide-type Li10GeP2S12 (LGPS)[26]; Arrhenius plots (b) and charge/discharge curves (c) for the ionic conductivities of the composite electrolytes[65]; (d) Schematic illustration of the in-situ synthesis of PEO-LGPS solid electrolyte[66]; Lattice model (e) and Arrhenius curves (f) of sulfide electrolyte Li6.25PS5.25Cl0.75[67]
CTMS—(3-chloropropyl) trimethoxysilane; CPEs—Composite polymer electrolytes; M—Molecular weight
图 6 (a) SiO2纳米颗粒嫁接在PEO主链卡通图[76];Li+在PEO-Mg2B2O5复合电解质中的迁移示意图(b)和充放电曲线图(c)[77];(d) 3种填料-聚合物界面几何结构图(上图)和PEO-Al2O3复合电解质合成示意图[78]
Figure 6. (a) Cartoon showing the SiO2 nanoparticles grafted onto the PEO backbone[76]; Schematics of Li+ migration in PEO-Mg2B2O5 composite electrolytes (b) and charge/discharge curves (c)[77]; (d) Schematics of composite solid polymer electrolyte with three types of geometrical structures of ceramic-polymer interface (upper image) and schematics of fabrication procedures of polymer-Al2O3 composite electrolyte(below image)[78]
SPE—Solid polymer electrolytes; AAO—Anodized aluminum oxide
图 8 (a) 固态LiFPO4 (LFP)/LLZO纳米线(PLLN)/Li电池示意图;(b) 熔合前后LFP与PLLN电解质横截面积及Fe元素mapping图;0.5 C/60℃ (c)和0.1 C/45℃ (d)下的循环性能[87]
Figure 8. (a) Schematic illustration of LiFPO4 (LFP)/LLZO nanowire (PLLN)/Li battery; (b) Cross-sectional images of LFP and PLLN electrolyte and the corresponding EDS mapping of Fe before and after the fused; Cycling performances at 0.5 C/60℃ (c) and 0.1 C/45℃ (d)[87]
PL—LiTFSI; PLLM—LLZO microparticles
图 10 全固态Li-S电池示意图(a)及其在0.1 mA·cm–2/50℃时充放电曲线(b)[95];碳纳米纤维(CNF)/S-PEO/LLTO双层结构设计示意图(c)、不同倍率下充放电曲线(d)和0.1 C/45℃下的循环性能(e)[56]
Figure 10. Schematic illustration (a) of Li-S battery and the corresponding discharge-charge curves at 0.1 mA·cm–2/50℃ (b)[95]; Schematic illustration of the carbon nanofiber (CNF)/S-PEO/LLTO bilayer structure design (c), the discharge-charge curves at various current densities (d), and cycling performance at 0.1 C/45℃ (e)[56]
SE—Solid-state electrolyte
图 11 (a) 锂-空气电池示意图[96];(b) PEO/LATP复合电解质基锂-空气电池[100];三维LLZO框架/聚合物制备流程(c)、LLZO颗粒(d)和LLZO/聚合物(e)的SEM图像及Arrhenius曲线(f)[101]
Figure 11. (a) Schematic representation of the Li-air battery[96]; (b) Schematic diagram of the PEO/LATP composite electrolyte based solid-state lithium-air battery[100]; Preparation procedure for the composite polymer electrolyte with 3D LLZO network (c), SEM images of 3D LLZO network grains (d) and PEO-LLZO hybrid electrolyte (e), and Arrhenius curves (f)[101]
表 1 聚合物-石榴石型陶瓷复合电解质性能
Table 1. Performance of polymer-garnet ceramic electrolyte
Composite electrolyte Conductivity/(S·cm−1) Li//Li cells Solid-state cells Ref. PVDF-LLZTO nanoparticles 5.0×10−4 — 140 mA·h·g−1 at 1.2 C; LCO//Li cell [28] PVDF-LLZTO (3D) 1.5×10−4 0.1 mA·cm−2, 200 h 168 mA·h·g−1 at 0.1 C; LFP//Li cell [29] PVDF-LLZTO nanofibers 1.2×10−4 0.5 mA·cm−2, 700 h 213 mA·h·g−1 at 0.2 C; NCA//Li cell [30] PVDF-LLZTO nanoparticles 2.1×10−4 3.0 mA·cm−2, 700 h 141 mA·h·g−1 at 0.1 C; LFP//Li cell [32] PVDF-LLTO nanowires 2.4×10−4 — — [33] Notes: LLTO—Li0.33La0.557TiO3; LCO—LiCoO2; LFP—LiFePO4; NCA—LiNi0.8Co0.15Al0.05O2. 表 2 聚合物-NASICON型陶瓷复合电解质性能
Table 2. Performance of polymer-NASICON ceramic electrolyte
Composite electrolyte Conductivity/
(S·cm−1)Electrochemical window Ref. PEG-CA/LATP/
LiClO4>10−4 at 60℃ ~5.0 V [37] PVDF-HFP/LAGP/
EMITFSI7.6×10−4 at 25℃ 4.8 V [39] PEO/LATP/LiClO4 1.6×10−4 at 60℃ — [40] LAGP-PEA/LiTFSI 1.3×10−4 at 25℃ — [43] PEO-PEG/LAGP/
LiClO41.7×10−4 at 25℃ — [44] PVDF/LATP/LiTFSI 3.3×10−4 at 20℃ — [45] PEO/LiTFSI+PAN/
LATP6.5×10−4 at 60℃ 4.2 V [46] PEO/LiTFSI+LATP/ PAN/LiTFSI 6.3×10−4 at 60℃ ~5.0 V [47] PEO-SN/LiTFSI+PAN/
LATP/LiTFSI1.3×10−4 at 25℃ 5.0 V [48] PET-PIL/LAGP/
LiTFSI7.8×10−5 at 30℃ 4.55 V [49] PEGDA/LiTFSI+
PAN/LAGP/LiTFSI3.7×10−4 at 25℃ 5.0 V [50] Notes: PEG-CA—Polyethylene glycol-cellulose acetate; HFP—
Hexafluoropropylene; PEO—Polyethylene oxide; PEA—Poly (ether-acrylate); PET—Polyethylene terephthalate; PIL—Polyme
rized ionic liquid; PEGDA—Polyethylene glycol diacrylate; LiTFSI—Lithium bis (trifluoromethanesulphonyl) imide; PAN—Polyacrylonitrile; SN—Succinonitrile; EMITFSI—1-ethyl-3-methylimidazolium triluoromethanesufonate.表 3 聚合物-钙钛矿型陶瓷复合电解质性能
Table 3. Performance of polymer-perovskite ceramic electrolyte
Composite electrolyte Conductivity/
(S·cm−1)Electrochemical window/V Ref. PEO/LLTO/LiTFSI 8.8×10−4 at 25℃ 4.5 [54] PEO/LLTO/LiTFSI 1.8×10−4 at 25℃ 4.5 [55] PEO/LLTO/LiClO4 2.3×10−4 at 60℃ 4.5 [56] PVDF/LLTO/LiTFSI 5.3×10−4 at 60℃ 5.1 [57] PAN/LLTO/LiTFSI 2.2×10−3 at 30℃ 5.1 [58] PEO/LLTO/LiTFSI 1.3×10−2 at 24℃ 3.8 [59] PVDF-HFP/LLTO/
LiTFSI1.2×10−4 at 25℃ 4.7 [60] PVDF/LLTO/LiClO4 5.8×10−4 at 25℃ 5.2 [61] -
[1] LARCHER D, TARASCON J M. Towards greener and more sustainable batteries for electrical energy storage[J]. Nature Chemistry, 2015, 7(1): 19-29. doi: 10.1038/nchem.2085 [2] YAGHOOBNEJAD H, MANTHIRAM A. Towards sustainable batteries[J]. Nature Sustainability, 2021, 5(4): 379-380. [3] LI W, LIU J, ZHAO D. Mesoporous materials for energy conversion and storage devices[J]. Nature Reviews Materials, 2016, 1(6): 1-17. [4] HOU J, LU L, WANG L, et al. Thermal runaway of lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes[J]. Nature Communications, 2020, 11(1): 5100. doi: 10.1038/s41467-020-18868-w [5] FENG X, REN D, HE X, et al. Mitigating thermal runaway of lithium-ion batteries[J]. Joule, 2020, 4(4): 743-770. doi: 10.1016/j.joule.2020.02.010 [6] WANG J, YAMADA Y, SODETAMA K, et al. Fire-extinguishing organic electrolytes for safe batteries[J]. Nature Energy, 2018, 3(1): 22-29. [7] 程俊, 黄蕊, 雷惊雷, 等. 电化学能源核心技术的关键科学问题[J]. 中国科学基金, 2020, 5(34): 350-357. doi: 10.16262/j.cnki.1000-8217.2020.03.021CHENG Jun, HUANG Rui, LEI Jinglei, et al. Key scientific questions in the core technologies of electrochemical energy[J]. National Natural Science Foundation of China, 2020, 5(34): 350-357. doi: 10.16262/j.cnki.1000-8217.2020.03.021 [8] ZHAO Q, STALIN S, ZHAO C, et al. Designing solid-state electrolytes for safe, energy-dense batteries[J]. Nature Reviews Materials, 2020, 5(3): 229-252. doi: 10.1038/s41578-019-0165-5 [9] GUO B, FU Y, WANG J, et al. Strategies and characterization methods for achieving high performance PEO-based solid-state lithium-ion batteries[J]. Chemical Communications, 2022, 58(59): 8182-8193. doi: 10.1039/D2CC02306G [10] SONG X, YU W, ZHOU S, et al. Enhancement of Mn-doped LiPON electrolyte for higher performance of all-solid-state thin film lithium battery[J]. Materials Today Physics, 2023, 33: 101037. doi: 10.1016/j.mtphys.2023.101037 [11] TAO B, REN C, LI H, et al. Thio-/LISICON and LGPS-type solid electrolytes for all-solid-state lithium-ion batteries[J]. Advance Functional Materials, 2022, 32(34): 2203551. doi: 10.1002/adfm.202203551 [12] ZHANG Y, HUANG J, SAITO N, et al. Layered perovskite lithium yttrium titanate as a low-potential and ultrahigh-rate anode for lithium-ion batteries[J]. Advanced Energy Materials, 2022, 12(31): 2200922. doi: 10.1002/aenm.202200922 [13] WOO S, KANG B. Superior compatibilities of a LISICON-type oxide solid electrolyte enable high energy density all-solid-state batteries[J]. Journal of Material Chemistry A, 2022, 10(43): 23185-23194. doi: 10.1039/D2TA05948G [14] LI Y, LI M, SUN Z, et al. Recent advance on NASICON electrolyte in solid-state sodium metal batteries[J]. Energy Storage Materials, 2023, 56(58): 582-599. [15] SUN F, YANG Y, ZHAO S, et al. Local Li+ framework regulation of a garnet-type solid-state electrolyte[J]. ACS Energy Letter, 2022, 7(8): 2835-2844. doi: 10.1021/acsenergylett.2c01432 [16] ZHAO S, WU Q, MA W, et al. Polyethylene oxide-based composites as solid-state polymer electrolytes for lithium metal batteries: A mini review[J]. Frontiers in Chemistry, 2020, 8: 640. doi: 10.3389/fchem.2020.00640 [17] LIANG J, CHEN D, ADAIR K, et al. Insight into prolonged cycling life of 4 V all-solid-state polymer batteries by a high-voltage stable binder[J]. Advanced Energy Materials, 2021, 11(1): 2002455. doi: 10.1002/aenm.202002455 [18] HUANG Y, ZENG J, LI S, et al. Conformational regulation of dielectric poly(vinylidene fluoride)-based solid-state electrolytes for efficient lithium salt dissociation and lithium-ion transportation[J]. Advanced Energy Materials, 2023, 13(15): 2203888. doi: 10.1002/aenm.202203888 [19] MI J, MA J, CHEN L, et al. Topology crafting of polyvinylidene difluoride electrolyte creates ultra-long cycling high-voltage lithium metal solid-state batteries[J]. Energy Storage Materials, 2022, 48: 375-383. doi: 10.1016/j.ensm.2022.02.048 [20] FU Y, YANG K, XUE S, et al. Surface defects reinforced polymer-ceramic interfacial anchoring for high-rate flexible solid-state batteries[J]. Advanced Functional Materials, 2023, 33(10): 2210845. doi: 10.1002/adfm.202210845 [21] FU C, HOMANN G, GRISSA R, et al. A polymerized-ionic-liquid-based polymer electrolyte with high oxidative stability for 4 and 5 V class solid-state lithium metal batteries[J]. Advanced Energy Materials, 2022, 12(27): 2200412. doi: 10.1002/aenm.202200412 [22] LIU Y, ZHAO Y, LU W, et al. PEO based polymer in plastic crystal electrolytes for room temperature high-voltage lithium metal batteries[J]. Nano Energy, 2021, 88: 106205. doi: 10.1016/j.nanoen.2021.106205 [23] ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Review, 2020, 49(23): 8790-8839. doi: 10.1039/D0CS00305K [24] CHENG Z, LIU T, ZHAO B, et al. Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries[J]. Energy Storage Materials, 2021, 34: 388-416. doi: 10.1016/j.ensm.2020.09.016 [25] YU Q, JIANG K, YU C, et al. Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries[J]. Chinese Chemical Letters, 2021, 32(9): 2659-2678. doi: 10.1016/j.cclet.2021.03.032 [26] LIU Y, HE P, ZHOU H. Rechargeable solid-sate Li-air and Li-S batteries: Materials, construction, and challenges[J]. Advanced Energy Materials, 2018, 8(4): 1701602. doi: 10.1002/aenm.201701602 [27] KRAVCHYK K, ZHANG H, OKUR F, et al. Li-garnet solid-state batteries with LLZO scaffolds[J]. Account of Materials Research, 2022, 3(4): 411-415. doi: 10.1021/accountsmr.2c00004 [28] ZHANG X, LIU T, ZHANG S, et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes[J]. Journal of the American Chemical Society, 2017, 139(39): 13779-13785. doi: 10.1021/jacs.7b06364 [29] ZHAO Y, YAN J, CAI W, et al. Elastic and well-aligned ceramic LLZO nanofiber-based electrolytes for solid-state lithium batteries[J]. Energy Storage Materials, 2019, 23: 306-313. doi: 10.1016/j.ensm.2019.04.043 [30] WU M, LIU D, QU D, et al. 3D coral-like LLZO/PVDF composite electrolytes with enhanced ionic conductivity and mechanical flexibility for solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(47): 52652-52659. [31] ZHAO N, KHOKHAR W, BI Z, et al. Solid garnet batteries[J]. Joule, 2019, 3(5): 1190-1199. doi: 10.1016/j.joule.2019.03.019 [32] ZHANG J, ZHAO N, ZHANG M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy, 2016, 28: 447-454. doi: 10.1016/j.nanoen.2016.09.002 [33] LIU W, LIU N, SUN J, et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers[J]. Nano Letter, 2015, 15(4): 2740-2745. doi: 10.1021/acs.nanolett.5b00600 [34] XI G, XIAO M, WANG S, et al. Polymer-based solid electrolytes: Material selection, design, and application[J]. Advanced Functional Materials, 2021, 31(9): 2007598. doi: 10.1002/adfm.202007598 [35] ZENG Y, OUYANG B, LIU J, et al. High-entropy mechanism to boost ionic conductivity[J]. Science, 2022, 378(6626): 1320-1324. doi: 10.1126/science.abq1346 [36] YANG K, CHEN L, MA J, et al. Progress and perspective of Li1+ x Al x Ti2− x (PO4)3 ceramic electrolyte in lithium batteries[J]. InfoMat, 2021, 3(11): 1195-1217. doi: 10.1002/inf2.12222 [37] MA Q, ZENG X, YUE J, et al. Viscoelastic and nonflammable interface design-enabled dendrite-free and safe solid lithium metal batteries[J]. Advanced Energy Materials, 2019, 9(13): 1803854. doi: 10.1002/aenm.201803854 [38] MOUSAVI T, SLATTERY I, JAGGER B, et al. Development of sputtered nitrogen-doped Li1+ x Al x Ge2− x (PO4)3 thin films for solid state batteries[J]. Solid State Ionics, 2021, 364: 115613. doi: 10.1016/j.ssi.2021.115613 [39] GUO Q, HAN Y, WANG H, et al. Flame retardant and stable Li1.5Al0.5Ge1.5(PO4)3-supported ionic liquid gel polymer electrolytes for high safety rechargeable solid-state lithium metal batteries[J]. The Journal of Physical Chemistry C, 2018, 122(19): 10334-10342. doi: 10.1021/acs.jpcc.8b02693 [40] YU X, MANTHIRAM A. A long cycle life, all-solid-state lithium battery with a ceramic-polymer composite electrolyte[J]. ACS Applied Energy Materials, 2020, 3(3): 2916-2924. [41] ZHAO Y, HUANG Z, CHEN S, et al. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries[J]. Solid State Ionics, 2016, 295: 65-71. [42] SHI X, MA N, WU Y, et al. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery[J]. Solid State Ionics, 2018, 325: 112-119. doi: 10.1016/j.ssi.2018.08.010 [43] LI A, LIAO X, ZHANG H, et al. Nacre-inspired composite electrolytes for load-bearing solid-state lithium-metal batteries[J]. Advanced Materials, 2020, 32(2): 1905517. doi: 10.1002/adma.201905517 [44] WANG X, ZHAI H, QIE B, et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte[J]. Nano Energy, 2019, 60: 205-212. doi: 10.1016/j.nanoen.2019.03.051 [45] XIA Y, WANG X, XIA X, et al. A newly designed composite gel polymer electrolyte based on poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for enhanced solid-state lithium-sulfur batteries[J]. Chemistry European Journal, 2017, 23(60): 15203-15209. doi: 10.1002/chem.201703464 [46] LI D, CHEN L, WANG T, et al. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(8): 7069-7078. [47] LIANG J, ZENG X, ZHANG X, et al. Engineering janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries[J]. Journal of the American Chemical Society, 2019, 141(23): 9165-9169. doi: 10.1021/jacs.9b03517 [48] YU X, LI J, MANTHIRAM A. Rational design of a laminated dual-polymer/polymer-ceramic composite electrolyte for high-voltage all-solid-state lithium batteries[J]. ACS Materials Letter, 2020, 2(4): 317-324. doi: 10.1021/acsmaterialslett.9b00535 [49] MA F, ZHANG Z, YAN W, et al. Solid polymer electrolyte based on polymerized ionic liquid for high performance all-solid-state lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 4675-4683. [50] DUAN H, FAN M, CHEN W, et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries[J]. Advanced Materials, 2019, 31(12): 1807789. doi: 10.1002/adma.201807789 [51] ZHU P, YAN C, ZHANG X, et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2018, 6(10): 4279-4285. doi: 10.1039/C7TA10517G [52] LIU W, LEE S, LIN D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires[J]. Nature Energy, 2017, 2(5): 17035. doi: 10.1038/nenergy.2017.35 [53] YANG T, ZHENG J, CHENG Q, Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: Mechanism of conductivity enhancement and role of doping and morphology[J]. ACS Applied Materials & Interfaces, 2017, 9(26): 21773-21780. [54] BAE J, LI Y, ZHANG J, et al. A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte[J]. Angewandte Chemie International Edition, 2018, 57(8): 2096-2100. doi: 10.1002/anie.201710841 [55] WANG X, ZHANG Y, ZHANG X, et al. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(29): 24791-24798. [56] ZHU P, YAN C, ZHU J, et al. Flexible electrolyte-cathode bilayer framework with stabilized interface for room-temperature all-solid-state lithium-sulfur batteries[J]. Energy Storage Materials, 2019, 17: 220-225. doi: 10.1016/j.ensm.2018.11.009 [57] LI B, SU Q, YU L, et al. Li0.35La0.55TiO3 nanofibers enhanced poly(vinylidene fluoride)-based composite polymer electrolytes for all-solid-state batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(45): 42206-42213. [58] BI J, MU D, WU B, et al. A hybrid solid electrolyte Li0.33La0.557TiO3/PAN membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries[J]. Journal of Materials Chemistry A, 2020, 8(17): 706-713. [59] LIU K, WU M, WEI L, et al. A composite solid electrolyte with a framework of vertically aligned perovskite for all-solid-state Li-metal batteries[J]. Journal of Membrane Science, 2020, 610: 118265. doi: 10.1016/j.memsci.2020.118265 [60] LI J, ZHU L, ZHANG J, et al. Approaching high performance PVDF-HFP based solid composite electrolytes with LLTO nanorods for solid-state lithium-ion batteries[J]. International Journal of Energy Research, 2021, 45(5): 7663-7674. doi: 10.1002/er.6347 [61] LI B, SU Q, YU L, et al. Biomimetic PVDF/LLTO composite polymer electrolyte enables excellent interface contact and enhanced ionic conductivity[J]. Applied Surface Science, 2021, 541: 148434. doi: 10.1016/j.apsusc.2020.148434 [62] WU J, LIU S, HAN F, et al. Lithium/sulfide all-solid-state batteries using sulfide electrolytes[J]. Advanced Materials, 2021, 33(6): 2000751. doi: 10.1002/adma.202000751 [63] JANG S, TATEYAMA Y, JALEM R, et al. High-throughput data-driven prediction of stable high-performance Na-ion sulfide solid electrolytes[J]. Advanced Functional Materials, 2022, 32(48): 2206036. doi: 10.1002/adfm.202206036 [64] PENG J, WANG X, LI H, et al. High-capacity, long-life iron fluoride all-solid-state lithium battery with sulfide solid electrolyte[J]. Advanced Energy Materials, 2023, 13(23): 2300706. doi: 10.1002/aenm.202300706 [65] ZHAO Y, WU C, PENG G, et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries[J]. Journal of Power Sources, 2016, 301: 47-53. doi: 10.1016/j.jpowsour.2015.09.111 [66] PAN K, ZHANG L, QIAN W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials, 2020, 32(17): 2000399. doi: 10.1002/adma.202000399 [67] LI D, CAO L, SHAO G, et al. A designer fast Li-ion conductor Li6.25PS5.25Cl0.75 and its contribution to the polyethylene oxide-based electrolyte[J]. Applied Surface Science, 2019, 493: 1326-1333. doi: 10.1016/j.apsusc.2019.07.041 [68] ZHENG J, WANG P, LIU H, et al. Interface-enabled ion conduction in Li10GeP2S12-poly(ethylene oxide) hybrid electrolytes[J]. ACS Applied Energy Materials, 2019, 2(2): 1452-1459. doi: 10.1021/acsaem.8b02008 [69] LIU S, LIU W, BA D, et al. Filler-integrated composite polymer electrolyte for solid-state lithium batteries[J]. Advanced Materials, 2023, 35(2): 2110423. doi: 10.1002/adma.202110423 [70] ZHANG Z, WANG X, LI X, et al. Review on composite solid electrolytes for solid-state lithium-ion batteries[J]. Materials Today Sustainability, 2023, 21: 100316. doi: 10.1016/j.mtsust.2023.100316 [71] FAN L, HE H, NAN C. Tailoring inorganic-polymer composites for the mass production of solid-state batteries[J]. Nature Reviews Materials, 2021, 6(11): 1003-1019. doi: 10.1038/s41578-021-00320-0 [72] LIANG H, WANG A, SONG Y, et al. Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: A review[J]. Nano-Micro Letter, 2023, 15(1): 42. doi: 10.1007/s40820-022-00996-1 [73] WESTON J E, STEELE B C H. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes[J]. Solid State Ionics, 1982, 7(1): 75-79. [74] DISSANAYAKE M, JAYATHILAKA P, BAKALAWALA R, et al. Effect of concentration and grain size of alumina filler on the ionic conductivity enhancement of the (PEO)9LiCF3SO3:Al2O3 composite polymer electrolyte[J]. Journal of Power Sources, 2003, 119: 409-414. [75] CHUNG S, WANG Y, PERSI L, et al. Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides[J]. Journal of Power Sources, 2001, 97: 644-648. [76] CHOUDHURY S, STALIN S, DENG Y, et al. Soft colloidal glasses as solid-state electrolytes[J]. Chemistry of Materials, 2018, 30(17): 5996-6004. doi: 10.1021/acs.chemmater.8b02227 [77] SHENG O, JIN C, LUO J, et al. Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance[J]. Nano Letter, 2018, 18(5): 3104-3112. doi: 10.1021/acs.nanolett.8b00659 [78] ZHANG X, XIE J, SHI F, et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity[J]. Nano Letter, 2018, 18(6): 3829-3838. doi: 10.1021/acs.nanolett.8b01111 [79] KIM S, CHART Y, NARAYANAN S, et al. Thin solid electrolyte separators for solid-state lithium-sulfur batteries[J]. Nano Letter, 2022, 22(24): 10176-10183. doi: 10.1021/acs.nanolett.2c04216 [80] LI Q, LI H, XIA Q, et al. Extra storage capacity in transition metal oxide lithium-ion batteries revealed by in situ magnetometry[J]. Nature Materials, 2021, 20(1): 76-83. doi: 10.1038/s41563-020-0756-y [81] WANG S, QU C, WEN J, et al. Progress of transition metal sulfides used as lithium-ion battery anodes[J]. Materials Chemistry Frontiers, 2023, 7: 2779-2808. doi: 10.1039/D2QM01200F [82] HOU T, LIU B, SUN X, et al. Covalent coupling-stabilized transition-metal sulfide/carbon nanotube composites for lithium/sodium-ion batteries[J]. ACS Nano, 2021, 15(4): 6735-6746. doi: 10.1021/acsnano.0c10121 [83] SUN L, LIU Y, SHAO R, et al. Recent progress and future perspective on practical silicon anode-based lithium-ion batteries[J]. Energy Storage Materials, 2022, 46: 482-502. doi: 10.1016/j.ensm.2022.01.042 [84] YU S, GUO B, ZENG T, et al. Graphene-based lithium-ion battery anode materials manufactured by mechanochemical ball milling process: A review and perspective[J]. Composites Part B: Engineering, 2022, 246: 110232. doi: 10.1016/j.compositesb.2022.110232 [85] ZHAO T, LIU C, MENG T, et al. Vacancy-clusters in-situ induced via microwave-irradiation enable high-durability and capacitor-level rate Li-ion storage[J]. Chemical Engineering Journal, 2023, 466: 143053. doi: 10.1016/j.cej.2023.143053 [86] CHEN S, ZHANG J, NIE L, et al. All-solid-state batteries with a limited lithium metal anode at room temperature using a garnet-based electrolyte[J]. Advanced Material, 2021, 33(1): 2002325. doi: 10.1002/adma.202002325 [87] WAN Z, LEI D, HE Y, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials, 2019, 29(1): 1805301. doi: 10.1002/adfm.201805301 [88] SHALEV O, LEIFER N, ROSY D, et al. Molecular layer deposition of alucone thin film on LiCoO2 to enable high voltage operation[J]. Batteries & Supercaps, 2021, 4(11): 1-11. [89] QIU J, LIU X, CHEN R, et al. Enabling stable cycling of 4.2 V high-voltage all-solid-state batteries with PEO-based solid electrolyte[J]. Advanced Functional Materials, 2020, 30(22): 1909392. doi: 10.1002/adfm.201909392 [90] HUANG Y, LIN L, ZHANG C, et al. Recent advances and strategies toward polysulfides shuttle inhibition for high-performance Li-S batteries[J]. Advanced Science, 2022, 9(12): 2106004. doi: 10.1002/advs.202106004 [91] HAN Z, GAO R, JIA Y, et al. Catalytic effect in Li-S batteries: From band theory to practical application[J]. Materials Today, 2022, 57: 84-120. doi: 10.1016/j.mattod.2022.05.017 [92] XING C, CHEN H, QIAN S, et al. Regulating liquid and solid-state electrolytes for solid-phase conversion in Li-S batteries[J]. Chem, 2022, 8(5): 1201-1230. doi: 10.1016/j.chempr.2022.01.002 [93] WU J, YE T, WANG Y, et al. Understanding the catalytic kinetics of polysulfide redox reactions on transition metal compounds in Li-S batteries[J]. ACS Nano, 2022, 16(10): 15734-15759. doi: 10.1021/acsnano.2c08581 [94] XIA J, HUA W, WANG L, et al. Boosting catalytic activity by seeding nanocatalysts onto interlayers to inhibit polysulfide shuttling in Li-S batteries[J]. Advanced Functional Materials, 2021, 31(26): 2101980. doi: 10.1002/adfm.202101980 [95] TAO X, LIU Y, CUI Y, et al. Solid-state lithium-sulfur batteries operated at 37℃ with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer[J]. Nano Letter, 2017, 17(5): 2967-2972. doi: 10.1021/acs.nanolett.7b00221 [96] HE P, ZHANG T, JIANG J, et al. Lithium-air batteries with hybrid electrolytes[J]. The Journal of Physical Chemistry Letters, 2016, 7(7): 1267-1280. doi: 10.1021/acs.jpclett.6b00080 [97] AHMED G, AWAN Z, BUTT F, et al. The study of different redox mediators for competent Li-air batteries[J]. Journal of Power Sources, 2022, 538(538): 231379. [98] PATHAK A, ADHIKARI P, CHOI W. Lithium-CO2 batteries and beyond[J]. Frontiers in Energy Research, 2023, 11: 1150737. doi: 10.3389/fenrg.2023.1150737 [99] WANG H, WANG X, LI F, et al. Fundamental understanding and construction of solid-state Li-air batteries[J]. International Journal of Energy Research, 2022, 2(5): 2200005. [100] KITAURA H, ZHOU H. Electrochemical performance of solid-state lithium-air batteries using carbon nanotube catalyst in the air electrode[J]. Advanced Energy Materials, 2012, 2(7): 889-894. doi: 10.1002/aenm.201100789 [101] SONG S, QIN X, RUAN Y, et al. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte[J]. Journal of Power Sources, 2020, 461: 228146. doi: 10.1016/j.jpowsour.2020.228146 -






 下载:
下载: