2D Zn(Bim)OAc MOF functionalized polyacrylonitrile composite separator for Li+ redistribution to achieve dendrite-free lithium metal batteries
-
摘要: 锂沉积/剥离过程中的枝晶形成生长和库仑效率降低导致电池循环寿命缩短,限制了锂金属电池(LMBs)的商业应用。通过静电纺丝结合真空过滤技术成功制备了具有调节Li+通量和提高Li+迁移数(tLi+)的二维苯并咪唑醋酸锌MOF功能化聚丙烯腈(Zn(Bim)OAc-PAN)复合隔膜,Zn(Bim)OAc纳米片的引入固定了阴离子,提高了离子电导率(2.13 mS/cm)和Li+迁移数(0.67)。同时,Zn(Bim)OAc纳米片上的孔隙和纳米片之间堆叠形成的纳米流体通道协同构筑了微纳孔道结构,降低了复合隔膜的孔径,使通过隔膜的Li+流分布均一,促进Li+在锂金属表面均匀沉积。因此,Zn(Bim)OAc-PAN隔膜组装的Li|LiFePO4电池表现出了更高的初始容量(146.6 mA·h/g)和更好的循环稳定性(300次循环后容量保留率为96.3%)。此外,Zn(Bim)OAc-PAN复合隔膜组装的Li|Li电池实现了在1 mA/cm2下长达1000 h的稳定循环,循环后的锂金属表面没有明显的锂枝晶生长。本文为通过隔膜调节Li+通量来提高锂金属电池性能提供了一种可行的策略。Abstract: The dendrite formation and growth during Li plating/stripping process, as well as the reduced coulomb efficiency, result in a shortened cycle life of lithium metal batterie (LMB), restricting its commercial application. 2D Zn(Bim)OAc MOF functionalized polyacrylonitrile (Zn(Bim)OAc-PAN) composite separator with regulating Li+ flux and increasing Li+ migration number was successfully prepared by electrospinning combined with vacuum filtration technology. The introduction of Zn(Bim)OAc nanosheets can fix the anion, improve the ionic conductivity (2.13 mS/cm) and Li+ migration number (0.67). Meanwhile, the pores on Zn(Bim)OAc nanosheets and the nanofluid channels between the stacked nanosheets synergistically construct a micro/nano pore structure, reducing the pore size, resulting in uniform Li+ flux distribution and promoting uniform Li+ deposition on the lithium metal surface. Therefore, as-prepared Zn(Bim)OAc-PAN separator assembled Li|LiFePO4 cells can also exhibit higher initial capacity (146.6 mA·h/g) and better cycle stability (96.3% capacity retention after 300 cycles) at 2 C. Besides, as-prepared Zn(Bim)OAc-PAN separator based Li|Li achieves a stable cycle up to 1000 h under 1 mA/cm2, and there was no obvious lithium dendrite growth on the Li anode surface after cycling. The reported approach provides a feasible strategy to improve the LMB performance by regulating the Li+ flux through separators.
-
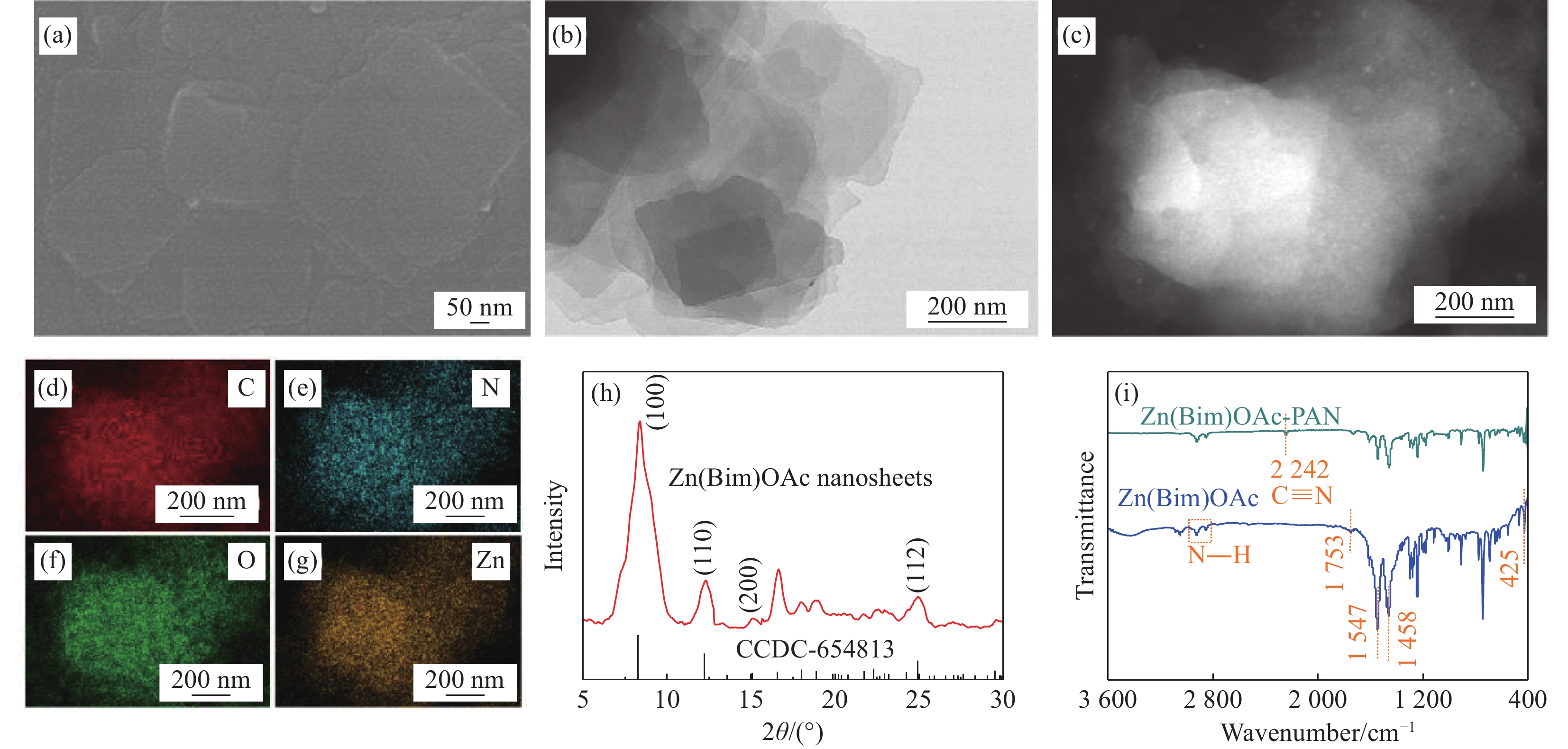
图 1 苯并咪唑醋酸锌(Zn(Bim)OAc)纳米片的SEM图像(a)、TEM图像((b), (c))、EDS元素分布图谱((d)~(g))、XRD图谱(h)及Zn(Bim)OAc-聚丙烯腈(PAN)和Zn(Bim)OAc的FTIR图谱(i)
Figure 1. SEM image (a), TEM images ((b), (c)), corresponding EDS elemental mapping ((d)-(g)) and XRD patterns (h) of the benzimidazole zinc acetate (Zn(Bim)OAc) nanosheets, FTIR spectra (i) of Zn(Bim)OAc-polyacrylonitrile (PAN) and Zn(Bim)OAc
图 3 Celgard (a)、PAN (b)和Zn(Bim)OAc-PAN (c)隔膜表面的液体电解质接触角;相关隔膜的交流阻抗谱(d)及高频区交流阻抗谱图(e)
Figure 3. Contact angles of liquid electrolyte on the surface of Celgard (a), PAN (b), Zn(Bim)OAc-PAN separators (c); AC impedance spectra (d) and plots at high-frequency for corresponding AC impedance spectra (e) of relevant separators
图 6 (a) 使用相关隔膜的Li|LiFePO4 (LFP)电池的倍率能力;(b) 使用Zn(Bim)OAc-PAN隔膜的Li|LFP电池在不同倍率下的充放电曲线;(c) 使用相关隔膜的Li|LFP电池在2 C时的放电容量;在300 次循环前(d)和后(e)的EIS (插图是等效电路);(f) Li|LFP电池的界面阻抗Rint拟合结果的比较
Rs—Solution resistance; Rct—Charge transfer resistance; CPE—Constant phase angle element
Figure 6. (a) Rate capability of Li|LiFePO4 (LFP) cells using relevant separators; (b) Charge-discharge curves of Li|LFP cell using Zn(Bim)OAc-PAN separator at different rates; (c) Discharge capacity at 2 C of the cells using relevant separators; EIS of before (d) and after (e) 300 cycles (Insets is the equivalent circuit); (f) Comparison of interface impedance Rint fitting results of the Li|LFP cells
图 8 相关隔膜组装的Li|Cu电池的库伦效率(CE)随循环圈数的对比(a)、第50次(b)和第100次(c)的电压曲线;(d)相关隔膜组装的Li|Li对称电池的循环性能;(e) Zn(Bim)OAc-PAN隔膜组装的Li|Li电池特定周期的电压曲线
Figure 8. Comparison of coulombic efficiency (CE) with number of cycles (a), voltage profiles of Li|Cu cells at 50th (b) and 100th (c) of Li|Cu cells with relevant separators; (d) Cycle performance of Li|Li symmetric cells with relevant separators; (e) Voltage profiles for specific cycles of the Li|Li cell with as-prepared Zn(Bim)OAc-PAN separator
表 1 相关隔膜的物理特性
Table 1. Physical properties of relevant separators
Sample Porosity/% Uptake/wt% Bulk resistance/Ω Ionic conductivity/(mS·cm−1) Celgard 41 117 1.27 0.78 PAN 75 263 2.10 1.26 Zn(Bim)OAc-PAN 84 583 1.05 2.13 -
[1] DING Z L, LI J L, LI J, et al. Interfaces: Key issue to be solved for all solid-state lithium battery technologies[J]. Journal of the Electrochemical Society,2020,167(7):070541. doi: 10.1149/1945-7111/ab7f84 [2] MA L B, CUI J, YAO S S, et al. Dendrite-free lithium metal and sodium metal batteries[J]. Energy Storage Materials,2020,27:522-554. doi: 10.1016/j.ensm.2019.12.014 [3] CHENG X B, ZHANG R, ZHAO C Z, et al. Toward safe lithium metal anode in rechargeable batteries: A review[J]. Chemical Reviews,2017,117(15):10403-10473. doi: 10.1021/acs.chemrev.7b00115 [4] XU W, WANG J L, DING F, et al. Lithium metal anodes for rechargeable batteries[J]. Energy & Environmental Science,2014,7(2):513-537. [5] ADENUSI H, CHASS G A, PASSERINI S, et al. Lithium batteries and the solid electrolyte interphase (SEI)-progress and outlook[J]. Advanced Energy Materials,2023,13(10):2203307. doi: 10.1002/aenm.202203307 [6] WANG D, ZHANG W, ZHENG W T, et al. Towards high-safe lithium metal anodes: Suppressing lithium dendrites via tuning surface energy[J]. Advanced Science,2017,4(1):1600168. doi: 10.1002/advs.201600168 [7] DORNBUSCH D A, HILTON R, LOHMAN S D, et al. Experimental validation of the elimination of dendrite short-circuit failure in secondary lithium-metal convection cell batteries[J]. Journal of the Electrochemical Society,2014,162(3):A262-A268. [8] ZHONG Y C, CHEN Y M, CHENG Y F, et al. Li alginate-based artificial SEI layer for stable lithium metal anodes[J]. ACS Applied Materials & Interfaces,2019,11(41):37726-37731. [9] WANG Z X, QI F L, YIN L C, et al. An anion-tuned solid electrolyte interphase with fast ion transfer kinetics for stable lithium anodes[J]. Advanced Energy Materials,2020,10(14):1903843. doi: 10.1002/aenm.201903843 [10] CHEN S J, XIANG Y X, ZHENG G R, et al. High-efficiency lithium metal anode enabled by a concentrated/fluorinated ester electrolyte[J]. ACS Applied Materials & Interfaces,2020,12(24):27794-27802. [11] ZOU P J, LIU J, HUANG Z G, et al. Phenylphosphonic acid as a grain-refinement additive for a stable lithium metal anode[J]. Chemical Communications,2022,58(91):12724-12727. doi: 10.1039/D2CC04504D [12] XIE Z K, WU Z J, AN X W, et al. 2-fluoropyridine: A novel electrolyte additive for lithium metal batteries with high areal capacity as well as high cycling stability[J]. Chemical Engineering Journal,2020,393:124789. doi: 10.1016/j.cej.2020.124789 [13] LIU W, MI Y Y, WENG Z, et al. Functional metal-organic framework boosting lithium metal anode performance via chemical interactions[J]. Chemical Science,2017,8(6):4285-4291. doi: 10.1039/C7SC00668C [14] ZHAO C Z, CHEN P Y, ZHANG R, et al. An ion redistributor for dendrite-free lithium metal anodes[J]. Science Advances,2018,4(11):eaat3446. doi: 10.1126/sciadv.aat3446 [15] FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials,2019,18(12):1278-1291. doi: 10.1038/s41563-019-0431-3 [16] WU B B, WANG S Y, LOCHALA J, et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries[J]. Energy & Environmental Science,2018,11(7):1803-1810. [17] YANG Q F, LI C L. Li metal batteries and solid state batteries benefiting from halogen-based strategies[J]. Energy Storage Materials,2018,14:100-117. doi: 10.1016/j.ensm.2018.02.017 [18] HE Y B, QIAO Y, CHANG Z, et al. The potential of electrolyte filled MOF membranes as ionic sieves in rechargeable batteries[J]. Energy & Environmental Science,2019,12(8):2327-2344. doi: 10.1039/C8EE03651A [19] SHEN L, WU H B, LIU F, et al. Anchoring anions with metal-organic framework-functionalized separators for advanced lithium batteries[J]. Nanoscale Horizons,2019,4(3):705-711. doi: 10.1039/C8NH00342D [20] ZHOU M L, ZHANG Z, XU J, et al. PDA modified commercial paper separator engineering with excellent lithiophilicity and mechanical strength for lithium metal batteries[J]. Journal of Electroanalytical Chemistry,2020,868:114195. doi: 10.1016/j.jelechem.2020.114195 [21] ZHANG T, YANG J, XU Z X, et al. Suppressing dendrite growth of a lithium metal anode by modifying conventional polypropylene separators with a composite layer[J]. ACS Applied Energy Materials,2020,3(1):506-513. doi: 10.1021/acsaem.9b01763 [22] LI C F, LIU S H, SHI C G, et al. Two-dimensional molecular brush-functionalized porous bilayer composite separators toward ultrastable high-current density lithium metal anodes[J]. Nature Communications,2019,10(1):1363. doi: 10.1038/s41467-019-09211-z [23] HAO Z D, ZHAO Q, TANG J D, et al. Functional separators towards the suppression of lithium dendrites for rechargeable high-energy batteries[J]. Materials Horizons,2021,8(1):12-32. doi: 10.1039/D0MH01167C [24] ZHAI Y Y, WANG X W, CHEN Y F, et al. Multiscale-structured polyvinylidene fluoride/polyacrylonitrile/vermiculite nanosheets fibrous membrane with uniform Li+ flux distribution for lithium metal battery [J]. Journal of Membrane Science, 2021, 621: 118996. [25] CHEN Y F, LI J H, JU Y, et al. Regulating Li-ion flux distribution via holey graphene oxide functionalized separator for dendrite-inhibited lithium metal battery[J]. Applied Surface Science,2022,592:153222. doi: 10.1016/j.apsusc.2022.153222 [26] LI Y J, ZHANG G, CHEN B, et al. Understanding the separator pore size inhibition effect on lithium dendrite via phase-field simulations[J]. Chinese Chemical Letters,2022,33(6):3287-3290. doi: 10.1016/j.cclet.2022.03.065 [27] PELED E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems-the solid electrolyte interphase model[J]. Journal of the Electrochemical Society,1979,126(12):2047-2051. doi: 10.1149/1.2128859 [28] CHEN X R, ZHAO B C, YAN C, et al. Review on Li deposition in working batteries: From nucleation to early growth[J]. Advanced Materials,2021,33(8):2004128. doi: 10.1002/adma.202004128 [29] 曹连胜, 赵超, 金欣, 等. 基于离子选择性迁移策略的动力/储能电池隔膜的研究进展[J]. 复合材料学报, 2021, 38(7):2025-2037. doi: 10.13801/j.cnki.fhclxb.20210114.002CAO Liansheng, ZHAO Chao, JIN Xin, et al. Research progress of power/energy storage battery separator based on selective ion migration strategy[J]. Acta Materiae Compositae Sinica,2021,38(7):2025-2037(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210114.002 [30] YANG L Y, CAO J H, CAI B R, et al. Electrospun MOF/PAN composite separator with superior electrochemical performances for high energy density lithium batteries[J]. Electrochimica Acta,2021,382:138346. doi: 10.1016/j.electacta.2021.138346 [31] HUANG D, LIANG C, CHEN L N, et al. MOF composite fibrous separators for high-rate lithium-ion batteries[J]. Journal of Materials Science,2021,56(9):5868-5877. doi: 10.1007/s10853-020-05559-6 [32] ZHANG C, SHEN L, SHEN J Q, et al. Anion-sorbent composite separators for high-rate lithium-ion batteries[J]. Advanced Materials,2019,31(21):1808338. doi: 10.1002/adma.201808338 [33] JU Y, LIU H Q, CHEN Y F, et al. An ultrathin Zn-BDC MOF nanosheets functionalized polyacrylonitrile composite separator with anion immobilization and Li+ redistribution for dendrite-free Li metal battery[J]. Composites Communications,2023,37:101449. doi: 10.1016/j.coco.2022.101449 [34] XUE F, KUMAR P, XU W Q, et al. Direct synthesis of 7 nm-thick zinc(II)-benzimidazole-acetate metal-organic framework nanosheets[J]. Chemistry of Materials,2018,30(1):69-73. doi: 10.1021/acs.chemmater.7b04083 [35] GAO Y, SANG X, CHEN Y F, et al. Polydopamine modification electrospun polyacrylonitrile fibrous membrane with decreased pore size and dendrite mitigation for lithium ion battery[J]. Journal of Materials Science,2020,55(8):3549-3560. doi: 10.1007/s10853-019-04218-9 [36] ZHAI Y Y, XIAO K, YU J Y, et al. Closely packed x-poly(ethylene glycol diacrylate) coated polyetherimide/poly(vinylidene fluoride) fiber separators for lithium ion batteries with enhanced thermostability and improved electrolyte wettability[J]. Journal of Power Sources,2016,325:292-300. doi: 10.1016/j.jpowsour.2016.06.050 [37] WANG H S, YU Z A, KONG X, et al. Liquid electrolyte: The nexus of practical lithium metal batteries[J]. Joule,2022,6(3):588-616. doi: 10.1016/j.joule.2021.12.018 [38] SUN B, ZHANG Q, XU W L, et al. A gradient topology host for a dendrite-free lithium metal anode[J]. Nano Energy,2022,94:106937. doi: 10.1016/j.nanoen.2022.106937 [39] LI X M. Poly[μ2-acetato-μ2-benzimidazolato-zinc (II)][J]. Acta Crystallographica, Section E: Structure Reports Online,2007,63(7):m1984. doi: 10.1107/S1600536807030309 [40] ZHAO K M, LIU S Q, YE G Y, et al. High-yield bottom-up synthesis of 2D metal-organic frameworks and their derived ultrathin carbon nanosheets for energy storage[J]. Journal of Materials Chemistry A,2018,6(5):2166-2175. doi: 10.1039/C7TA06916B [41] 张红涛, 胡昊, 顾波, 等. 聚偏氟乙烯-沸石复合锂电隔膜的制备及性能[J]. 复合材料学报, 2017, 34(3):625-631. doi: 10.13801/j.cnki.fhclxb.20160612.002ZHANG Hongtao, HU Hao, GU Bo, et al. Preparation and performances of PVDF-zeolite composite separator for lithium-ion batteries[J]. Acta Materiae Compositae Sinica,2017,34(3):625-631(in Chinese). doi: 10.13801/j.cnki.fhclxb.20160612.002 [42] CHOI J H, LEE C H, YU J H, et al. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix[J]. Journal of Power Sources,2015,274:458-463. doi: 10.1016/j.jpowsour.2014.10.078 [43] WEI L Y, DENG N P, JU J G, et al. ZnF2 doped porous carbon nanofibers as separator coating for stable lithium-metal batteries[J]. Chemical Engineering Journal,2021,424:130346. doi: 10.1016/j.cej.2021.130346 [44] HOU Z, ZHANG J L, WANG W H, et al. Towards high-performance lithium metal anodes via the modification of solid electrolyte interphases[J]. Journal of Energy Chemistry,2020,45:7-17. doi: 10.1016/j.jechem.2019.09.028 [45] CHEN H, PEI A, LIN D C, et al. Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode[J]. Advanced Energy Materials,2019,9(22):1900858. doi: 10.1002/aenm.201900858 -






 下载:
下载: