Preparation and properties of corrosion inhibited poly(o-toluidine)-graphene oxide-based anticorrosive materials
-
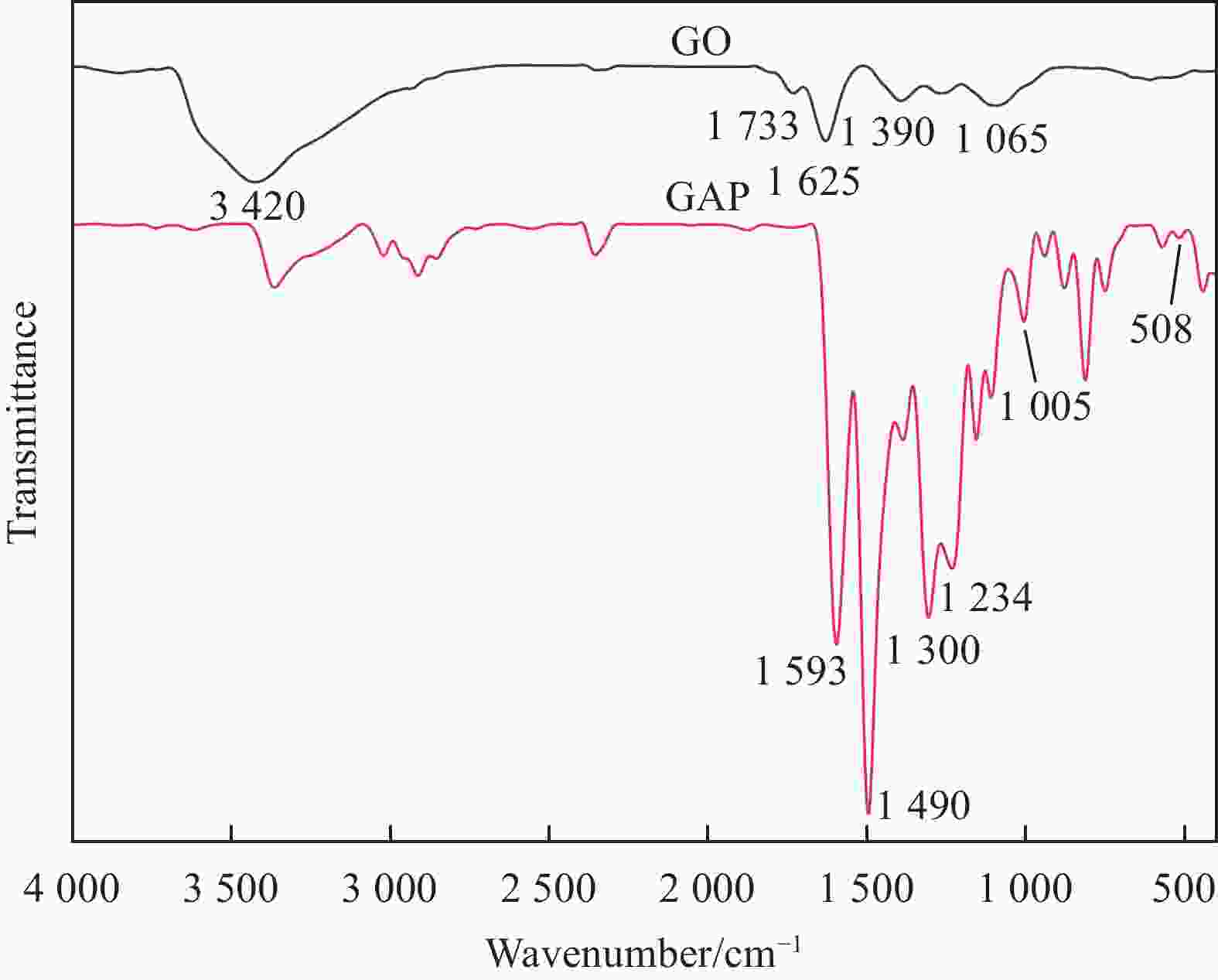
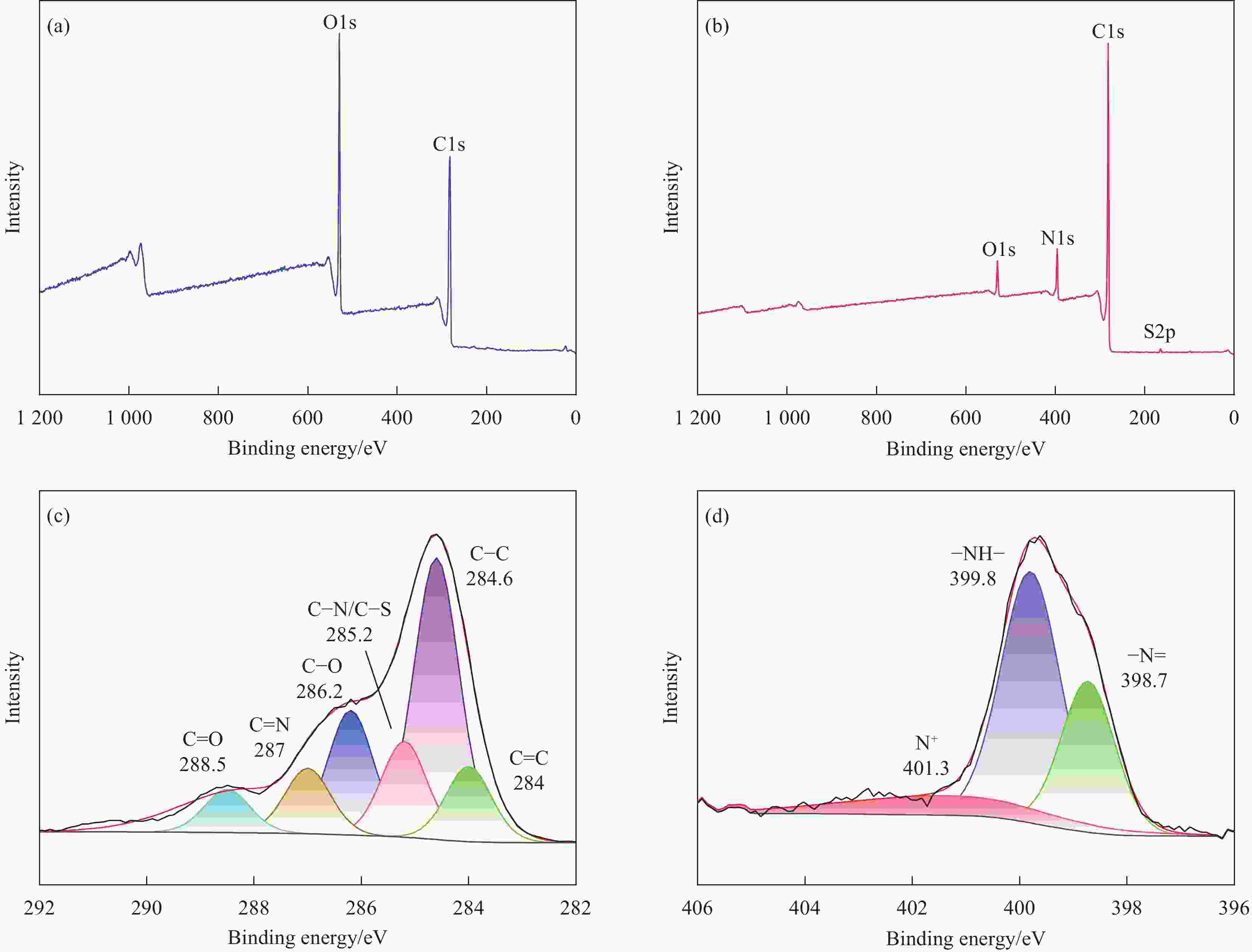
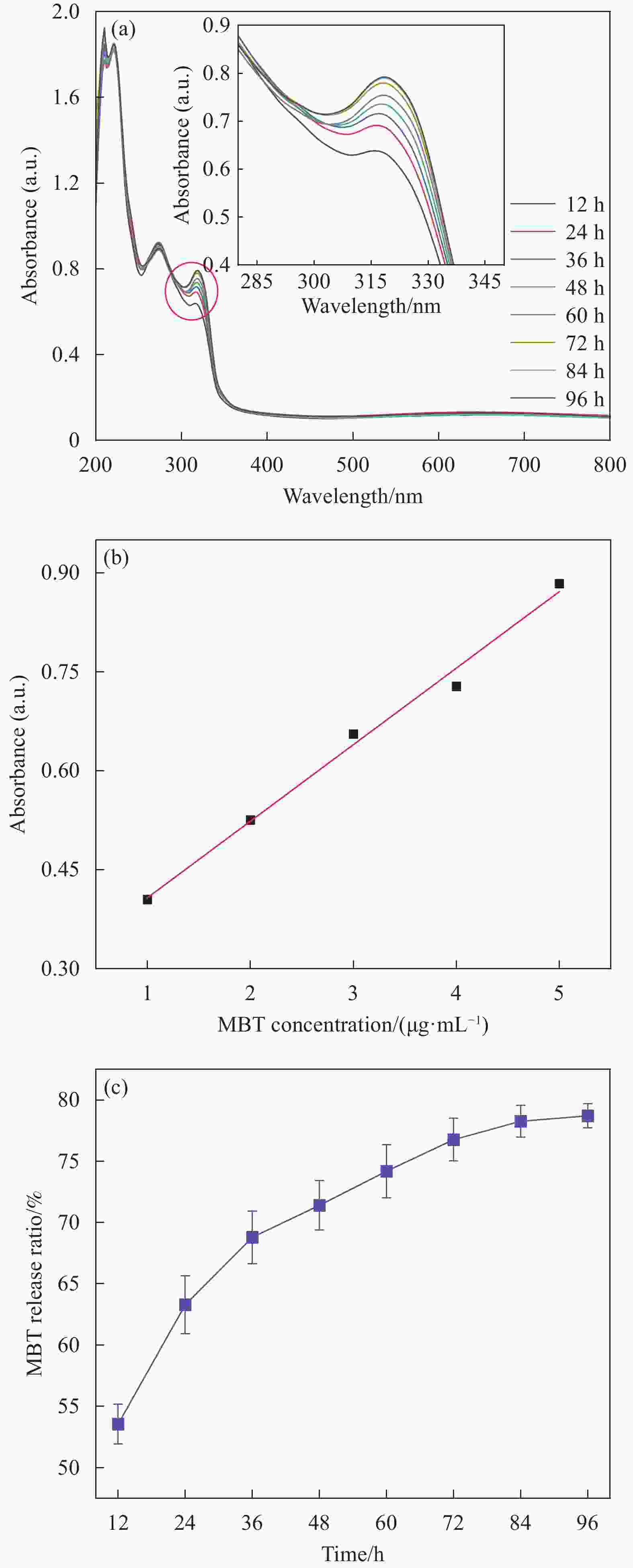
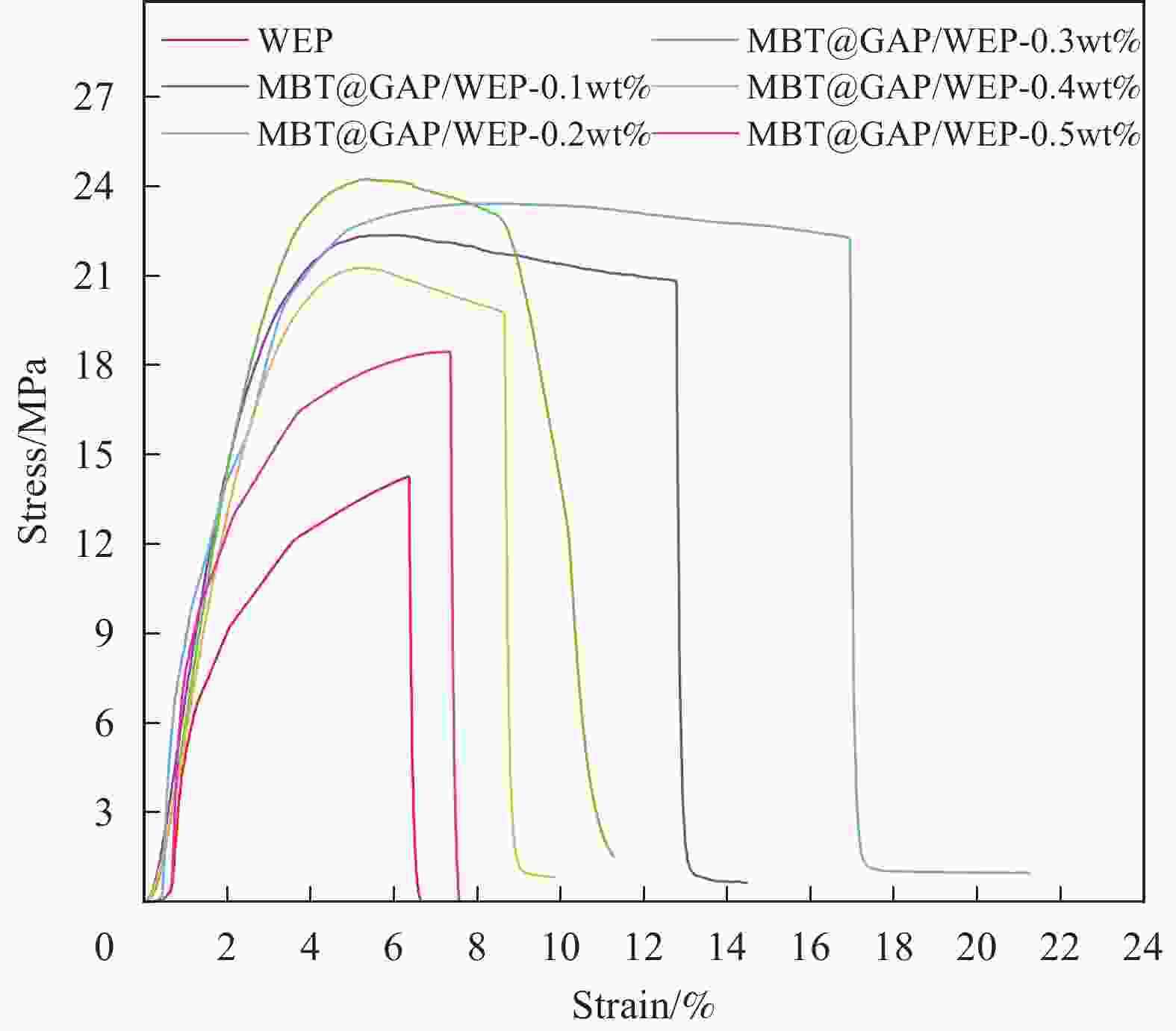
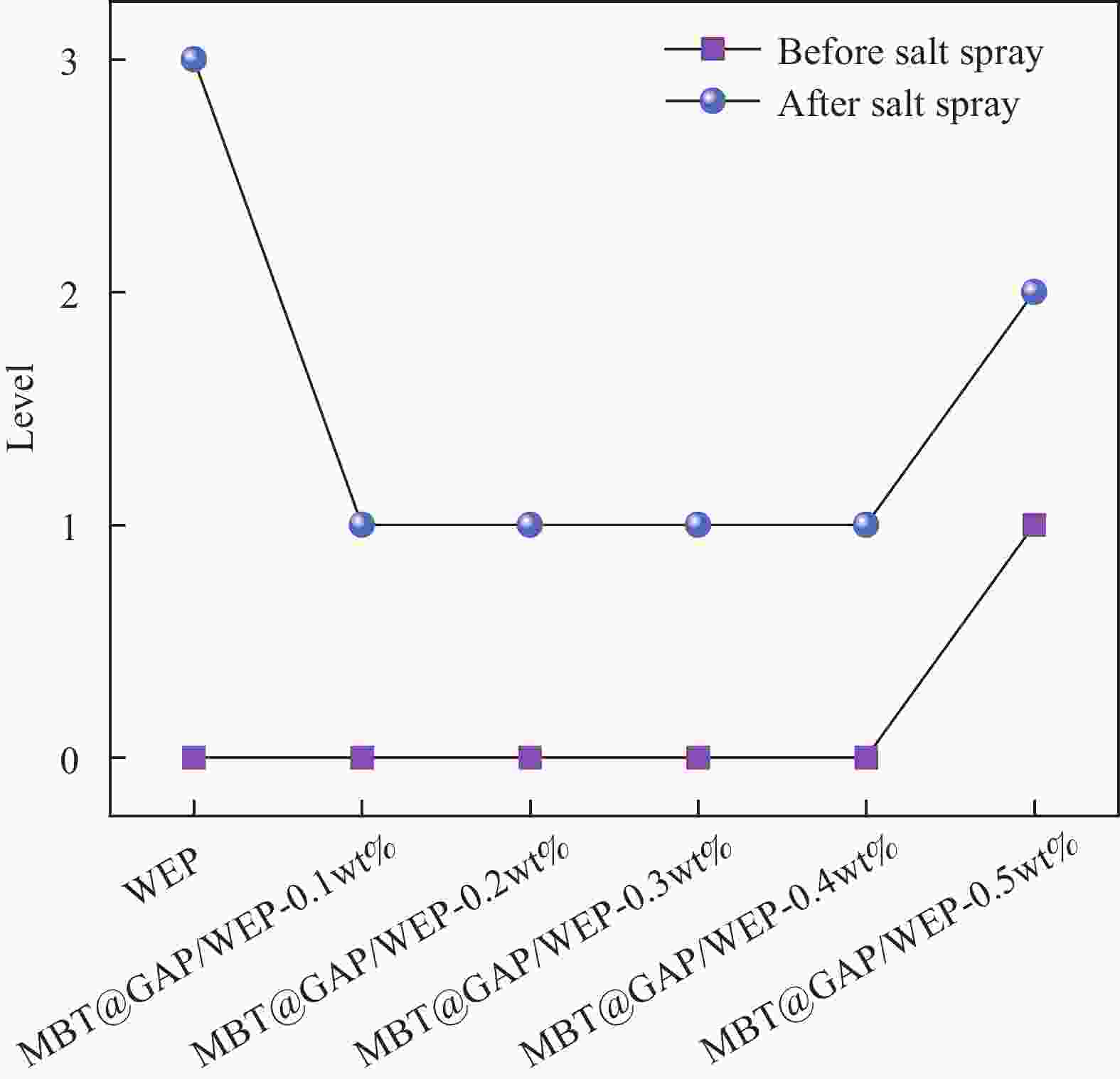
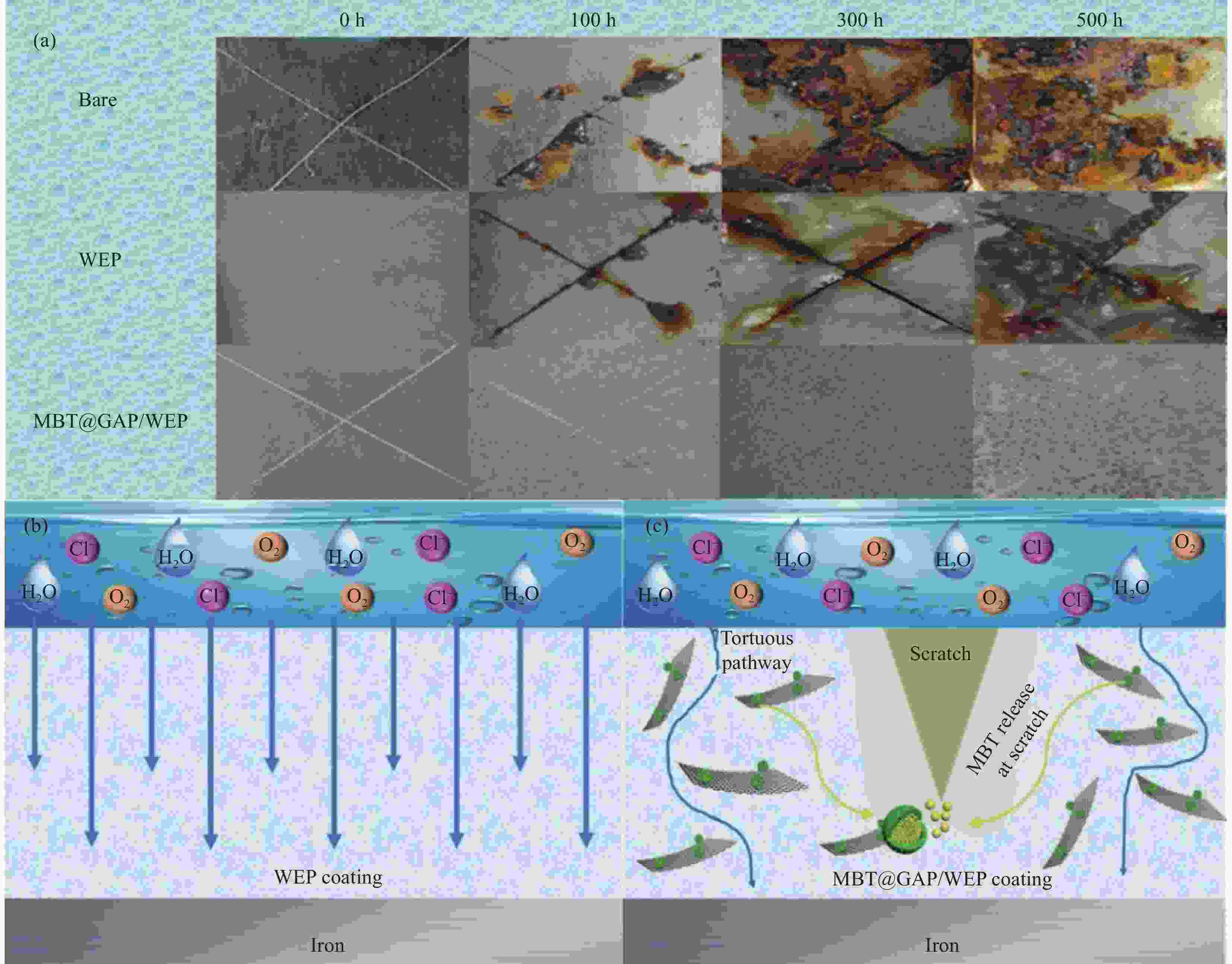
摘要: 为开发缓蚀剂高效利用的新途径,选取氧化石墨烯为基材、聚邻甲苯胺微胶囊为壁材、缓蚀剂2-巯基苯并噻唑为芯材,制备了缓蚀型聚邻甲苯胺-氧化石墨烯基防腐材料,并将其作为填料用于水性环氧树脂涂层(WEP)的改性。通过FTIR、XRD、XPS和SEM等对材料进行了结构和形貌的表征,采用紫外可见光谱对缓蚀剂的释放行为进行分析,采用万能试验机、电化学测试和盐雾实验对涂层的拉伸性能和防腐性能进行了评价。结果表明:缓蚀剂成功包覆于聚邻甲苯胺微胶囊内部,并通过共价键方式将微胶囊连接在改性氧化石墨烯表面,使缓蚀剂得到了充分利用,提高了涂层的拉伸性能、自修复性能及对腐蚀介质的屏蔽性能。紫外可见光谱测试结果表明,微胶囊在人工破损96 h后,内部缓蚀剂的释放量达78%;拉伸性能测试结果表明,与纯WEP相比,当填料加入量为0.3wt%时,涂层应力从14.281 MPa增加到24.25 MPa;SEM结果表明,被划伤的涂层在常温下放置10 h后自修复;电化学测试和盐雾实验结果表明,涂层腐蚀电位从−0.6216 V提高到−0.1554 V,腐蚀电流密度从4.271×10−7 A·cm−2减小到1.016×10−11 A·cm−2,阻抗模量可达到1.5757×109 Ω·cm2,在盐雾500 h后仍表现出较好的防腐性能。Abstract: In order to develop a new way of efficient utilization of corrosion inhibitors, a corrosion inhibited poly(o-toluidine)-graphene oxide-based anticorrosive materials was prepared by using graphene oxide as the substrate, poly(o-toluidine) microcapsules as the wall material and 2-mercaptobenzothiazole as the corrosion inhibitor as the core material, and it was used as the filler for the modification of waterborne epoxy resin coating (WEP). The structure and morphology of the coating were characterized by FTIR, XRD, XPS and SEM. The release behavior of the corrosion inhibitor was analyzed by UV-Vis spectroscopy. The tensile property and anti-corrosion properties of the coating were evaluated by universal testing machine, electrochemical test and salt spray test. The results show that the corrosion inhibitor is successfully coated inside poly(o-toluidine) microcapsules, and the microcapsules are connected to the surface of the modified graphene oxide by covalent bond, so that the corrosion inhibitor is fully utilized, and the tensile property, self-healing properties and shielding properties of the coating against corrosive media are improved. UV-vis spectrum test results show that the release of corrosion inhibitor in microcapsules reaches 78% after 96 h of artificial damage. The tensile property test results show that, compared with pure WEP, the coating stress increases from 14.281 MPa to 24.25 MPa when the filler content is 0.3wt%. SEM results show that the scratched coating self-healing after 10 h at room temperature. The electrochemical test and salt spray test results show that the corrosion potential of the coating increases from −0.6216 V to −0.1554 V, the corrosion current density decreases from 4.271×10−7 A·cm−2 to 1.016×10−11 A·cm−2, and the impedance modulus can reach 1.5757×109 Ω·cm2. After 500 h of salt spray, the corrosion resistance is still good.
-
Key words:
- graphene oxide /
- poly(o-toluidine) /
- microcapsule /
- water-based epoxy resin /
- anticorrosive coating
-
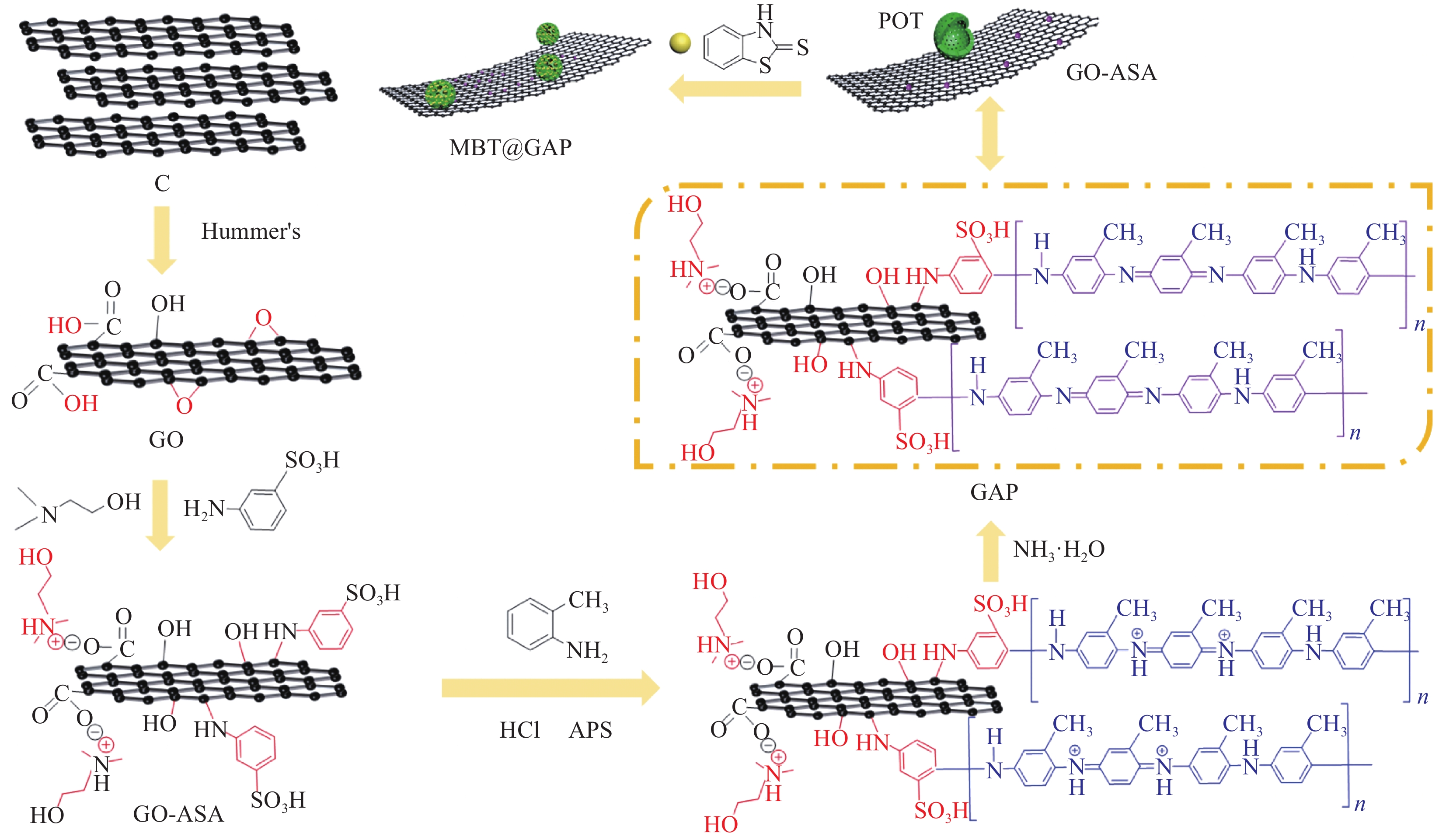
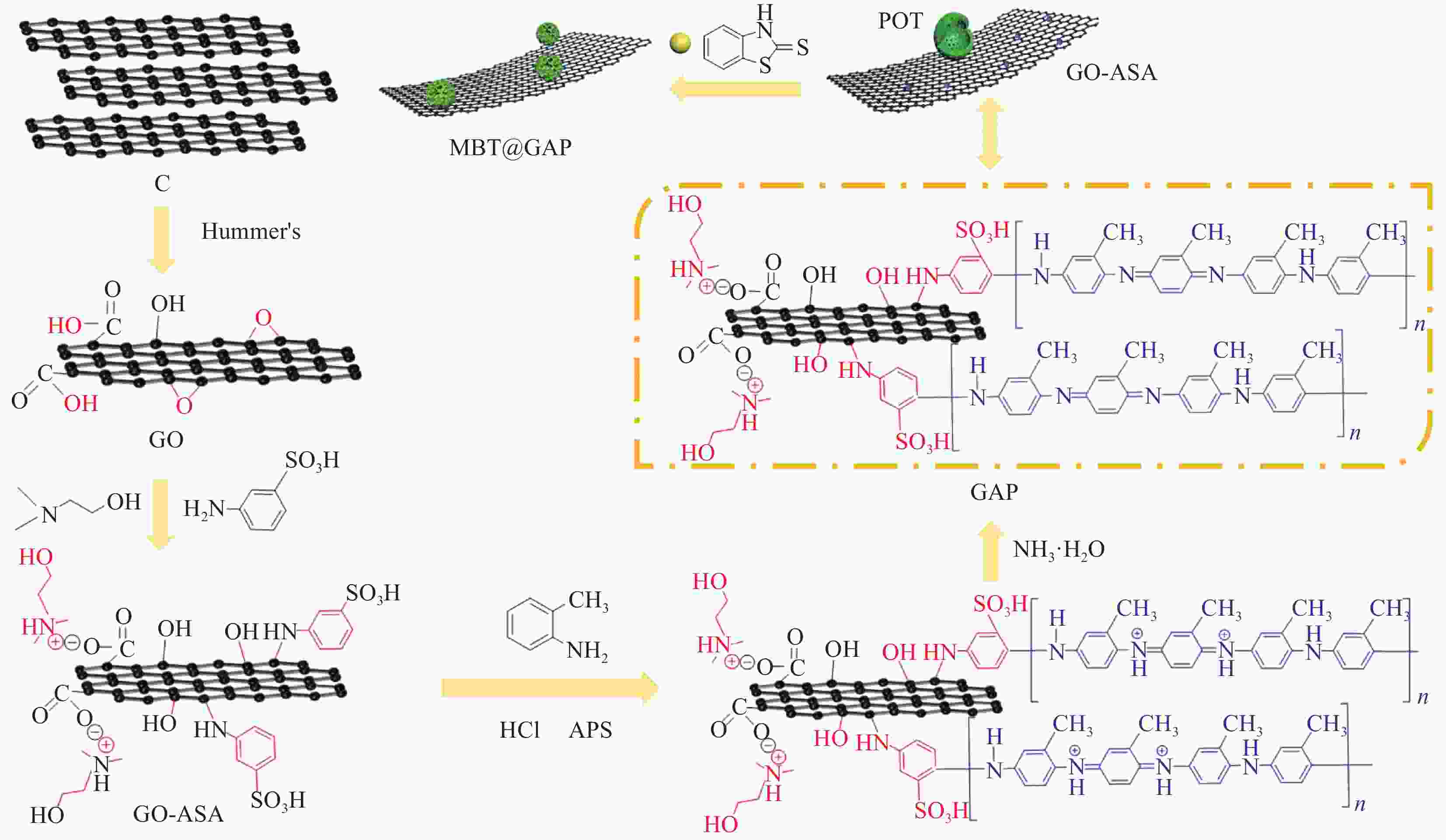
图 1 缓蚀型聚邻甲苯胺-氧化石墨烯基(MBT@GAP)复合材料的制备过程
Figure 1. Preparation process of corrosion inhibited poly(o-toluidine)-graphene oxide-based (MBT@GAP) composite materials
C—Graphite; GO—Graphene oxide; ASA—3-aminobenzenesulfonic acid; APS—Ammonium persulphate; POT—Poly(o-toluidine); MBT—2-mercaptobenzothiazole; GAP—POT-GO
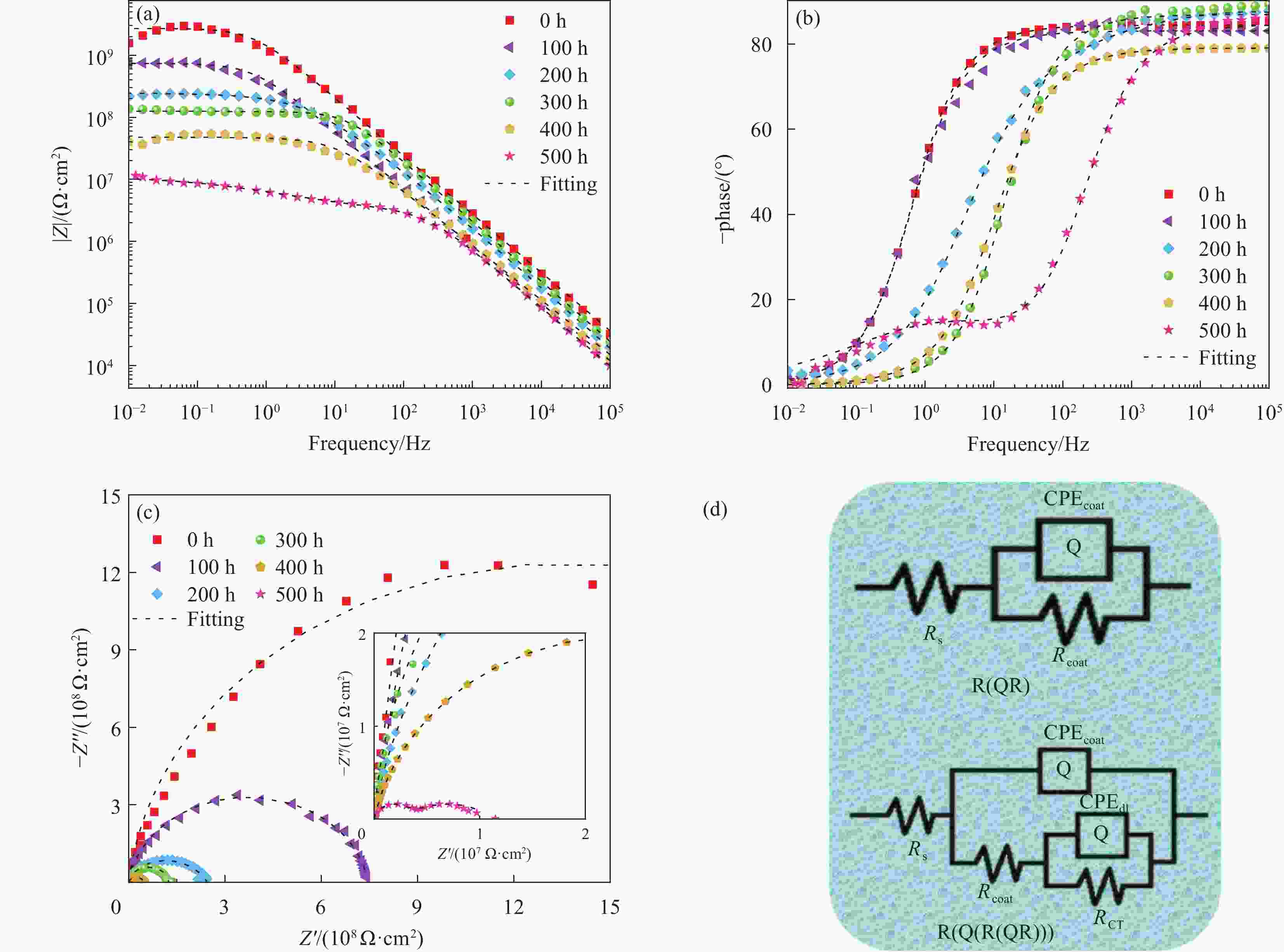
图 11 MBT@GAP/WEP-0.3wt%涂层不同盐雾时间下的阻抗模量曲线(a)、相位角曲线(b)、Nyquist图(c)、等效电路模型(d)
Figure 11. Impedance modulus curves (a), phase angle curves (b), Nyquist diagram (c), electrical equivalent circuit models (d) of MBT@GAP/WEP-0.3wt% coating under different salt spray time
Rcoat—Coating resistance; CPEcoat—Coating non-ideal capacitance; CPEdl—Double layer non-ideal capacitor; RCT—Charge transfer resistance; Rs—Solution resistance
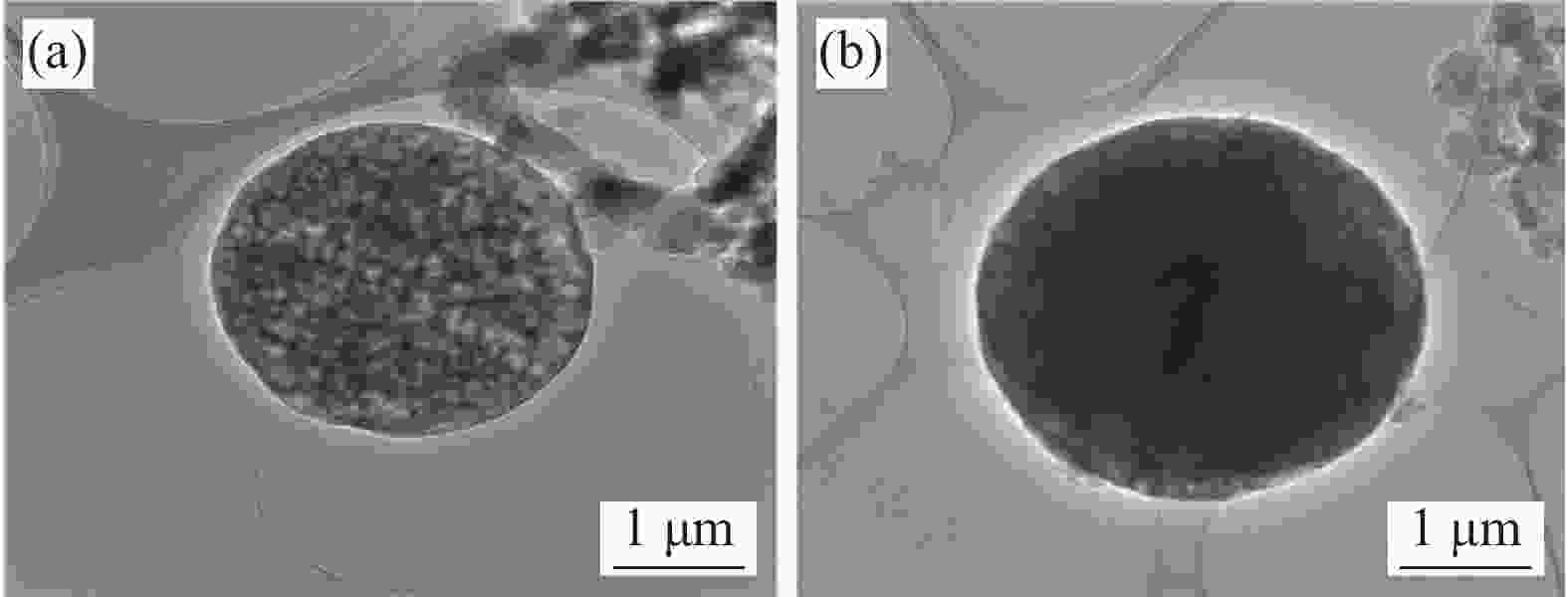
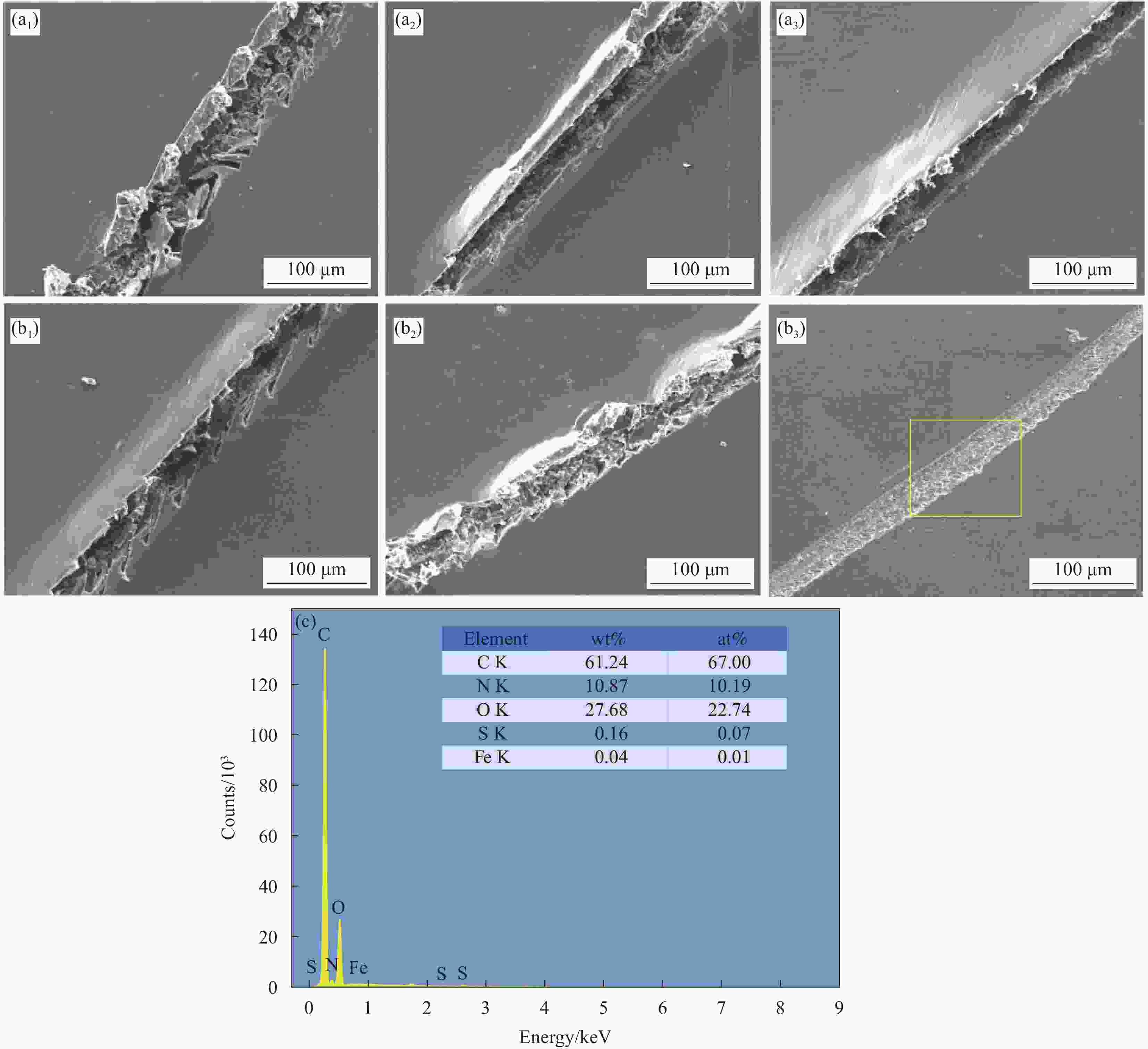
图 13 WEP涂层划伤后0 h (a1)、5 h (a2)、10 h (a3)和MBT@GAP/WEP-0.3wt%涂层划伤后0 h (b1)、5 h (b2)、10 h (b3)的SEM图像;(c) MBT@GAP/WEP-0.3wt%复合涂层划痕自修复后的EDS谱图
Figure 13. SEM images of 0 h (a1), 5 h (a2), 10 h (a3) after scratch of WEP coating and 0 h (b1), 5 h (b2), 10 h (b3) after scratch of MBT@GAP/WEP-0.3wt% coating; (c) EDS spectrum of MBT@GAP/WEP-0.3wt% composite coating scratches after self-healing
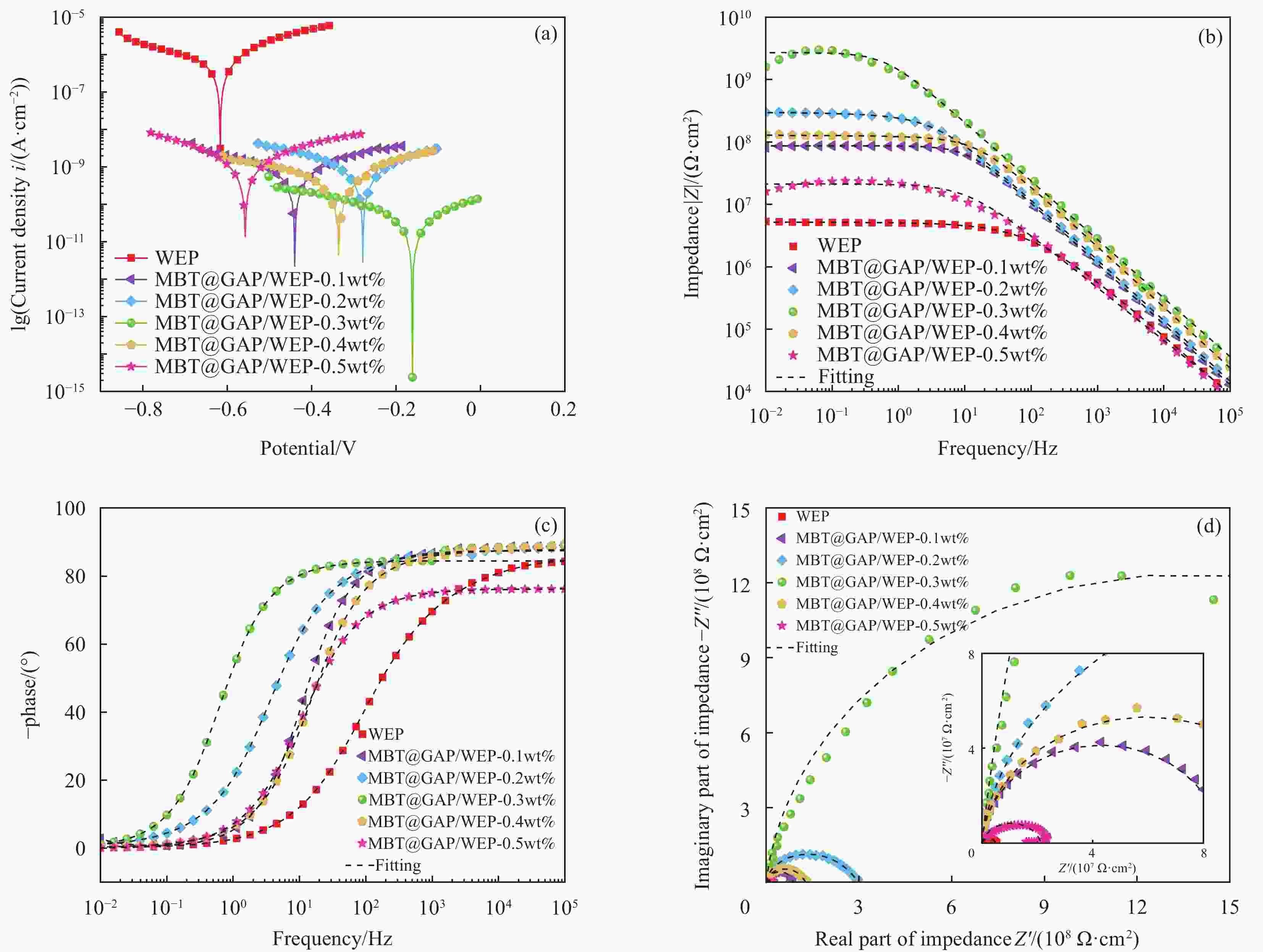
表 1 不同MBT@GAP添加量时复合涂层极化曲线参数
Table 1. Polarization curve parameters of composite coating with different contents of MBT@GAP
Ecorr/V Icorr/(A·cm−2) βa/(V·dec−1) βc/(V·dec−1) Rp/(Ω·cm2) WEP −0.6216 4.271×10−7 0.1781 0.2112 9.823×104 MBT@GAP/WEP-0.1wt% −0.4380 3.150×10−10 0.1962 0.1877 1.322×108 MBT@GAP/WEP-0.2wt% −0.2828 1.937×10−10 0.1397 0.2002 1.845×108 MBT@GAP/WEP-0.3wt% −0.1554 1.016×10−11 0.1212 0.0644 1.797×109 MBT@GAP/WEP-0.4wt% −0.3204 2.262×10−10 0.1453 0.2212 1.683×108 MBT@GAP/WEP-0.5wt% −0.5481 6.507×10−10 0.1957 0.1579 5.832×107 Notes: Ecorr—Corrosion potential; Icorr—Corrosion current density; βa—Anode slope; βc—Cathode slope; Rp—Polarization resistance. 表 2 不同MBT@GAP添加量时复合涂层交流阻抗谱图拟合参数
Table 2. Fitting parameters of alternating current impedance spectra of composite coating with different contents of MBT@GAP
Sample Rcoat/(Ω·cm2) CPEcoatγ/(Ω−1·cm−2·sn) n WEP 4.967×106 9.129×10−10 0.8288 MBT@GAP/WEP-0.1wt% 8.643×107 2.816×10−10 0.8915 MBT@GAP/WEP-0.2wt% 2.781×108 1.107×10−10 0.9185 MBT@GAP/WEP-0.3wt% 2.837×109 9.922×10−11 0.9388 MBT@GAP/WEP-0.4wt% 1.234×108 2.015×10−10 0.9101 MBT@GAP/WEP-0.5wt% 2.132×107 3.313×10−10 0.8667 Notes: n—Empirical index of CPEcoat; γ—Proportional factor. 表 3 MBT@GAP/WEP-0.3wt%复合涂层随盐雾时间变化的电化学阻抗拟合参数
Table 3. Electrochemical impedance fitting parameters for MBT@GAP/WEP-0.3wt% composite coatings with salt spray time
Salt spray time/h Rcoat/(Ω·cm2) CPEcoatγ/(Ω−1·cm−2·sn) n RCT/(Ω·cm2) CPEdlγ/(Ω−1·cm−2·sn') n' 0 2.837×109 9.922×10−11 0.9388 — — — 100 7.760×108 3.725×10−10 0.9225 — — — 200 2.454×108 4.339×10−10 0.9981 — — — 300 1.243×108 1.024×10−10 0.9662 — — — 400 4.885×107 5.424×10−10 0.8778 — — — 500 8.169×106 5.606×10−10 0.8401 3.111×106 8.138×10−8 0.4673 Note: n'—Empirical index of CPEdl. -
[1] WILDS N. Corrosion under insulation[M]//EL-SHERIK A M. Trends in Oil and Gas Corrosion Research and Technologies. Cambridge: Woodhead Publishing, 2017: 409-429. [2] XAVIER J R. Effect of surface modified WO3 nanoparticle on the epoxy coatings for the adhesive and anticorrosion properties of mild steel[J]. Journal of Applied Polymer Science,2020,137(5):48323. doi: 10.1002/app.48323 [3] SARI M G, RAMEZANZADEH B. Epoxy composite coating corrosion protection properties reinforcement through the addition of hydroxyl-terminated hyperbranched polyamide non-covalently assembled graphene oxide platforms[J]. Construction and Building Materials,2020,234:117421. doi: 10.1016/j.conbuildmat.2019.117421 [4] OU B L, WANG Y W, LU Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials[J]. Polymer-Plastics Technology and Materials,2021,60(6):601-625. doi: 10.1080/25740881.2020.1819317 [5] CAI G Y, XIAO S, DENG C M, et al. CeO2 grafted carbon nanotube via polydopamine wrapping to enhance corrosion barrier of polyurethane coating[J]. Corrosion Science,2021,178:109014. doi: 10.1016/j.corsci.2020.109014 [6] DAGDAG O, HSISSOU R, BERISHA A, et al. Polymeric-based epoxy cured with a polyaminoamide as an anticorrosive coating for aluminum 2024-T3 surface: Experimental studies supported by computational modeling[J]. Journal of Bio- and Tribo-Corrosion,2019,5(3):58. doi: 10.1007/s40735-019-0251-7 [7] ATTA A M, MOHAMED N H, ROSTOM M, et al. New hydrophobic silica nanoparticles capped with petroleum paraffin wax embedded in epoxy networks as multifunctional steel epoxy coatings[J]. Progress in Organic Coatings,2019,128:99-111. doi: 10.1016/j.porgcoat.2018.12.018 [8] LU F S, LIU C Q, CHEN Z H, et al. Polypyrrole-functionalized boron nitride nanosheets for high-performance anti-corrosion composite coating[J]. Surface Coatings Technology,2021,420:127273. doi: 10.1016/j.surfcoat.2021.127273 [9] YEGANEH M, ASADI N, OMIDI M, et al. An investigation on the corrosion behavior of the epoxy coating embedded with mesoporous silica nanocontainer loaded by sulfamethazine inhibitor[J]. Progress in Organic Coatings,2019,128:75-81. doi: 10.1016/j.porgcoat.2018.12.022 [10] YAN H, LI W, LI H, et al. Ti3C2 MXene nanosheets toward high-performance corrosion inhibitor for epoxy coating[J]. Progress in Organic Coatings,2019,135:156-167. doi: 10.1016/j.porgcoat.2019.06.013 [11] CAO X K, PAN J L, CAI G Y, et al. A chemically robust and self-healing superhydrophobic polybenzoxazine coating without fluorocarbon resin modification: Fabrication and failure mechanism[J]. Progress in Organic Coatings,2022,163:106630. doi: 10.1016/j.porgcoat.2021.106630 [12] SAMIEE R, RAMEZANZADEH B, MAHDAVIAN M, et al. Designing a non-hazardous nano-carrier based on graphene oxide@polyaniline-praseodymium (III) for fabrication of the active/passive anti-corrosion coating[J]. Journal of Hazardous Materials,2020,398:123136. doi: 10.1016/j.jhazmat.2020.123136 [13] KEYVANI A, YEGANEH M, REZAEYAN H. Application of mesoporous silica nanocontainers as an intelligent host of molybdate corrosion inhibitor embedded in the epoxy coated steel[J]. Progress in Natural Science: Materials International,2017,27(2):261-267. doi: 10.1016/j.pnsc.2017.02.005 [14] KASAEIAN M, GHASEMI E, RAMEZANZADEH B, et al. Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM)[J]. Corrosion Science,2018,145:119-134. doi: 10.1016/j.corsci.2018.09.023 [15] JIANG D, XIA X C, HOU J, et al. A novel coating system with self-reparable slippery surface and active corrosion inhibition for reliable protection of Mg alloy[J]. Chemical Engineering Journal,2019,373:285-297. doi: 10.1016/j.cej.2019.05.046 [16] LU H, ZHANG S T, LI W H, et al. Synthesis of graphene oxide-based sulfonated oligoanilines coatings for synergistically enhanced corrosion protection in 3.5% NaCl solution[J]. ACS Applied Materials & Interfaces,2017,9(4):4034-4043. [17] HAO Y S, SUN W, JIANG L L, et al. Self-healing effect of epoxy coating containing mesoporous polyaniline hollow spheres loaded with benzotriazole[J]. Progress in Organic Coatings,2021,159:106445. doi: 10.1016/j.porgcoat.2021.106445 [18] MOHAMMADZADEH A, GHAFOURI TALEGHANI H, LASHKENARI M S. Preparation and comparative study of anticorrosion nanocomposites of polyaniline/graphene oxide/clay coating[J]. Journal of Materials Research and Technology,2021,13:2325-2335. doi: 10.1016/j.jmrt.2021.05.098 [19] 张青青, 陈亚鑫, 刘仁, 等. 基于聚苯胺微胶囊的双重自修复防腐涂层[J]. 高分子学报, 2023, 54(5):720-730.ZHANG Qingqing, CHEN Yaxin, LIU Ren, et al. Dual-action self-healing anticorrosive coating based on polyaniline microcapsules[J]. Acta Polymerica Sinica,2023,54(5):720-730(in Chinese). [20] DONG J H, PAN W H, LUO J, et al. Synthesis of inhibitor-loaded polyaniline microcapsules with dual anti-corrosion functions for protection of carbon steel[J]. Electrochimica Acta, 2020, 364: 137299. [21] ZHANG Y J, LI M Y, WEN J, et al. Preparation of polyaniline encapsulated acrylic resin microcapsules and its active corrosion protection of coating for magnesium alloy[J]. Arabian Journal of Chemistry,2023,16(10):105129. doi: 10.1016/j.arabjc.2023.105129 [22] CAO Y, YUAN X W, WANG X, et al. Synthesis and controlled release kinetics of pH-sensitive hollow polyaniline microspheres encapsuled with the corrosion inhibitor[J]. Journal of Molecular Liquids, 2021, 342: 117497. [23] LIANG X, LI X J, TANG Y, et al. Hyperbranched epoxy resin-grafted graphene oxide for efficient and all-purpose epoxy resin modification[J]. Journal of Colloid and Interface Science,2021,611:105-117. [24] TAHERI N N, RAMEZANZADEH B, MAHDAVIAN M, et al. In-situ synthesis of Zn doped polyaniline on graphene oxide for anti-corrosive reinforcement of epoxy coating[J]. Journal of Industrial and Engineering Chemistry,2018,63:322-339. doi: 10.1016/j.jiec.2018.02.033 [25] AN H R, LIU K B, WANG S Q, et al. Enhanced corrosion resistance of waterborne epoxy coatings by polyaniline nanorods and nitrogen and fluorine dual-doped graphene oxide composites[J]. ACS Applied Nano Materials,2023,6(14):13250-13259. doi: 10.1021/acsanm.3c01964 [26] LIU S Y, LIU L, GUO H X, et al. Electrochemical polymerization of polyaniline-reduced graphene oxide composite coating on 5083Al alloy: Role of reduced graphene oxide[J]. Electrochemistry Communications,2019,98:110-114. doi: 10.1016/j.elecom.2018.12.004 [27] ZHU K, LI X R, WANG H H, et al. Electrochemical and anti-corrosion behaviors of water dispersible graphene/acrylic modified alkyd resin latex composites coated carbon steel[J]. Journal of Applied Polymer Science,2017,134(11):44445. [28] 中国国家标准化管理委员会. 漆膜一般制备法: GB/T 1727—2021[S]. 北京: 中国标准出版社, 2021.Standardization Administration of the People's Republic of China. General methods for preparation of coating films: GB/T 1727—2021[S]. Beijing: Standards Press of China, 2021(in Chinese). [29] 邹明明, 李小瑞, 沈一丁, 等. 改性氧化石墨烯/聚苯胺防腐材料的制备及性能[J]. 精细化工, 2018, 35(5):891-900. doi: 10.13550/j.jxhg.20170370ZOU Mingming, LI Xiaorui, SHEN Yiding, et al. Preparation and properties of modified graphene oxide/polyaniline anticorrosive materials[J]. Fine Chemicals,2018,35(5):891-900(in Chinese). doi: 10.13550/j.jxhg.20170370 [30] WANG J J, SU W H, ZHANG J M, et al. Improving the volumetric specific capacitance of flexible polyaniline electrode: Solution casting method and effect of reduced graphene oxide sheets[J]. Science China Materials,2021,64(3):571-580. doi: 10.1007/s40843-020-1472-3 [31] ZHOU C, HONG M, YANG Y, et al. Engineering sulfonated polyaniline molecules on reduced graphene oxide nanosheets for high-performance corrosion protective coatings[J]. Applied Surface Science,2019,484(1):663-675. [32] PARANGUSAN H, BHADRA J, AHMAD Z, et al. Investigation of the structural, optical and gas sensing properties of PANI coated Cu-ZnS microsphere composite[J]. RSC Advances,2020,10(45):26604-26612. doi: 10.1039/D0RA04991C [33] PIRHADY TAVANDASHTI N, GHORBANI M, SHOJAEI A, et al. Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects[J]. Corrosion Science,2016,112:138-149. doi: 10.1016/j.corsci.2016.07.003 [34] VETHANATHAN S J K, ABOORVAKANI R, MADHU K U. Yttrium doped ZnO nanofillers reinforced epoxy coating for anticorrosion application[J]. Inorganic Chemistry Communications,2022,144:109929. doi: 10.1016/j.inoche.2022.109929 [35] MENG Y B, GAO Y F, LI J Y, et al. Preparation and characterization of cross-linked waterborne acrylic/PTFE composite coating with good hydrophobicity and anticorrosion properties[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2022,653:129872. doi: 10.1016/j.colsurfa.2022.129872 [36] DUAN H L, JI J W, CAO C C, et al. Enhanced anti-corrosion performance of carbon steels via CeO2@BNNSs/epoxy resin composite coatings[J]. Macromolecular Chemistry and Physics, 2023, 224(11): 2300006. [37] DONG H Y, ZHAN Y Q, CHEN Y W, et al. Fabrication of hydrophobic and enhanced anticorrosion performance of epoxy coating through the synergy of functionalized graphene oxide and nano-silica binary fillers[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2023,664:131086. doi: 10.1016/j.colsurfa.2023.131086 [38] LI J Y, ZHU K, FU Z L. A waterborne uniform graphene oxide-epoxy complex with enhanced anticorrosive properties enabled by intercalation polymerization[J]. Journal of Polymer Engineering,2023,43(5):443-453. doi: 10.1515/polyeng-2022-0295 -





 下载:
下载: