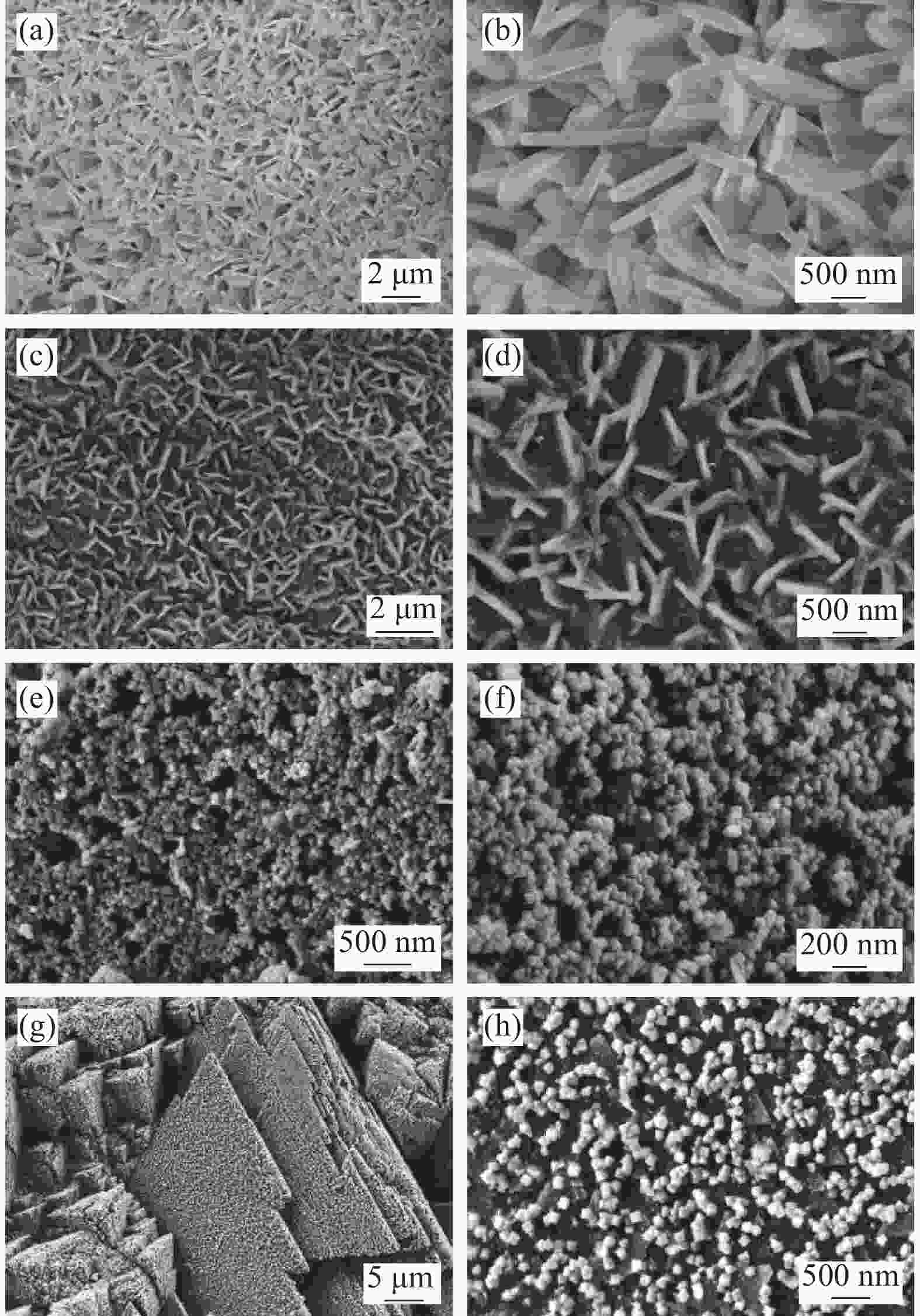
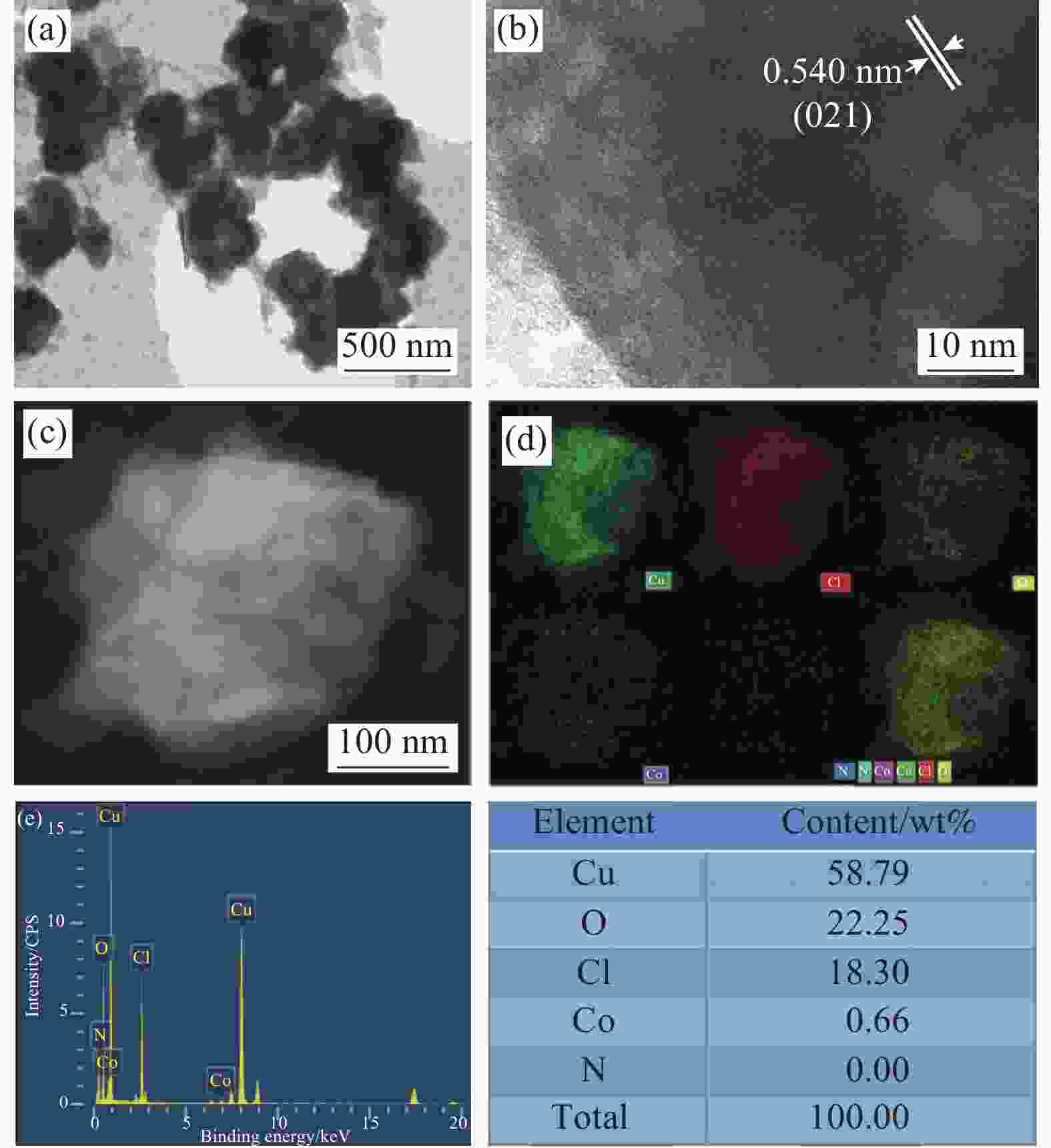
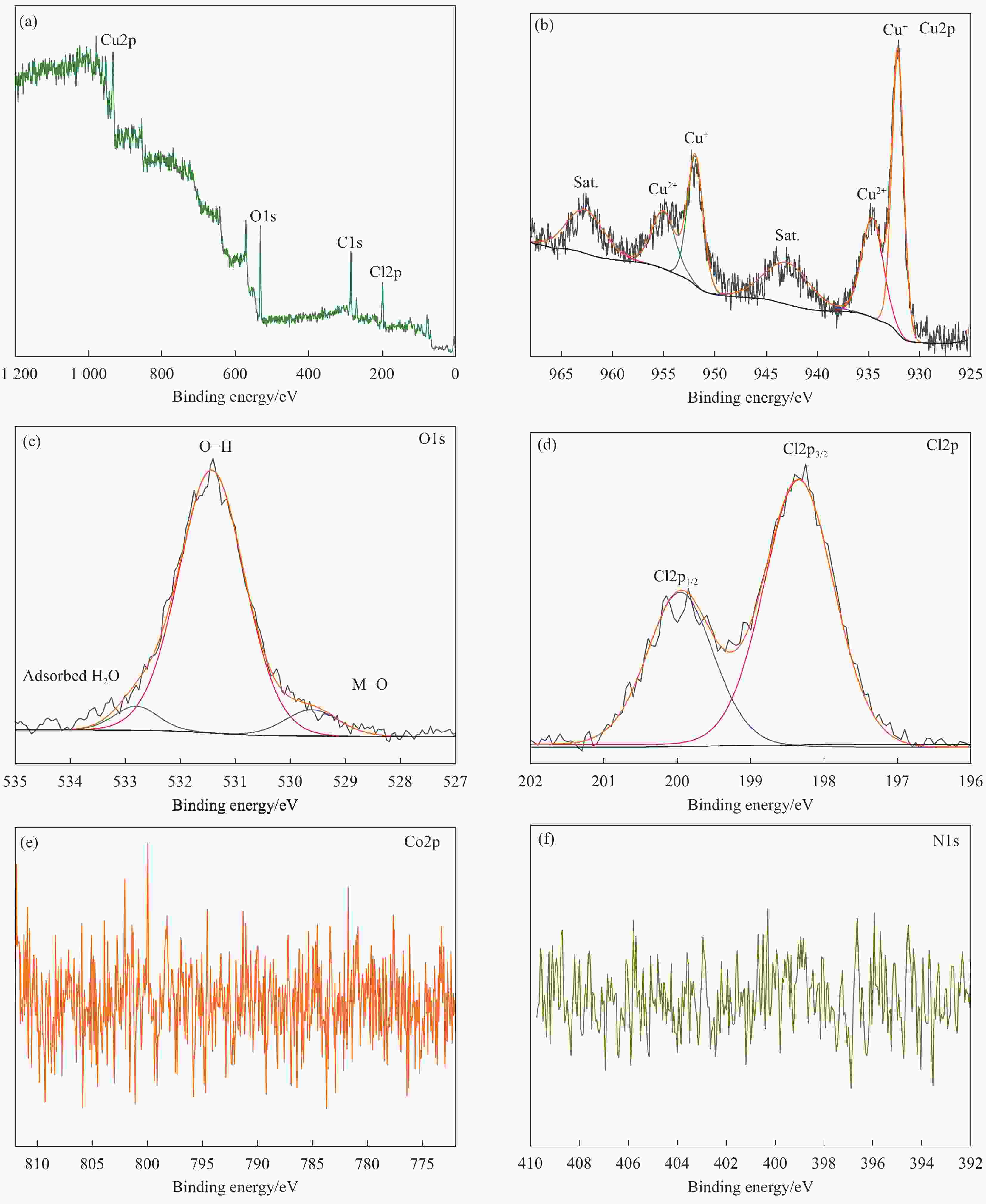
Synthesis and electrocatalytic oxygen evolution performance of cobalt doped copper-based composites
-
摘要: 铜基纳米材料在电催化方面受到广泛关注,但其存在催化活性低和稳定性差的问题,探索简单高效的策略解决上述问题具有重要的实际意义。本文在室温条件下,采用Co-MOF材料在CuCl2溶液中水解刻蚀策略成功在泡沫镍基底上构筑了钴掺杂的碱式氯化铜/氯化亚铜复合材料。通过改变Co-MOF在CuCl2溶液中的水解刻蚀时间,从而调控物种和复合物的形貌结构。最优催化剂仅需238 mV的过电位便能够驱动100 mA·cm−2的电流密度。经过50 h的稳定性测试,电流密度几乎没有下降,表明其具有良好的稳定性。优异的电催化析氧反应(OER)性能可归属于Co原子的掺杂优化了Cu原子周围电子环境,激活碱式氯化铜和氯化亚铜的催化活性及CuCl2对泡沫镍的刻蚀增加了活性位点。本文为铜基电催化材料的制备和电催化OER活性增强提供了新的思路和策略。Abstract: Copper-based nanomaterials have received much attention in electrocatalysis, but they suffer from low catalytic activity, unstable structures, and poor stability, and it is of great practical importance to explore simple and efficient strategies to solve these problems. In this study, a Co-MOF material was used to successfully construct cobalt-doped Cu2Cl(OH)3/CuCl composite materials on a nickel foam substrate through a hydrolysis-etching strategy in a CuCl2 solution at room temperature. By varying the hydrolysis-etching time of Co-MOF in the CuCl2 solution, the morphology and structure of the species and composites were controlled. The optimized catalyst only requires an overpotential of 238 mV to drive a current density of 100 mA·cm−2. After 50 h of stability testing, the current density hardly decreases, indicating excellent stability. The excellent electrocatalytic oxygen evolution reaction (OER) performance can be attributed to the cobalt atom doping, which optimizes the electronic environment around the copper atoms, activating the catalytic activity of Cu2Cl(OH)3 and CuCl, as well as the CuCl2 etching of the nickel foam, which increases the active sites. This study provides new ideas and strategies for the preparation of copper-based electrocatalytic materials and enhancing their electrocatalytic OER activity.
-
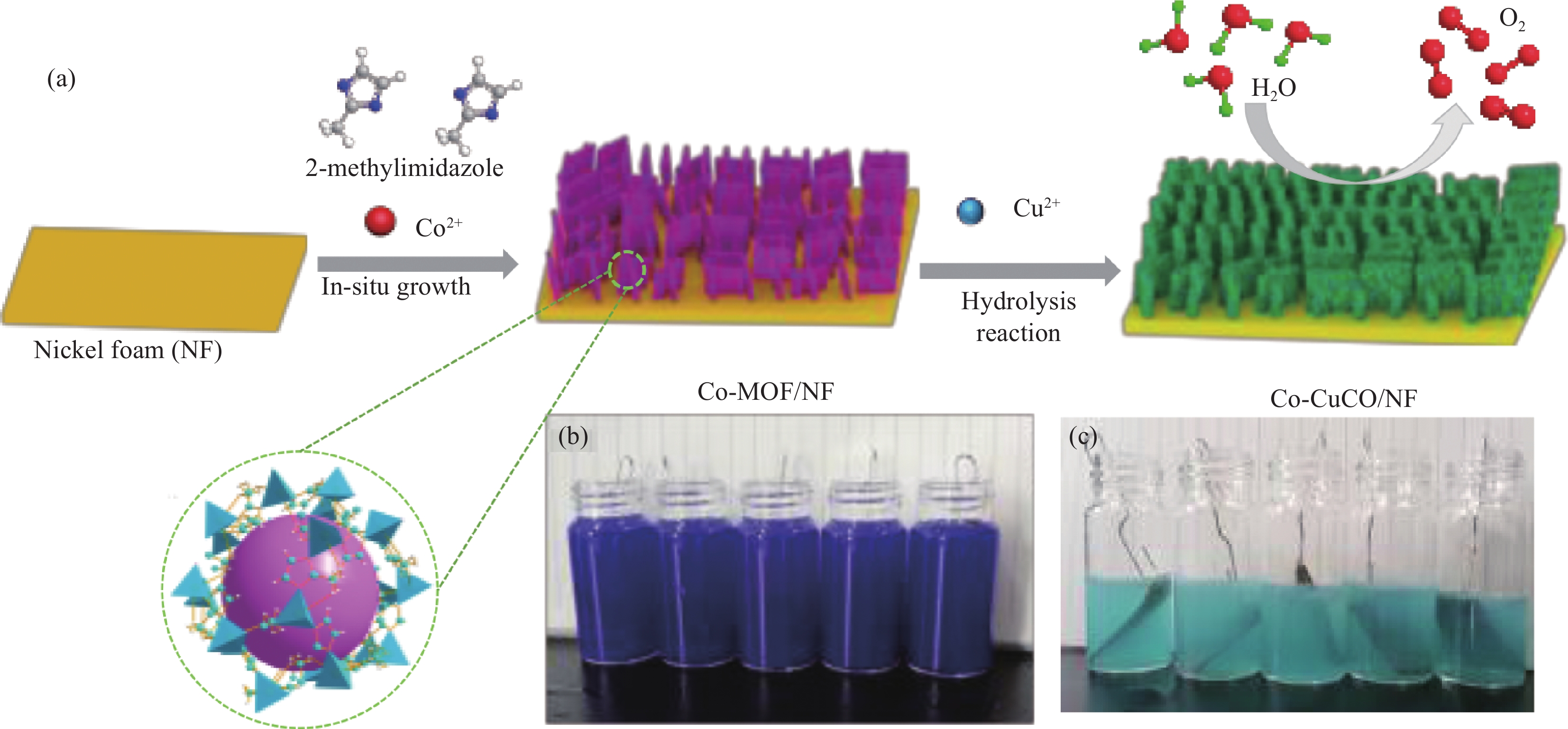
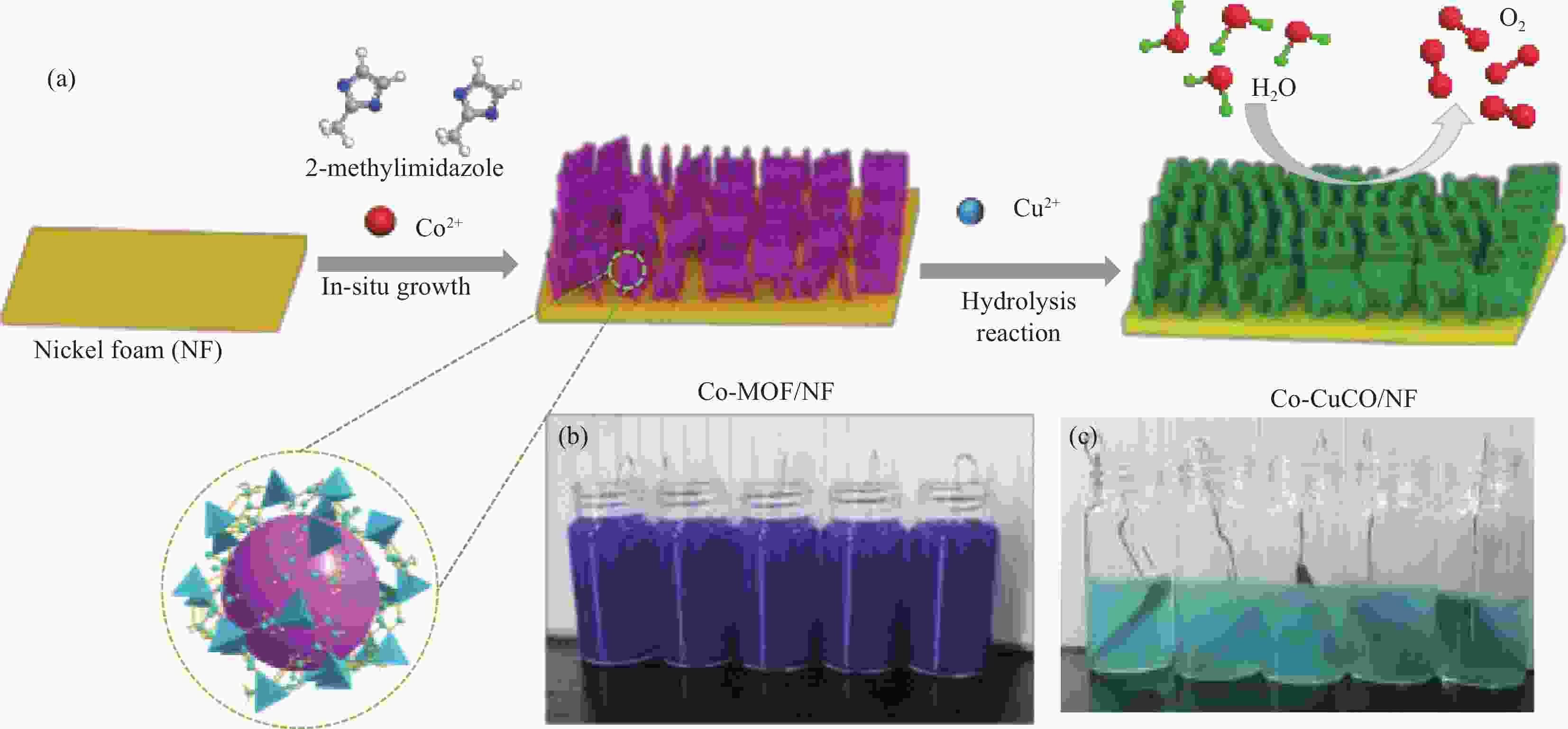
图 1 钴掺杂的碱式氯化铜/氯化亚铜(Co-CuCO)/泡沫镍(NF)的制备过程示意图和Co-MOF结构示意图(a)、反应过程中的Co-MOF/NF照片(b)和Co-CuCO/NF的照片(c)
Figure 1. Schematic diagram of the preparation of cobalt-doped Cu2Cl(OH)3/CuCl (Co-CuCO)/NF and the structure diagram of Co-MOF (a), digital photographs of Co-MOF/NF (b) and Co-CuCO/NF (c) during the reaction
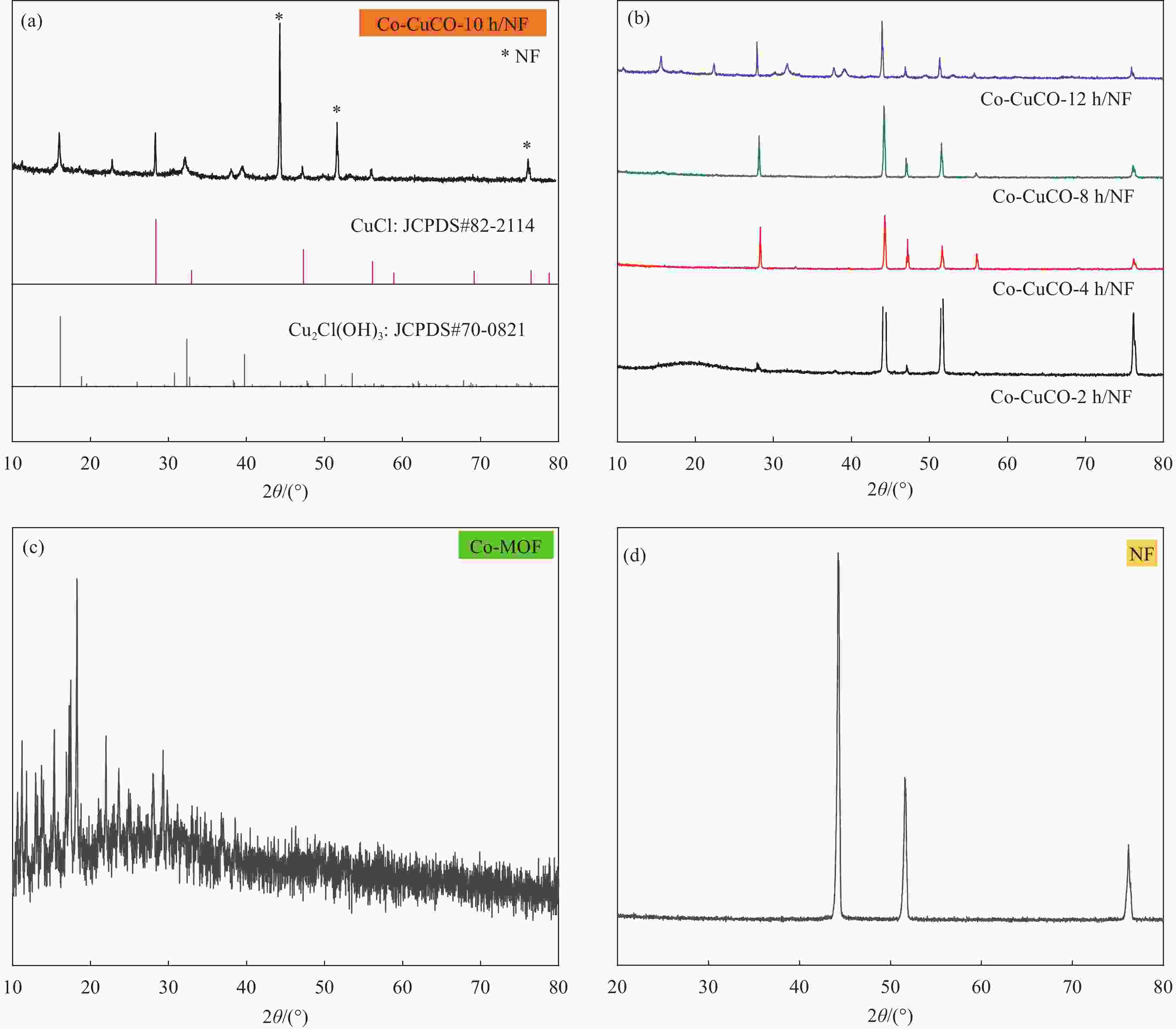
图 2 (a) Co-CuCO-10 h/NF复合材料的XRD图谱;(b) Co-CuCO-2 h/NF、Co-CuCO-4 h/NF、Co-CuCO-8 h/NF、Co-CuCO-12 h/NF复合材料的XRD图谱;Co-MOF (c)和裸泡沫镍(d)的XRD图谱
Figure 2. (a) XRD patterns of Co-CuCO-10 h/NF composites; (b) XRD patterns of Co-CuCO-2 h/NF, Co-CuCO-4 h/NF, Co-CuCO-8 h/NF, Co-CuCO-12 h/NF composites; XRD patterns of Co-MOF powder sample (c) and bare nickel foam (d)
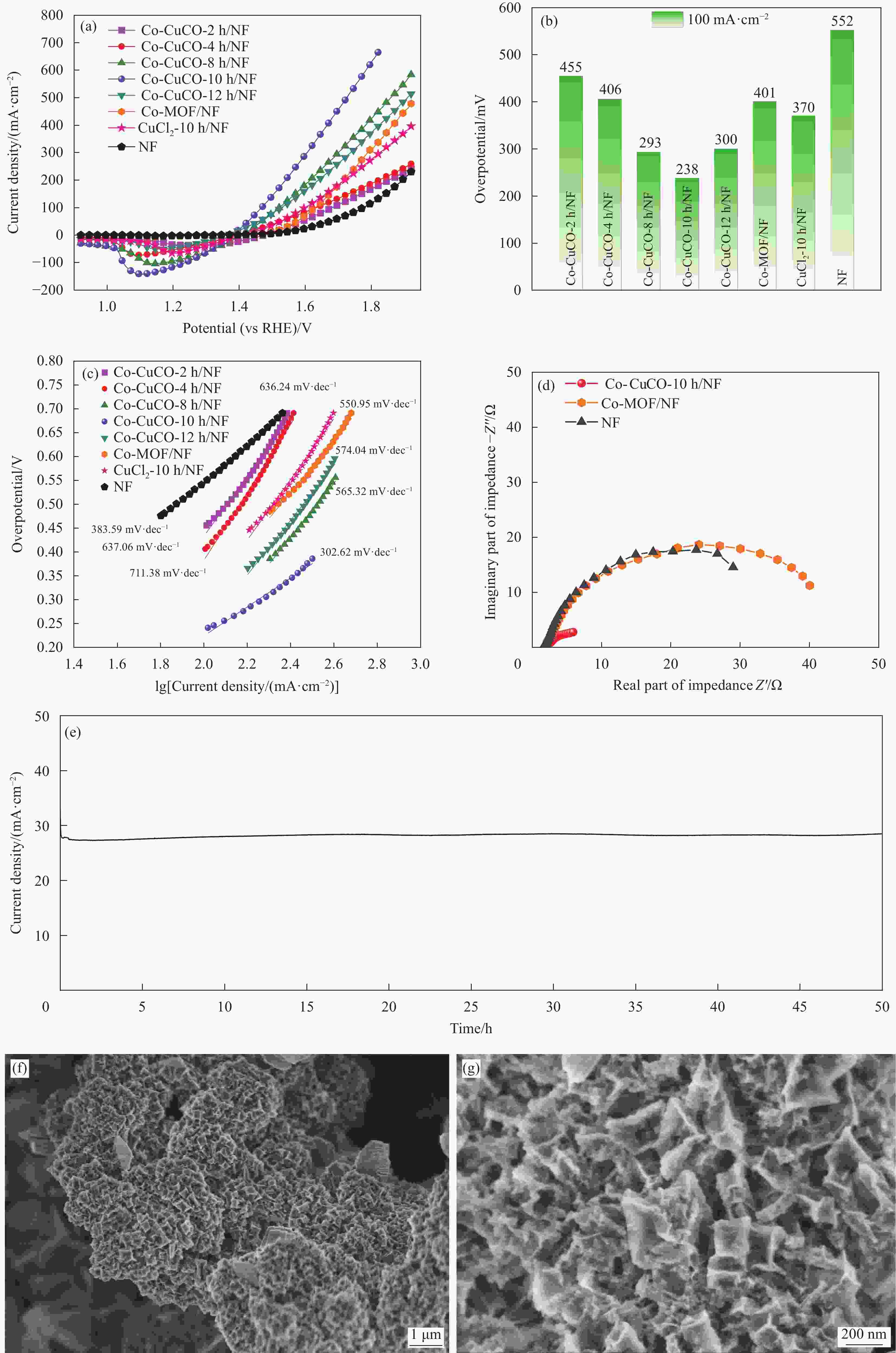
图 6 Co-CuCO-2 h/NF、Co-CuCO-4 h/NF、Co-CuCO-8 h/NF、Co-CuCO-10 h/NF、Co-CuCO-12 h/NF、CuCl2-10 h/NF、Co-MOF/NF和NF的极化曲线(a)、在电流密度100 mA·cm−2下的过电位比较(b)和Tafel斜率(c);(d) Co-CuCO-10 h/NF、Co-MOF/NF和NF的电化学阻抗谱;Co-CuCO-10 h/NF电极的稳定性测试(e)和反应50 h之后的SEM图像((f), (g))
Figure 6. LSV polarization curves (a), overpotentials at a current density of 100 mA·cm−2 (b) and Tafel slopes (c) of Co-CuCO-2 h/NF, Co-CuCO-4 h/NF, Co-CuCO-8 h/NF, Co-CuCO-10 h/NF, Co-CuCO-12 h/NF, CuCl2-10 h/NF, Co-MOF/NF, and NF; (d) Nyquist plots for Co-CuCO-10 h/NF, Co-MOF/NF, and NF; Stability test (e) and SEM images ((f), (g)) after 50 h of reaction for the Co-CuCO-10 h/NF electrode
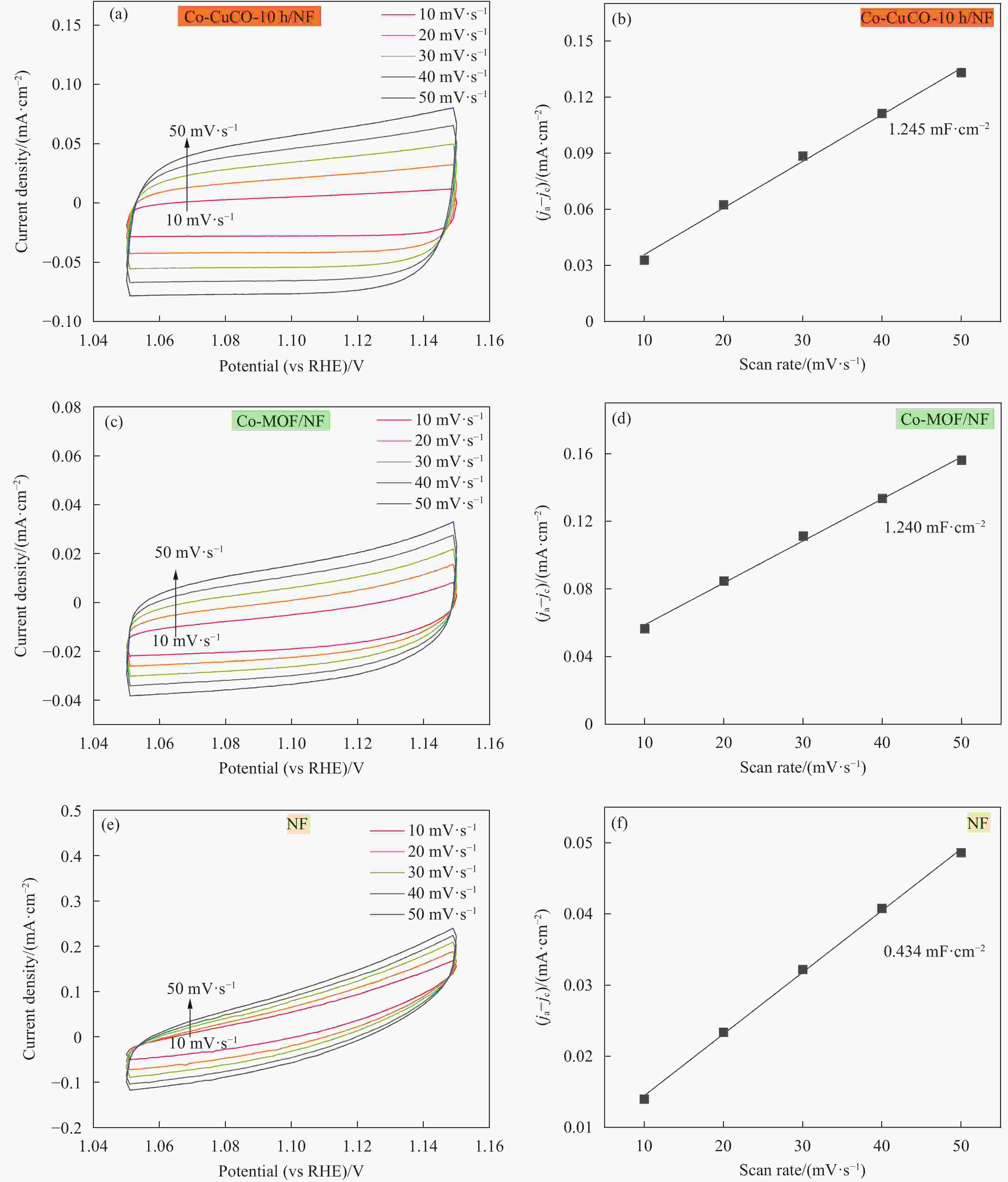
图 7 Co-CuCO-10 h/NF ((a), (b))、Co-MOF/NF ((c), (d))和NF ((e), (f))在非法拉第电位区间的循环伏安曲线和相应的双电层电容曲线
Figure 7. Cyclic voltammetry curves in the non-Faradaic potential region and corresponding the capacitive current densities plotted against scan rate of Co-CuCO-10 h/NF ((a), (b)), Co-MOF/NF ((c), (d)) and NF ((e), (f))
ja—Anodic current density; jc—Cathodic current density
表 1 刻蚀不同时间所制备的复合材料的组分
Table 1. Components of composites prepared by etching for different time
Abbreviation of
sample nameReaction time/h Component Co-CuCO-2 h/NF 2 CuCl, Co-MOF Co-CuCO-4 h/NF 4 CuCl, Co-MOF Co-CuCO-8 h/NF 8 CuCl, Co-MOF,Cu2Cl(OH)3 Co-CuCO-10 h/NF 10 CuCl, Cu2Cl(OH)3 Co-CuCO-12 h/NF 12 CuCl, Cu2Cl(OH)3 Note: NF—Nickel foam. -
[1] 赖嘉俊, 李潇潇, 曾传旺, 等. 富氧空位铁基复合材料的制备及其电催化析氢性能[J]. 复合材料学报, 2023, 40(5):2827-2835. doi: 10.13801/j.cnki.fhclxb.20220704.003LAI Jiajun, LI Xiaoxiao, ZENG Chuanwang, et al. Preparation and electro catalytic hydrogen evolution performance of iron-based composites with rich oxygen vacancies[J]. Acta Materiae Compositae Sinica,2023,40(5):2827-2835(in Chinese). doi: 10.13801/j.cnki.fhclxb.20220704.003 [2] WAN L, XU Z, XU Q, et al. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis[J]. Energy Environmental Science,2023,16(4):1384-1430. doi: 10.1039/D3EE00142C [3] SHI Y, ZHANG B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction[J]. Chemical Society Reviews,2016,45(6):1781. doi: 10.1039/C6CS90013E [4] WU Z P, LU X F, ZANG S Q, et al. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction[J]. Advanced Functional Materials,2020,30(15):1910274. doi: 10.1002/adfm.201910274 [5] 李垒, 欧阳汶俊, 郑泽锋, 等. 基于Pt@TiO2催化剂光-热协同催化甲醇-水液相重整甲醇制氢[J]. 催化学报, 2022, 43(5):1258-1266. doi: 10.1016/S1872-2067(21)63963-3LI Lei, OUYANG Wenjun, ZHENG Zefeng, et al. Synergetic photocatalytic and thermocatalytic reforming of methanol for hydrogen production based on Pt@TiO2 catalyst[J]. Chinese Journal of Catalysis,2022,43(5):1258-1266(in Chinese). doi: 10.1016/S1872-2067(21)63963-3 [6] JI L, ZHENG H, WEI Y, et al. Temperature-controlled fabrication of Co-Fe-based nanoframes for efficient oxygen evolution[J]. Science China Materials,2022,65:431-441. [7] FENG Y F, XU C Y, HU E L, et al. Construction of hierarchical FeP/Ni2P hollow nanospindles for efficient oxygen evolution[J]. Journal of Materials Chemistry A,2018,6(29):14103-14111. doi: 10.1039/C8TA03933J [8] WANG Y, WANG S, MA L Z, et al. Competitive coordination-oriented monodispersed ruthenium sites in conductive MOF/LDH hetero-nanotree catalysts for efficient overall water splitting in alkaline media[J]. Advanced Materials,2022,34(12):2107488. doi: 10.1002/adma.202107488 [9] 王田田, 王宇, 梁文进, 等. CoWO4/NC复合材料制备及其电催化析氧性能[J]. 复合材料学报, 2023, 40(11):6194-6201.WANG Tiantian, WANG Yu, LIANG Wenjin, et al. Preparation and electrocatalytic oxygen evolution performance of CoWO4/NC composites[J]. Acta Materiae Compositae Sinica,2023,40(11):6194-6201(in Chinese). [10] 李筱霏, 赵恩德, 彭少波, 等. MOF衍生CoSe2基电催化剂的制备及其电解水性能研究进展[J]. 复合材料学报, 2023, 40(8): 4374-4389.LI Xiaofei, ZHAO Ende, PENG Shaobo, et al. Research progress of synthesis of metal organic framework derived CoSe2-based electrocatalysts for overall water splitting[J]. Acta Materiae Compositae Sinica, 2023, 40(8): 4374-4389(in Chinese). [11] ABOUSERIE A, EL-NAGAR A, HEYNE B. Facile synthesis of hierarchical CuS and CuCo2S4 structures from an ionic liquid precursor for electrocatalysis applications[J]. ACS Applied Materials Interfaces, 2020, 12(47): 52560-52570. [12] SAAD A, LIU D Q, WU Y C, et al. Ag nanoparticles modified crumpled borophene supported Co3O4 catalyst showing superior oxygen evolution reaction (OER) performance[J]. Applied Catalysis B: Environmental,2021,298:120529. doi: 10.1016/j.apcatb.2021.120529 [13] ZHU J, ZI S, ZHANG N, et al. Surface reconstruction of covellite CuS nanocrystals for enhanced OER catalytic performance in alkaline solution[J]. Small, 2023, 19(37): 2301762. [14] OUYANG Q, CHENG S, YANG C, et al. Vertically grown p-n heterojunction FeCoNi LDH/CuO arrays with modulated interfacial charges to facilitate the electrocatalytic oxygen evolution reaction[J]. Journal of Materials Chemistry A,2022,10(22):11938-11947. doi: 10.1039/D1TA09892F [15] YANG Y, YANG Q N, YANG Y B, et al. Enhancing water oxidation of Ru single atoms via oxygen-coordination bonding with NiFe layered double hydroxide[J]. ACS Catalysis,2023,13(4):2771-2779. doi: 10.1021/acscatal.2c05624 [16] TANG J, RUAN Q, YU H, et al. Activating Co(OH)2 active sites by coupled with V2O5 to boost highly efficient oxygen evolution reaction[J]. Advanced Sustainable Systems, 2023, 7(5): 2200473. [17] CHEN F W, ZHANG S N, LI J J, et al. Precursor-mediated synthesis of interconnected ultrathin NiFe-layered double hydroxides nanosheets for efficient oxygen evolution electrocatalysis[J]. Materials Letters,2022,309:131470. doi: 10.1016/j.matlet.2021.131470 [18] MA Y, CHU J, LI Z, et al. Homogeneous metal nitrate hydroxide nanoarrays grown on nickel foam for efficient electrocatalytic oxygen evolution[J]. Small,2018,14(52):1803783. doi: 10.1002/smll.201803783 [19] MA N, FEI C, WANG J, et al. Fabrication of NiFe-MOF/cobalt carbonate hydroxide hydrate heterostructure for a high performance electrocatalyst of oxygen evolution reaction[J]. Journal of Alloys Compounds,2022,917:165511. doi: 10.1016/j.jallcom.2022.165511 [20] WANG F, LIU B, LIN Z, et al. Constructing partially amorphous borate doped iron-nickel nitrate hydroxide nanoarrays by rapid microwave activation for oxygen evolution[J]. Applied Surface Science,2022,592:153245. doi: 10.1016/j.apsusc.2022.153245 [21] GUO B, DING Y, HUO H, et al. Recent advances of transition metal basic salts for electrocatalytic oxygen evolution reaction and overall water electrolysis[J]. Nano-Micro Letters,2023,15:57. doi: 10.1007/s40820-023-01038-0 [22] WU Y Y, LI Y, YUAN M K, et al. Operando capturing of surface self-reconstruction of Ni3S2/FeNi2S4 hybrid nanosheet array for overall water splitting[J]. Chemical Engineering Journal,2022,427:131944. doi: 10.1016/j.cej.2021.131944 [23] GE S Y, XIE R K, HUANG B, et al. A robust chromium-iridium oxide catalyst for high-current-density acidic oxygen evolution in proton exchange membrane electrolyzers[J]. Energy & Environmental Science, 2023, 16(9): 3734-3742. [24] 施伟东, 尉兵, 肖立松, 等. 表面金属铜修饰氯化亚铜/泡沫镍复合材料的制备方法及其应用: CN, 201810855956[P]. 2018-12-18.SHI Weidong, WEI Bing, XIAO Lisong, et al. Preparation method and application of surface metal copper-modified cuprous chloride/foam nickel composite material: CN, 201810855956[P]. 2018-12-18(in Chinese). [25] WAN L, ZHOU Q X, WANG X, et al. Cu2O nanocubes with mixed oxidation-state facets for (photo)catalytic hydrogenation of carbon dioxide[J]. Nature Catalysis,2019,2:889-898. doi: 10.1038/s41929-019-0338-z [26] 和佳乐, 王菊琳. 初始pH值和Cl−浓度对CuCl水解的影响[J]. 中国腐蚀与防护学报, 2018, 38(4):397-402.HE Jiale, WANG Julin. Impacts of initial pH and Cl− concentration on nantokite hydrolysis[J]. Journal of Chinese Society for Corrosion and Protection,2018,38(4):397-402(in Chinese). [27] ZHAO Y, GAO Y X, CHEN Z, et al. Trifle Pt coupled with NiFe hydroxide synthesized via corrosion engineering to boost the cleavage of water molecule for alkaline water-splitting[J]. Applied Catalysis B: Environmental,2023,297:120395. [28] HUANG Y, LI F, ZHANG X, et al. Cu vacancy engineering on facet dependent CuO to enhance water oxidation efficiency[J]. International Journal of Hydrogen Energy,2022,47(15):9261-9272. doi: 10.1016/j.ijhydene.2021.12.267 [29] SILVA O D, SINGH M, MAHASIVAM S, et al. Importance of phase purity in two-dimensional β-Co(OH)2 for driving oxygen evolution[J]. ACS Applied Nano Materials, 2022, 5(9): 12209-12216. [30] MENG C, LIN M, SUN X, et al. Laser synthesis of oxygen vacancy-modified CoOOH for highly efficient oxygen evolution[J]. Chemical Communications, 2019, 55(20): 2904-2907. [31] XU H T, SONG D H, LI J, et al. Chlorine-assisted synthesis of CuCo2S4@(Cu, Co)2Cl(OH)3 heterostructures with an efficient nanointerface for electrocatalytic oxygen evolution[J]. Journal of Colloid and Interface Science, 2021, 601: 437-445. -





 下载:
下载: