Effect of Cr(VI) on photocatalytic of xanthate and synergistic mechanism
-
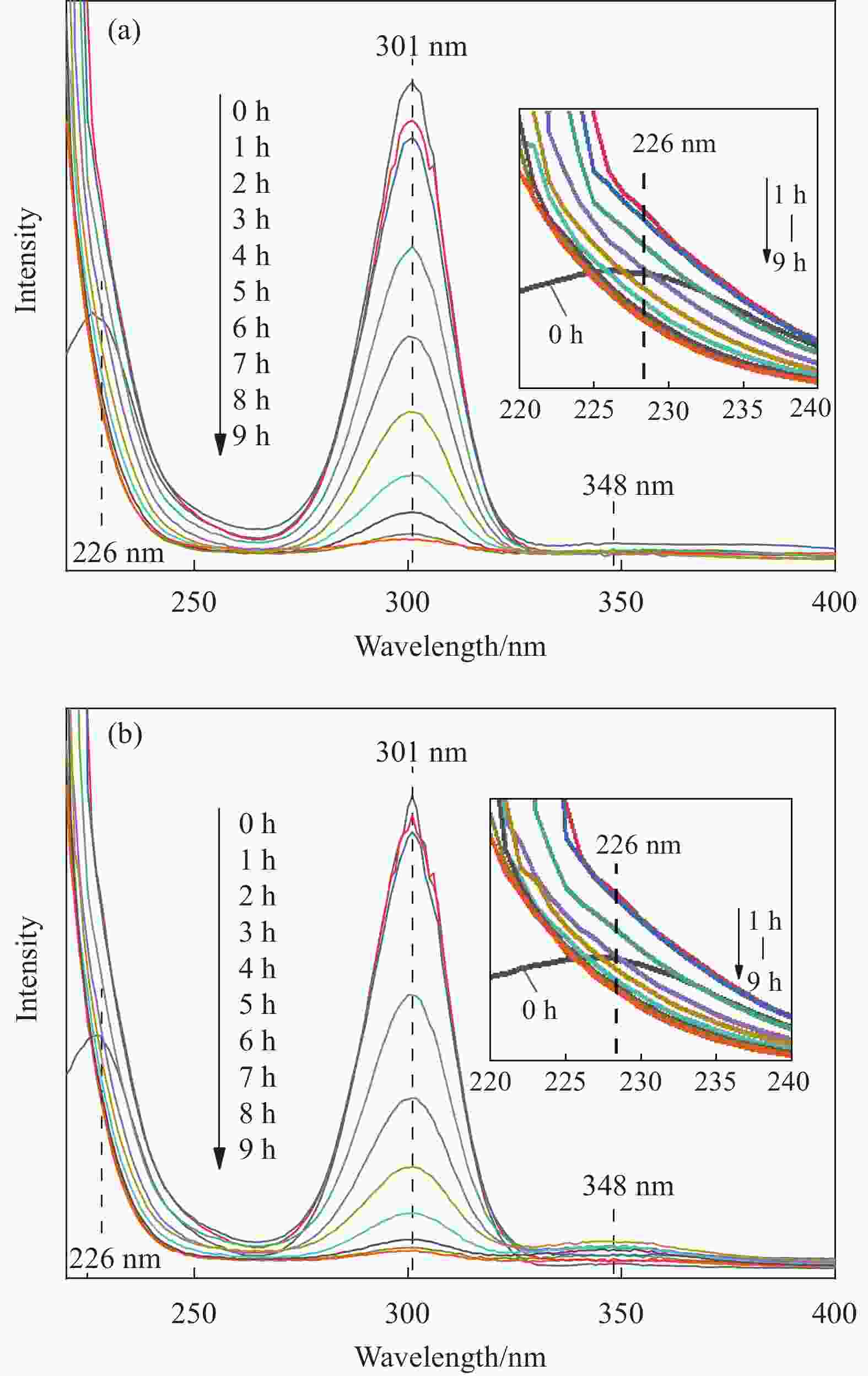
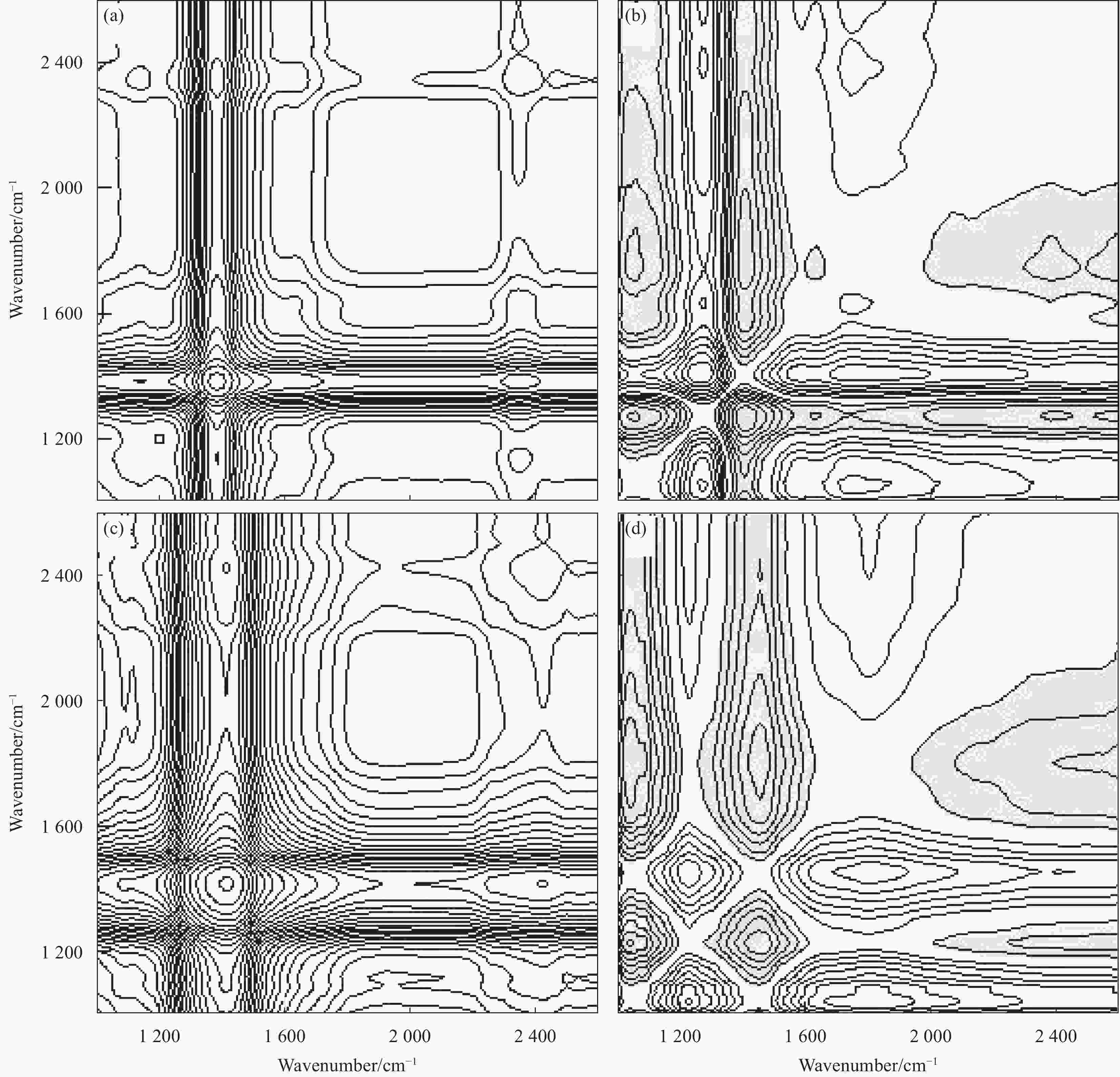
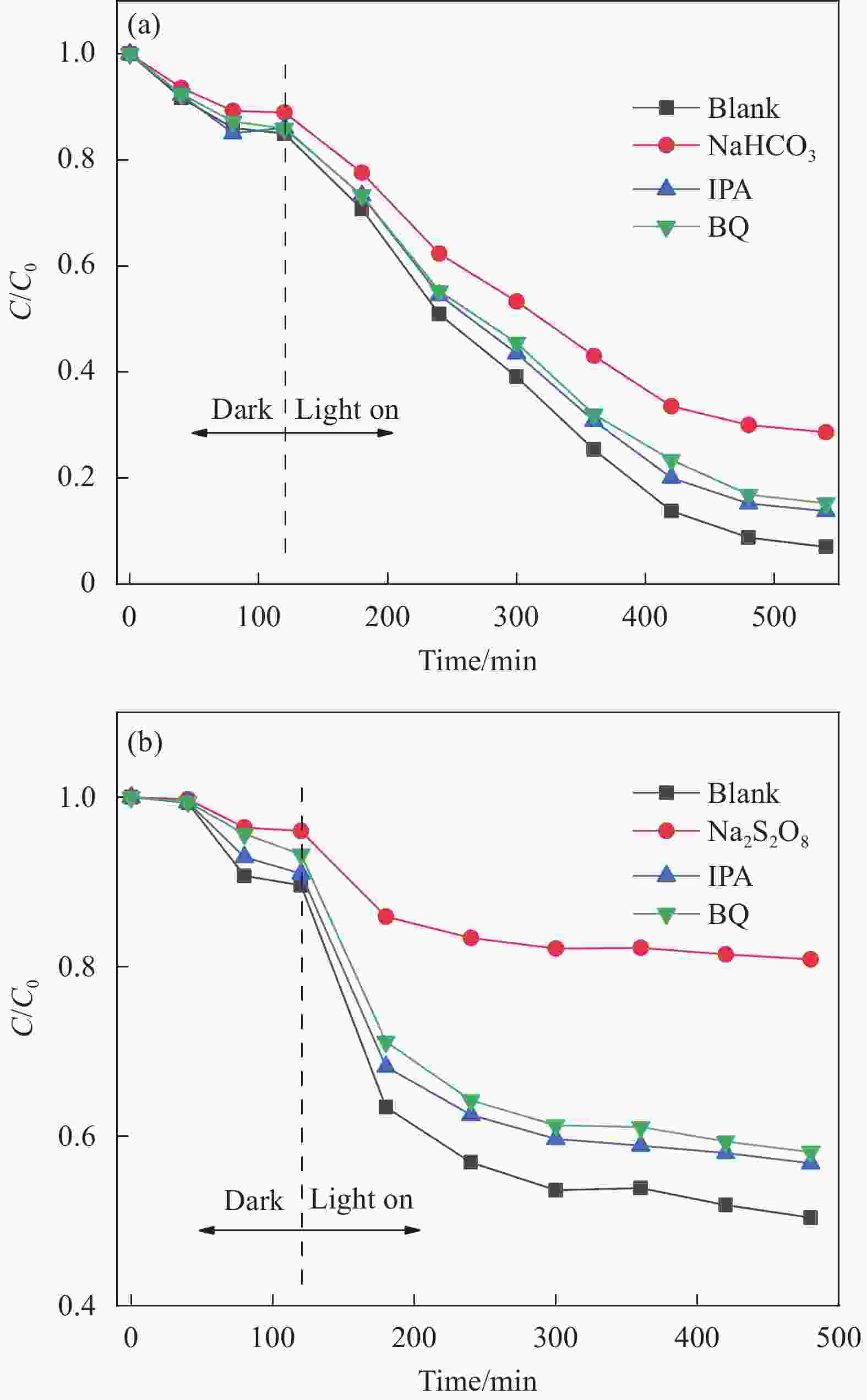
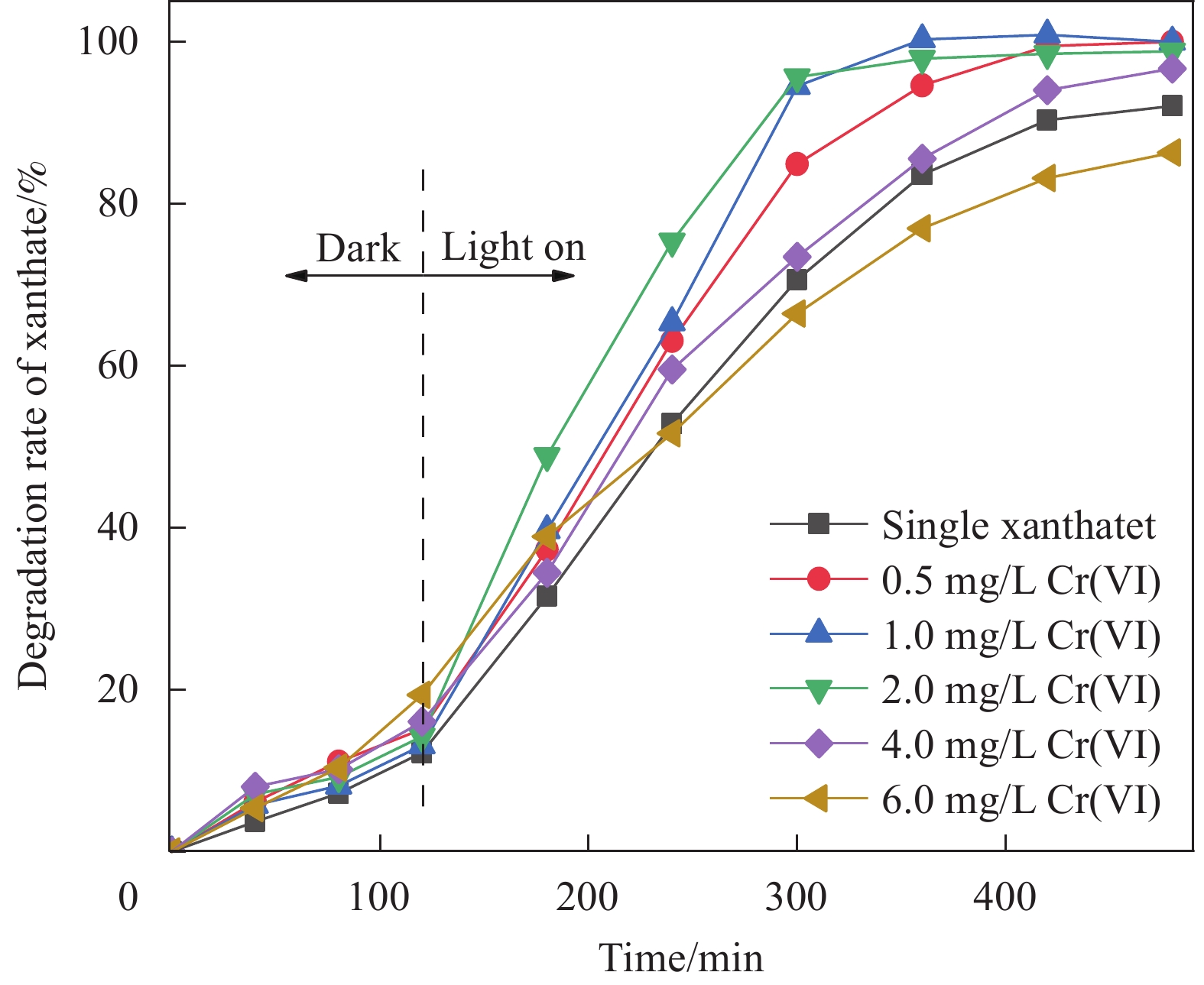
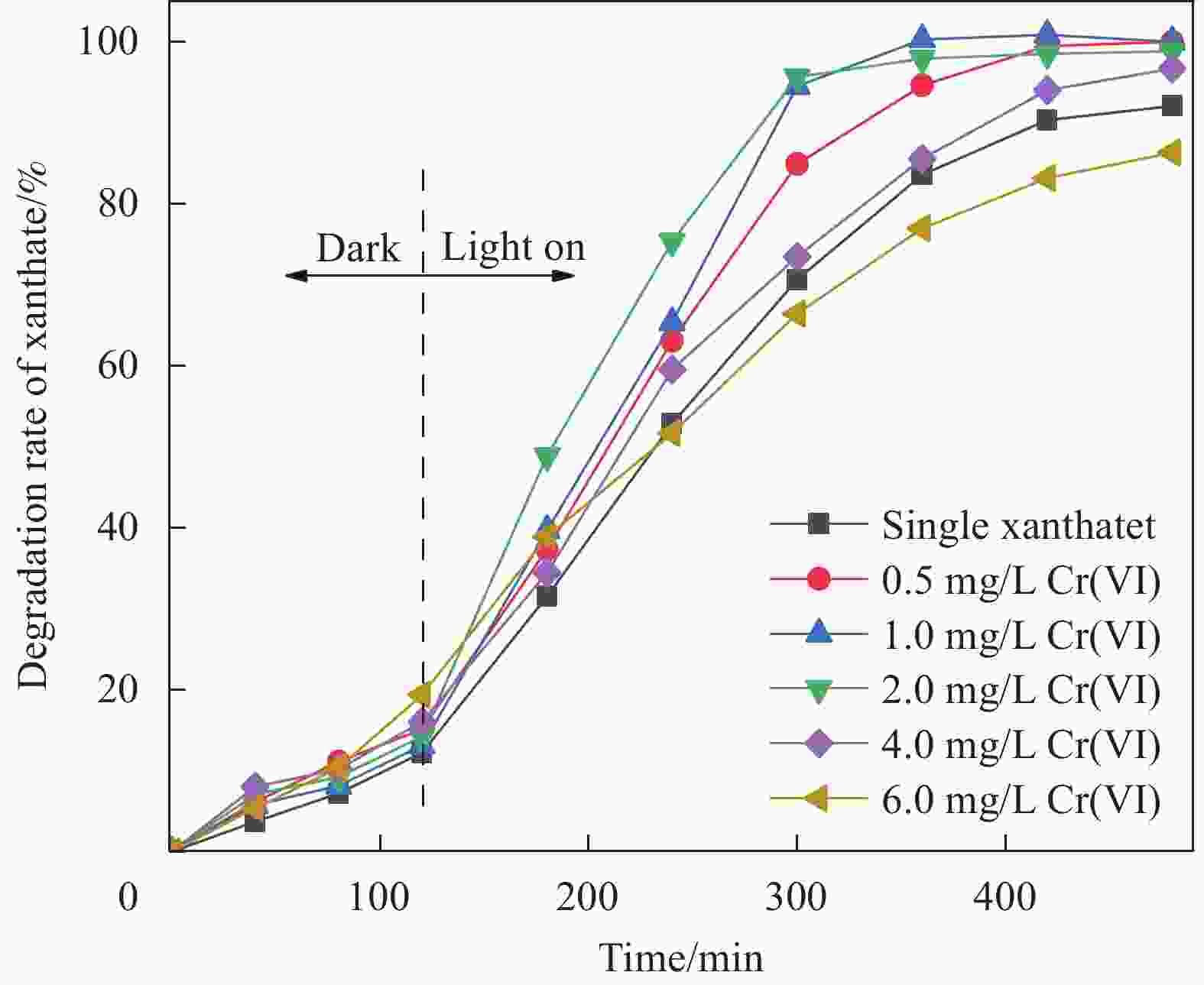
摘要: 为进一步研究黄药和Cr(VI)共存体系中,Cr(VI)对黄药光降解性能的影响以及两者协同作用机制,本文以煤矸石/钒酸铋(CG/BiVO4)为光催化剂,黄药和Cr(VI)共存体系为研究对象,通过光催化活性测试以及紫外光谱、红外光谱、离子色谱和猝灭实验等技术手段,深入研究黄药光氧化和Cr(VI)光还原过程以及两者之间的协同作用机制。结果表明,在黄药和Cr(VI)的共存体系中,两者之间存在显著的协同效应,当黄药浓度为25 mg/L、pH=7、催化剂投加量为1.5 g/L、Cr(VI)浓度为2.0 mg/L、反应480 min时,CG/BiVO4对黄药和Cr(VI)的去除率均达最佳,分别是98.81%和88.80%;基于响应面法预测得到共存体系中黄药的降解率为94.79%,与实际降解率相差 3.82%,该响应面模型可预测共存体系下黄药的降解过程;共存体系中黄药的C=S振动优先发生变化,其次为C—O—C、S—H、S—C—S和丁基,光反应3 h形成中间产物过黄药(ROCSSO−),7 h的转化率最高为97.94%;协同作用机制研究发现,Cr(VI)光还原会迅速捕捉光生e−,黄药光降解会大量消耗h+,两者在光反应过程中不断消耗光生电子和空穴,一方面可抑制光生电子和空穴对的复合,延长光生电子空穴对的寿命;另一方面光生电子-空穴对的快速消耗,加速了光能向化学能的转化,提高了可见光利用率的同时生成大量光生电子空穴对,进而促使黄药和Cr(VI)的协同去除。Abstract: In order to further study the effect of Cr(VI) on the photodegradation of xanthate and its synergistic mechanism in the co-existing system of xanthate and Cr(VI), the photocatalyst of coal gangue/bismuth vanadate (CG/BiVO4) was used, xanthate and Cr(VI) coexisting systems were studied by photocatalytic activity test, UV, FTIR, ion chromatography and quenching experiments, the photooxidation of xanthate and photoreduction of Cr(VI) and their synergistic mechanism were explored. The results show that there is a significant synergistic effect between the photo-oxidation of xanthate and photo-reduction of Cr(VI) in the co-existence system of xanthate and Cr(VI). Then, at the xanthate of 25 mg/L and the pH value of 7, with the dosage of catalyst being 1. 5 g/L, at the Cr(VI) of 2.0 mg/L, the removal rate of xanthate and Cr(VI) by CG/BiVO4 are the best during a period of 480 min, reaching 98.81% and 88.80% respectively. The predicted degradation rate of xanthate is 94.79% by response surface methodology, lower 3.82% than the actual degradation rate, which indicates that the model can be used to predict the degradation of xanthate in the co-existing system. In the co-existing system, the vibration of C=S is changed first, followed by C—O—C, S—H, S—C—S, butyl, and the intermediate product peroxy xanthate (ROCSSO−) is formed after the visible light illumination 3 h , the highest conversion of sulfur is reached 97.94% after the visible light illumination 7 h. The synergistic mechanism analysis shows that the photogenerated e− are rapidly captured in the photo-reduction of Cr(VI) and photogenerated h+ are consumed by xanthate photodegradation. The photogenic electron and hole pairs are consumed, on the one hand, due to the inhibition of photogenerated electrons and holes recombination, the lifetime of photogenerated electron and hole pairs are prolonged, on the other hand, the rapid consumption of photogenerated electron-hole pairs accelerates the conversion of light energy to chemical energy, generating a large number of photogenerated electron-hole pairs, therefor promoting the synergistic removal of xanthate and Cr(VI) .
-
Key words:
- Cr(VI) /
- xanthatet /
- photocatalysis /
- synergistic mechanism /
- degradation /
- removal
-
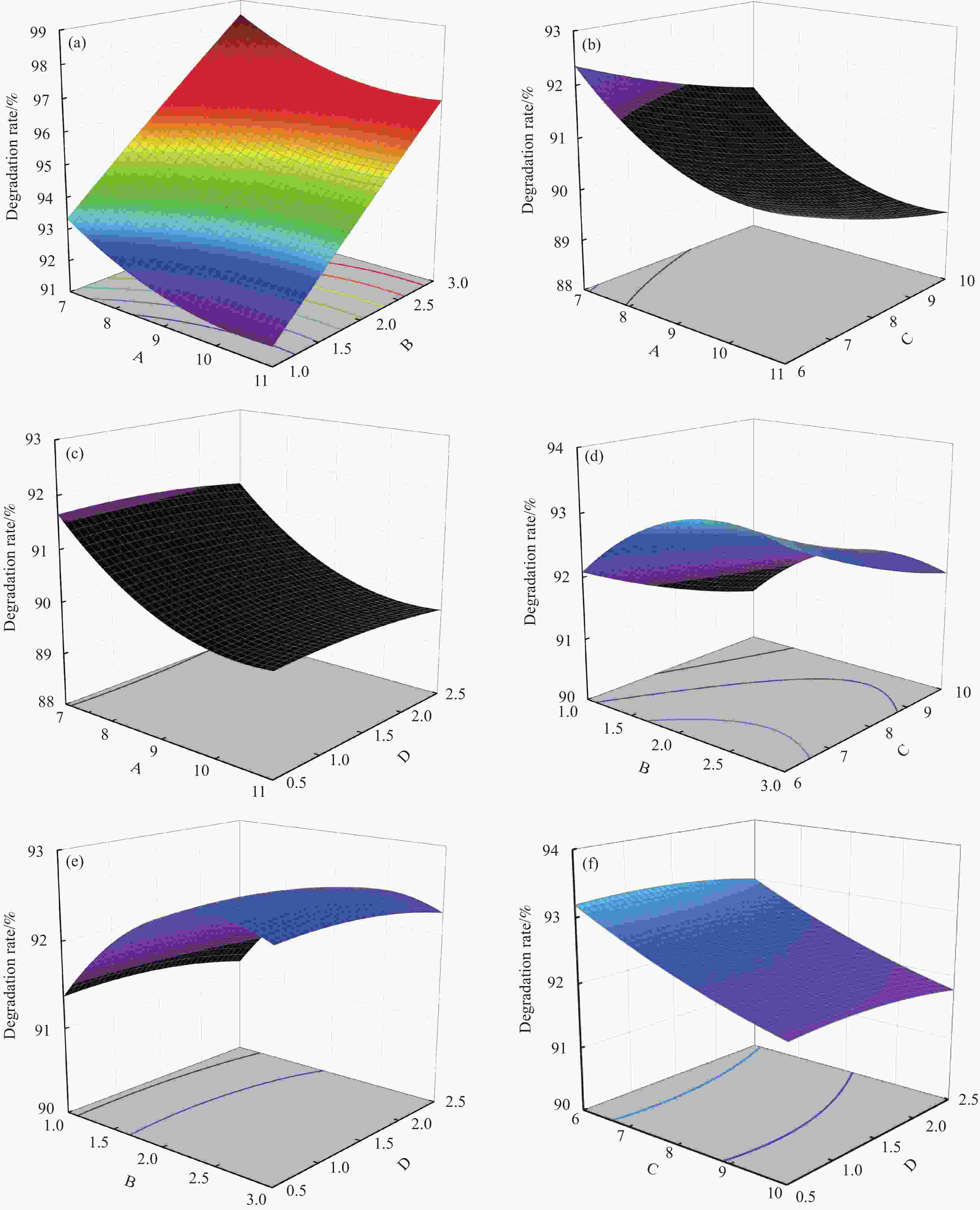
表 1 影响CG/BiVO4光降解黄药的因素及水平
Table 1. Factors and levels affecting CG/BiVO4 photodegradation of xanthate
Factor Lever −1 0 +1 pH 7 9 11 m/(g·L-1) 1 2 3 C0/(mg·L−1) 6 8 10 CCr/(mg·L−1) 0.5 1.5 2.5 Notes: m—Catalyst dosage; C0—Initial concentration of xanthate; CCr—Concentration of Cr(VI). 表 2 共存体系中优化CG/BiVO4光降解黄药实验回归模型的方差分析
Table 2. Variance analysis of regression model in optimizing CG/BiVO4 photodegradation of xanthate in co-existing system
Source SS DF Mean square F value P value prob>F Model 22.73 14 1.62 236.55 <0.0001 A 9.36 1 9.36 1364.23 <0.0001 B 3.47 1 3.47 505.12 <0.0001 C 4.38 1 4.38 638.19 <0.0001 D 0.12 1 0.12 16.91 0.0011 AB 6.75 1 6.75 9.87 0.9527 AC 0 1 0 0 1.0000 AD 6.75 1 6.75 9.87 0.9527 BC 0 1 0 0 1.0000 BD 0 1 0 0 1.0000 CD 6.75 1 6.75 9.87 0.9527 A2 2.12 1 2.12 309.12 <0.0001 B2 2.12 1 2.12 309.03 <0.0001 C2 0.072 1 0.072 10.55 0.0058 D2 0.066 1 0.066 9.56 0.0080 Residual 0.096 14 18.63 Lack of fit 0.089 10 24.06 4.74 0.0736 Pure error 20.3 4 5.05 Cor total 22.83 28 Notes: A—Initial pH of the reaction; B—Catalyst dosage; C—Initial concentration of xanthate; D—Cr(VI) concentration; SS—Sum of squares; DF—Degree of freedom; F value—Ratio of the mean square to the residual term; P value prob—Influence degree value of each factor. -
[1] 郑永兴, 黄宇松, 吕晋芳, 等. 有色金属选矿废水处理研究现状与进展[J]. 矿产综合利用, 2023(240):177-183, 190.ZHENG Yongxing, HUANG Yusong, LYU Jinfang, et al. Research status and progress on the treatment of non-ferrous metal dressing wastewater[J]. Comprehensive Utilization of Mineral Resources,2023(240):177-183, 190(in Chinese). [2] ROY S, DATTA A, REHANI S. Flotation of copper sulphide from copper smelter slag using multiple collectors and their mixtures[J]. International Journal of Mineral Processing,2015,143:43-49. doi: 10.1016/j.minpro.2015.08.008 [3] 张文茜, 张婧, 王海洋, 等. 选矿废水中黄药的处理方法研究进展[J]. 广东化工, 2022, 49(20):130-132.ZHANG Wenqian, ZHANG Jing, WANG Haiyang, et al. Research progress on the treatment of xanthate in mineral processing wastewater[J]. Guangdong Chemical,2022,49(20):130-132(in Chinese). [4] 吴吉昀, 冯博, 陈燕, 等. 粉煤灰-硅藻土复合材料对选矿废水中Cr(VI)的吸附行为研究[J]. 矿冶工程, 2022, 42(4):125-129. doi: 10.3969/j.issn.0253-6099.2022.04.029WU Jiyun, FENG Bo, CHEN Yan, et al. Adsorption behavior of fly ash-diatomite composite for Cr(VI) in mineral processing wastewater[J]. Mining and Metallurgical Engineering,2022,42(4):125-129(in Chinese). doi: 10.3969/j.issn.0253-6099.2022.04.029 [5] MOHAMED R M, IBRAHIM F M. Vsible light photocataly-tic reduction of nitrobenzene using Ag/Bi2MoO6 nanocomposite[J]. Journal of Industrial and Engineering Chemistry,2015,22:28-33. doi: 10.1016/j.jiec.2014.06.021 [6] 张明慧, 崔石岩, 刘凤春, 等. 石墨烯-TiO2复合材料可见光催化降解乙黄药[J]. 金属矿山, 2020(2):129-133.ZHANG Minghui, CUI Shiyan, LIU Fengchun, et al. Photocatalytic degradation of ethyl xanthateby graphite-TiO2 composite under visible light irradiation[J]. Metal Mines,2020(2):129-133(in Chinese). [7] WANG J C, REN J, YAO H C, et al. Synergistic photocatalysis of Cr(VI) reduction and 4-chlorophenol degradation over hydroxylated-Fe2O3 under visible light irradiation[J]. Journal of Hazardous Materials,2016,311:11-19. doi: 10.1016/j.jhazmat.2016.02.055 [8] 唐双, 张雪乔, 蒋莉萍, 等. 煤矸石/BiVO4复合光催化剂的制备及其对黄药废水的降解[J]. 复合材料学报, 2023, 40(12): 6703-6717.TANG Shuang, ZHANG Xueqiao, JIANG Liping, et al. Preparation of coal gangue/BiVO4 composite photocatalyst and its degradation of xanthate wastewater [J]. Acta Mate-riae Compositae Sinica, 2023, 40(12): 6703-6717(in Chinese). [9] SHEN Q, ZHANG Y H, FAN Y J, et al. On-line in situ ATR-FTIR study on the adsorption behavior of heptyl xanthate on ZnO and Cu(II) activated ZnO surface[J]. Transactions of Nonferrous Metals Society of China,2022,32(7):2370-2378. doi: 10.1016/S1003-6326(22)65953-3 [10] 李云红, 林雪梅, 张伟亚. 二苯碳酰二肼分光光度法测定实验废水中六价铬含量的研究[J]. 环境科学与管理, 2022, 47(8):111-115. doi: 10.3969/j.issn.1673-1212.2022.08.024LI Yunhong, LIN Xuemei, ZHANG Weiya. Determination of hexavalent chromium in experimental wastewater by diphenylcarbazide spectrophotometer[J]. Environmental Science and Management,2022,47(8):111-115(in Chinese). doi: 10.3969/j.issn.1673-1212.2022.08.024 [11] ZHU C S, LI J Y, CHAI Y K, et al. Synergistic Cr(VI) reduction and chloramphenicol degradation by the visible-light-induced photocatalysis of CuInS2: Performance and reaction mechanism[J]. Frontiers in Chemistry, 2022, 10: 964008. [12] ZHAO X, DU P, CAI Z, et al. Photocatalysis of bisphenol A by an easy-settling titania/titanate composite: Effects of water chemistry factors, degradation pathway and theore-tical calculation[J]. Environmental Pollution,2018,232:580-590. doi: 10.1016/j.envpol.2017.09.094 [13] 孙思琦, 黄齐茂. 新型黄原酸盐重金属离子螯合剂的合成[J]. 工业水处理, 2020, 40(11):41-44.SUN Siqi, HUANG Qimao. Synthesis of a new xanthate heavy metal ion chelating agent[J]. Industrial Water Treatment,2020,40(11):41-44(in Chinese). [14] 曹阳, 王楷, 王湖坤. 重金属捕集剂丁基黄药处理电镀废水的研究[J]. 电镀与精饰, 2014, 36(10):43-46. doi: 10.3969/j.issn.1001-3849.2014.10.011CAO Yang, WANG Kai, WANG Hukun. Study on the treatment of electroplating wastewater with butyl xanthate[J]. Plating and Finishing,2014,36(10):43-46(in Chinese). doi: 10.3969/j.issn.1001-3849.2014.10.011 [15] D.W.贝宁, 俞继华. 黄药的水毒性与环境结局[J]. 国外选矿快报, 1999(18):18-20.D.W.Benin, YU Jihua. Water toxicity and environmental consequences of xanthate[J]. Foreign Mineral Processing Express,1999(18):18-20(in Chinese). [16] LUO L, WANG G, WANG Z, et al. Optimization of Fenton process on removing antibiotic resistance genes from excess sludge by single-factor experiment and response surface methodology[J]. Science of the Total Environment,2021,788:147889. doi: 10.1016/j.scitotenv.2021.147889 [17] LI M, ZHAO G, LIU J, et al. Optimization of ultrasound-assisted extraction of peony seed oil with response surface methodology and analysis of fatty acid[J]. Agricultural Research, 2021, 10: 543-555. [18] 邓萍. Gaussian软件在有机化合物波谱解析教学中的应用(II)−吲哚紫外光谱及其跃迁轨道的可视化[J]. 化学教育, 2017, 38(8):66-68.DENG Ping. Application of Gaussian software in the teaching of spectral analysis of organic compounds (II)—Visualization of indole ultraviolet spectra and their transition trajectories[J]. Chemistry Education,2017,38(8):66-68(in Chinese). [19] 刘嘉友, 聂倩倩, 俞和胜, 等. 卷心菜状Bi2WO6光催化降解黄药废水[J]. 金属矿山, 2020(524):122-128.LIU Jiayou, NIE Qianqian, YU Hesheng, et al. Photocatalytic degradation of xanthate wastewater[J]. Metal Mines,2020(524):122-128(in Chinese). [20] HU C, TANG Y, JIMMY C Y, et al. Photocatalytic degradation of cationic blue X-GRL adsorbed on TiO2/SiO2 photocatalyst[J]. Applied Catalysis B: Environmental,2003,40(2):131-140. doi: 10.1016/S0926-3373(02)00147-9 [21] RENZI C, GUILLARD C, HERRMANN J M, et al. Effects of methanol, formamide, acetone and acetate ions on phenol disappearance rate and aromatic products in UV-irra-diated TiO2 aqueous suspensions[J]. Chemosphere,1997,35(4):819-826. doi: 10.1016/S0045-6535(97)00203-8 [22] GUO Y, CUI K, HU M, et al. Fe(III) ions enhanced catalytic properties of (BiO)2CO3 nanowires and mechanism study for complete degradation of xanthate[J]. Chemosphere,2017,181:190-196. doi: 10.1016/j.chemosphere.2017.04.069 [23] HAO F P, SILVESTER E, SENIOR G D. Spectroscopic characterization of ethyl xanthate oxidation products and analysis by ion interaction chromatography[J]. Analytical Chemistry,2000,72(20):4836-4845. doi: 10.1021/ac991277o [24] 刘楚玉, 黄自力, 袁晨光, 等. 磁性活性炭的制备及其对选矿废水中丁基黄药的去除研究[J]. 矿冶工程, 2022, 42(3):70-75. doi: 10.3969/j.issn.0253-6099.2022.03.016LIU Chuyu, HUANG Zili, YUAN Chenguang, et al. Preparation of magnetic activated carbon and its removal of butyl xanthate from mineral processing wastewater[J]. Mining and Metallurgical Engineering,2022,42(3):70-75(in Chinese). doi: 10.3969/j.issn.0253-6099.2022.03.016 [25] 葛东来, 范迎菊, 尹龙, 等. 连续在线原位ATR-FTIR技术测定介孔CuAl2O4对黄药的吸附[J]. 物理化学学报, 2013, 29(2): 371-376.GE Donglai, FAN Yingju, YIN Long, et al. Continuous on-line in situ ATR-FTIR determination of CuAl2O4 adsorption on xanthate[J]. Journal of Physical Chemistry, 2013, 29(2): 371-376(in Chinese). [26] MORITA S, SHINZAWA H, TSENKOVA R, et al. Computational simulations and a practical application of moving-window two-dimensional correlation spectroscopy[J]. Journal of Molecular Structure,2006,799(1-3):111-120. doi: 10.1016/j.molstruc.2006.03.023 [27] DOU L, MA D, CHEN J, et al. F127-assisted hydrothermal preparation of BiOI with enhanced sunlight-driven photocatalytic activity originated from the effective separation of photo-induced carriers[J]. Solid State Sciences,2019,90:1-8. doi: 10.1016/j.solidstatesciences.2019.01.010 [28] GUO M, XIANG H, TANG S, et al. Effect of pyrolysis tempe-rature on structure and photocatalytic properties of biochar-coupled BiVO4[J]. Journal of Environmental Chemical Engineering,2022,10(2):107255. doi: 10.1016/j.jece.2022.107255 [29] JAWAD A, LU X, CHEN Z, et al. Degradation of chlorophenols by supported Co-Mg-Al layered double hydrotalcite with bicarbonate activated hydrogen peroxide[J]. The Journal of Physical Chemistry A,2014,118(43):10028-10035. doi: 10.1021/jp5085313 [30] 杨宏剑, 薛秀玲, 付旺. 过硫酸盐氧化剂对Al0/O2/H+体系降解TC的协同作用[J]. 环境化学, 2020, 39(9):2584-2592. doi: 10.7524/j.issn.0254-6108.2019062305YANG Hongjian, XUE Xiuling, FU Wang. Synergistic effect of persulfate oxidant on the degradation of TC in Al0/O2/H+ system[J]. Environmental Chemistry,2020,39(9):2584-2592(in Chinese). doi: 10.7524/j.issn.0254-6108.2019062305 [31] PAN L, WAN Z, FENG Q, et al. Biofilm response and removal via the coupling of visible-light-driven photocataly-sis and biodegradation in an environment of sulfamethoxazole and Cr(VI)[J]. Journal of Environmental Sciences,2022,122(12):50-61. [32] 李官超, 祝瑄, 滕青, 等. TiO2@芽孢杆菌光催化性能研究[J]. 金属矿山, 2021(8):186-195.LI Guanchao, ZHU Xuan, TENG Qing, et al. Photocatalytic activity of TiO2@bacillus[J]. Metal Mines,2021(8):186-195(in Chinese). [33] 崔陪陪, 胡芸, 黄倩倩, 等. MIL-101光催化剂对Cr(VI)-RhB复合污染的定向分离及其高效光催化协同处理[J]. 化工进展, 2018, 37(7):2860-2866.CUI Peipei, HU Yun, HUANG Qianqian, et al. Directional separation of Cr(VI)-RhB compound pollution by MIL-101 photocatalyst and its high efficient photocatalytic synergistic treatment[J]. Progress in Chemical Engineering,2018,37(7):2860-2866(in Chinese). [34] 李红艳, 李玉鉴, 崔建国, 等. rGO/TNTs光催化剂协同降解水中Cr(VI)与苯酚的性能[J]. 工业水处理, 2019, 39(10):32-36. doi: 10.11894/iwt.2019-0166LI Hongyan, LI Yujian, CUI Jianguo, et al. Photocatalytic degradation of Cr(VI) and phenol in water by rGO/TNTs photocatalyst[J]. Industrial Water Treatment,2019,39(10):32-36(in Chinese). doi: 10.11894/iwt.2019-0166 [35] 陈运双, 马瑞雪, 蒋潇宇, 等. TiO2/蒙脱土复合材料光催化降解丁基黄药性能研究[J]. 金属矿山, 2022(5):212-220.CHEN Yunshuang, MA Ruixue, JIANG Xiaoyu, et al. Photocatalytic degradation of butyl xanthate by TiO2/montmorillonite composite[J]. Metal Mines,2022(5):212-220(in Chinese). -





 下载:
下载: