Preparation of magnetic carbon nanotubes and their application in tumor cell photo-thermal therapy and magnetic resonance imaging
-
摘要: 肿瘤是目前最主要的致死原因之一,实现对肿瘤的精准和非侵入性高效诊疗具有重要意义。以具有极高长径比、易于穿透细胞膜并具有优异生物相容性的碳纳米管(CNTs)作为载体,以乙酰丙酮铁为铁源,通过溶剂热法在其表面原位生长具有超顺磁特性的四氧化三铁纳米粒子(Fe3O4 NPs),制备了具有优异水分散稳定性的磁性碳纳米管复合纳米材料。结果表明该磁性碳纳米管具有较高的近红外光热转换性能,在50 μg·mL−1浓度下808 nm激光照射10 min即可升温至48.6℃,且具有良好的光热稳定性。细胞及成像实验结果表明该复合纳米材料具有较好的生物相容性并对人宫颈癌细胞(HeLa)具有优异的光热杀伤效果,在体外模拟肿瘤微环境中磁共振成像(MRI) T2弛豫率r2可达215.61 mmol−1·L·s−1,表明制备的磁性碳纳米管具有出色的生物安全性、磁性和光热特性,有望用于磁靶向的肿瘤光热疗与磁共振成像的一体化诊疗。
-
关键词:
- 碳纳米管 /
- 磁性Fe3O4纳米粒子 /
- 光热治疗 /
- 磁共振成像 /
- 肿瘤细胞
Abstract: Tumors are one of the leading causes of death in the world, and achieving precise and non-invasive efficient diagnosis and treatment of tumors is of great significance. We used carbon nanotubes (CNTs) with extremely high aspect ratio, easy to penetrate cell membrane and excellent biocompatibility as carriers, and acetylacetone iron as iron source, to synthesize magnetic carbon nanotube composite nanomaterials with excellent water dispersion stability by in situ growing superparamagnetic ferric oxide nanoparticles (Fe3O4 NPs) on their surface through solvothermal method. The results showed that the magnetic carbon nanotubes had high near-infrared photothermal conversion performance, and could reach 48.6℃ in 10 min under 808 nm laser irradiation at a concentration of 50 μg·mL−1, and had good photothermal stability. Cell and imaging experiments showed that the composite nanomaterials had good biocompatibility and excellent photothermal killing effect on human cervical cancer cells (HeLa). In vitro simulated tumor microenvironment, the magnetic resonance imaging (MRI) T2 relaxation rate r2 of the magnetic carbon nanotubes was up to 215.61 mmol−1·L·s−1, indicating that the prepared magnetic carbon nanotubes had outstanding biosafety, magnetism and photothermal properties, and were expected to be applied to the integration of magnetic targeted tumor photothermal therapy and magnetic resonance imaging. -
图 1 (a) 羧化截短的多壁碳纳米管(cut-MWNTs)的TEM图像;(b) Fe3O4-MWNTs复合纳米材料(M@Fe3O4)的TEM图像;(c) 局部放大的M@Fe3O4 TEM图像;(d) M@Fe3O4表面Fe3O4纳米粒子粒径统计(200个颗粒);(e) cut-MWNTs和M@Fe3O4的表面zeta电势;(f) M@Fe3O4水分散液在磁场作用下聚集;(g) M@Fe3O4水分散液放置5天前后的光学照片
Figure 1. (a) TEM image of carboxylated truncated multi-walled carbon nanotubes (cut-MWNTs); (b) TEM image of Fe3O4-MWNTs (M@Fe3O4); (c) TEM image of locally magnified M@Fe3O4; (d) Particle size statistics of iron tetroxide nanoparticles on the surface of M@Fe3O4 (200 particles); (e) Surface zeta potential of cut-MWNTs and M@Fe3O4; (f) M@Fe3O4 aqueous dispersions aggregated under magnetic field; (g) Photographs of M@Fe3O4 aqueous dispersion at 0 day and 5 days
图 2 (a) cut-MWNTs和M@Fe3O4的紫外可见吸收光谱;(b) M@Fe3O4的XRD图谱;(c) M@Fe3O4的XPS全谱;(d) M@Fe3O4的XPS Fe2p谱;(e) M@Fe3O4的磁滞回线
Figure 2. (a) UV-visible absorption spectrum of cut-MWNTs and M@Fe3O4; (b) XRD spectrum of M@Fe3O4; (c) XPS survey spectrum of M@Fe3O4; (d) XPS Fe2p spectrum of M@Fe3O4; (e) Magnetic hysteresis curves measured for M@Fe3O4
图 3 光热性能:(a) M@Fe3O4在不同浓度和激光辐照功率下的光热升温曲线(A:150 μg·mL−1、1.5 W·cm−2;B:100 μg·mL−1、1.5 W·cm−2;C:50 μg·mL−1、1.5 W·cm−2;D:50 μg·mL−1、1.0 W·cm−2;E:20 μg·mL−1、0.5 W·cm−2);(b) cut-MWNTs和M@Fe3O4在808 nm激光下的光热升温曲线,浓度为50 μg·mL−1,激光功率为1.5 W·cm−2;(c) M@Fe3O4在1.5 W·cm−2下的循环升温-降温曲线
Figure 3. Photothermal properties: (a) Photothermal heating curves of M@Fe3O4 at different concentrations and laser irradiation power (A: 150 μg·mL−1, 1.5 W·cm−2; B: 100 μg·mL−1, 1.5 W·cm−2; C: 50 μg·mL−1, 1.5 W·cm−2; D: 50 μg·mL−1, 1.0 W·cm−2; E: 20 μg·mL−1, 0.5 W·cm−2); (b) Photothermal heating curves of cut-MWNTs and M@Fe3O4 at 808 nm laser, 50 μg·mL−1, Laser power is 1.5 W·cm−2; (c) Cyclic heating-cooling curve of M@Fe3O4 at 1.5 W·cm−2
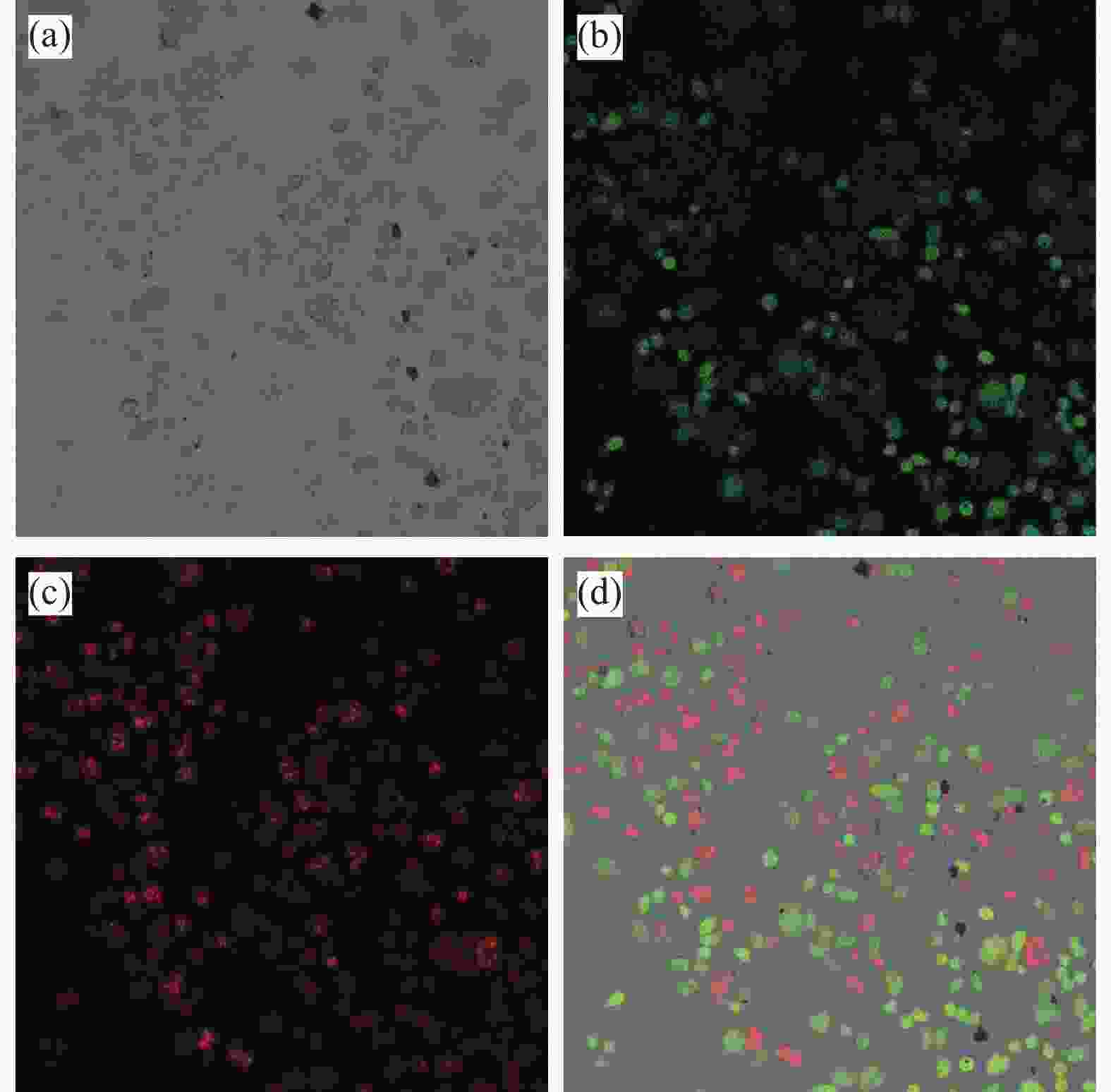
图 6 M@Fe3O4与HeLa细胞共孵育经过活死细胞双染后的共聚焦扫描图像:(a) 明场图像;(b) 活细胞图像;(c) 死细胞图像;(d) 明场和暗场叠加图像
Figure 6. Confocal scanning images of M@Fe3O4 coincubated with HeLa cells after double staining with live-dead cells: (a) Bright field image; (b) Live cell image; (c) Dead cell image; (d) Bright field and dark field superimposed image
-
[1] SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2020[J]. CA: A Cancer Journal for Clinicians,2020,70(1):7-30. doi: 10.3322/caac.21590 [2] JANG B, KWON H, KATILA P, et al. Dual delivery of biological therapeutics for multimodal and synergistic cancer therapies[J]. Advanced Drug Delivery Reviews,2016,98:113-133. doi: 10.1016/j.addr.2015.10.023 [3] MENG Z Q, WEI F, WANG R H, et al. NIR-laser-switched in vivo smart nanocapsules for synergic photothermal and chemotherapy of tumors[J]. Advanced Materials,2016,28(2):245-253. doi: 10.1002/adma.201502669 [4] JORDAN M A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin[J]. Current Medicinal Chemistry-Anti-Cancer Agents,2002,2(1):1-17. doi: 10.2174/1568011023354425 [5] MANSOORI B, MOHAMMADI A, DAVUDIAN S, et al. The different mechanisms of cancer drug resistance: A brief review[J]. Advanced Pharmaceutical Bulletin,2017,7(3):339-348. doi: 10.15171/apb.2017.041 [6] CHEN G Y, QIU H L, PRASAD P N, et al. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics[J]. Chemical Reviews,2014,114(10):5161-5214. doi: 10.1021/cr400425h [7] YUAN Y Y, LIU J, LIU B. Conjugated-polyelectrolyte-based polyprodrug: Targeted and image guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source[J]. Angewandte Chemie International Edition in English,2014,53(28):7163-7168. [8] DING Y X, XU Y J, YANG W Z, et al. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy[J]. Nano Today,2020,35:100970. doi: 10.1016/j.nantod.2020.100970 [9] SHI J J, KANTOFF P W, WOOSTER R, et al. Cancer nanomedicine: Progress, challenges and opportunities[J]. Nature Reviews Cancer,2017,17(1):20-37. doi: 10.1038/nrc.2016.108 [10] DING J X, CHEN J J, GAO L Q, et al. Engineered nanomedicines with enhanced tumor penetration[J]. Nano Today, 2019, 29: 100800. [11] LIU Y J, BHATTARAI P, DAI Z F, et al. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer[J]. Chemical Society Reviews,2019,48(7):2053-2108. doi: 10.1039/C8CS00618K [12] ABIOLA T T, RIOUX B, TOLDO J M, et al. Towards developing novel and sustainable molecular light-to-heat converters[J]. Chemical Science,2021,12(46):15239-15252. doi: 10.1039/D1SC05077J [13] WANG J P, SUN J Y, WANG Y H, et al. Gold nanoframeworks with mesopores for Raman-photoacoustic imaging and photo-chemo tumor therapy in the second near-infrared biowindow[J]. Advanced Functional Materials, 2020, 30(9): 1908825. [14] ZHOU Z G, SUN Y N, SHEN J C, et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy[J]. Biomaterials,2014,35(26):7470-7478. doi: 10.1016/j.biomaterials.2014.04.063 [15] XI D M, XIAO M, CAO J F, et al. NIR light-driving barrier-free group rotation in nanoparticles with an 88.3% photothermal conversion efficiency for photothermal therapy[J]. Advanced Materials, 2020, 32(11): 1907855. [16] MODUGNO G, MÉNARD-MOYON C, PRATO M, et al. Carbon nanomaterials combined with metal nanoparticles for theranostic applications[J]. British Journal of Pharmacology,2015,172(4):975-991. [17] GU Z J, ZHU S A, YAN L A, et al. Graphene-based smart platforms for combined cancer therapy[J]. Advanced Materials,2019,31(9):1800662. doi: 10.1002/adma.201800662 [18] RYU T K, BAEK S W, KANG R H, et al. Selective photothermal tumor therapy using nanodiamond-based nanoclusters with folic acid[J]. Advanced Functional Materials,2016,26(35):6428-6436. doi: 10.1002/adfm.201601207 [19] LYU Z Q, HE S J, WANG Y F, et al. Noble metal nanomaterials for NIR-triggered photothermal therapy in cancer[J]. Advanced Healthcare Materials,2021,10(6):2001806. doi: 10.1002/adhm.202001806 [20] LI S S, GU K, WANG H, et al. Degradable holey palladium nanosheets with highly active 1D nanoholes for synergetic phototherapy of hypoxic tumors[J]. Journal of the American Chemical Society,2020,142(12):5649-5656. [21] ZHANG Y J, SHA R, ZHANG L, et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer[J]. Nature Communications,2018,9:4236. doi: 10.1038/s41467-018-06529-y [22] JIANG Y Y, LI J C, ZHEN X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: A comparative study[J]. Advanced Materials,2018,30(14):1705980. doi: 10.1002/adma.201705980 [23] LI Y W, LIU Z T, MA Y F, et al. Semiconducting nanocomposite with AIEgen-triggered enhanced photoluminescence and photodegradation for dual-modality tumor imaging and therapy[J]. Advanced Functional Materials,2019,29(38):1903733. doi: 10.1002/adfm.201903733 [24] LI J C, YU X R, JIANG Y Y, et al. Second near-infrared photothermal semiconducting polymer nanoadjuvant for enhanced cancer immunotherapy[J]. Advanced Materials,2021,33(4):2003458. doi: 10.1002/adma.202003458 [25] WANG J, SU X Q, ZHAO P X, et al. Cancer photothermal therapy based on near infrared fluorescent CdSeTe/ZnS quantum dots[J]. Analytical Methods,2021,13(45):5509-5515. doi: 10.1039/D1AY01635K [26] UZHYTCHAK M, SMOLKOVÁ B, LUNOVA M, et al. Lysosomal nanotoxicity: Impact of nanomedicines on lysosomal function.[J]. Advanced Drug Delivery Reviews,2023,197:114828. doi: 10.1016/j.addr.2023.114828 [27] LIU H J, LI C W, QIAN Y, et al. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window[J]. Biomaterials,2020,232:119700. doi: 10.1016/j.biomaterials.2019.119700 [28] JENA P V, ROXBURY D, GALASSI T V, et al. A carbon nanotube optical reporter maps endolysosomal lipid flux[J]. ACS Nano,2017,11(11):10689-10703. doi: 10.1021/acsnano.7b04743 [29] JENA P V, SAFAEE M M, HELLER D A, et al. DNA-carbon nanotube complexation affinity and photoluminescence modulation are independent[J]. ACS Applied Materials & Interfaces,2017,9(25):21397-21405. [30] WANG D Q, MENG L J, FEI Z F, et al. Multi-layered tumor-targeting photothermal-doxorubicin releasing nanotubes eradicate tumors in vivo with negligible systemic toxicity[J]. Nanoscale,2018,10(18):8536-8546. doi: 10.1039/C8NR00663F [31] ZHANG M, WANG W T, WU F, et al. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice[J]. Carbon,2017,123:70-83. doi: 10.1016/j.carbon.2017.07.032 [32] MENG L J, XIA W J, LIU L, et al. Golden single-walled carbon nanotubes prepared using double layer polysaccharides bridge for photothermal therapy[J]. ACS Applied Materials & Interfaces,2014,6(7):4989-4996. doi: 10.1021/am406031n [33] WANG D Q, HOU C, MENG L J, et al. Stepwise growth of gold coated cancer targeting carbon nanotubes for the precise delivery of doxorubicin combined with photothermal therapy[J]. Journal of Materials Chemistry B,2017,5(7):1380-1387. doi: 10.1039/C6TB02755E [34] WANG D Q, REN Y B, SHAO Y P, et al. Facile preparation of doxorubicin-loaded and folic acid-conjugated carbon nanotubes@poly(N-vinyl pyrrole) for targeted synergistic chemo-photothermal cancer treatment[J]. Bioconjugate Chemistry,2017,28(11):2815-2822. doi: 10.1021/acs.bioconjchem.7b00515 [35] QIU Y Z, TONG S, ZHANG L L, et al. Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions[J]. Nature Communications,2017,8:15594. doi: 10.1038/ncomms15594 [36] SAADAT M, MANSHADI M K D, MOHAMMADI M, et al. Magnetic particle targeting for diagnosis and therapy of lung cancers[J]. Journal of Controlled Release,2020,10(328):776-791. [37] ZHOU Z J, SHEN Z Y, CHEN X Y, et al. Tale of two magnets: An advanced magnetic targeting system[J]. ACS Nano,2020,14(1):7-11. [38] BHATTACHARYA S, KIRAN RAJ M, PRIYADARSHANI J, et al. Targeting magnetic nanoparticles in physiologically mimicking tissue microenvironment[J]. ACS Applied Materials & Interfaces,2022,14(28):31689-31701. doi: 10.1021/acsami.2c07246 [39] HAIMOV-TALMOUD E, HAREL Y, SCHORI H, et al. Magnetic targeting of mTHPC to improve the selectivity and efficiency of photodynamic therapy[J]. ACS Applied Materials & Interfaces,2019,11(49):45368-45380. doi: 10.1021/acsami.9b14060 [40] PALANISAMY S, WANG Y M. Superparamagnetic iron oxide nanoparticulate system: Synthesis, targeting, drug delivery and therapy in cancer[J]. Dalton Transactions,2019,48(26):9490-9515. doi: 10.1039/C9DT00459A [41] JING X N, ZHI Z, ZHANG N, et al. Multistage tumor microenvironment-responsive theranostic nanopeanuts: Toward multimode imaging guided chemo-photodynamic therapy[J]. Chemical Engineering Journal,2020,385:123893. doi: 10.1016/j.cej.2019.123893 [42] JING X N, XU Y Z, LIU D M, et al. Intelligent nanoflowers: A full tumor microenvironment-responsive multimodal cancer theranostic nanoplatform[J]. Nanoscale,2019,11(33):15508-15518. doi: 10.1039/C9NR04768A -






 下载:
下载: