β-cyclodextrin modified magnetic palm fiber biochar for highly efficient Pb(II) removal from water
-
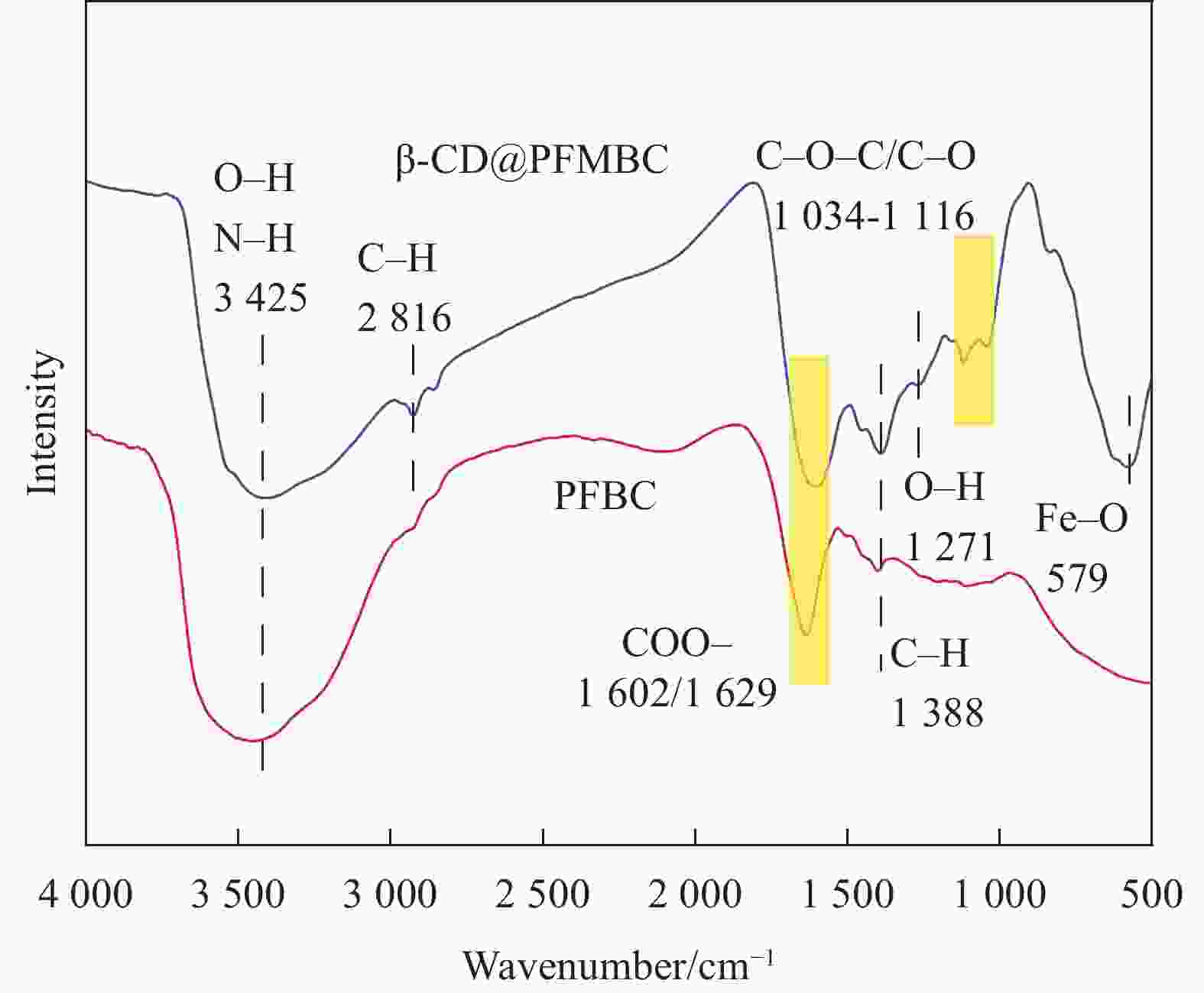
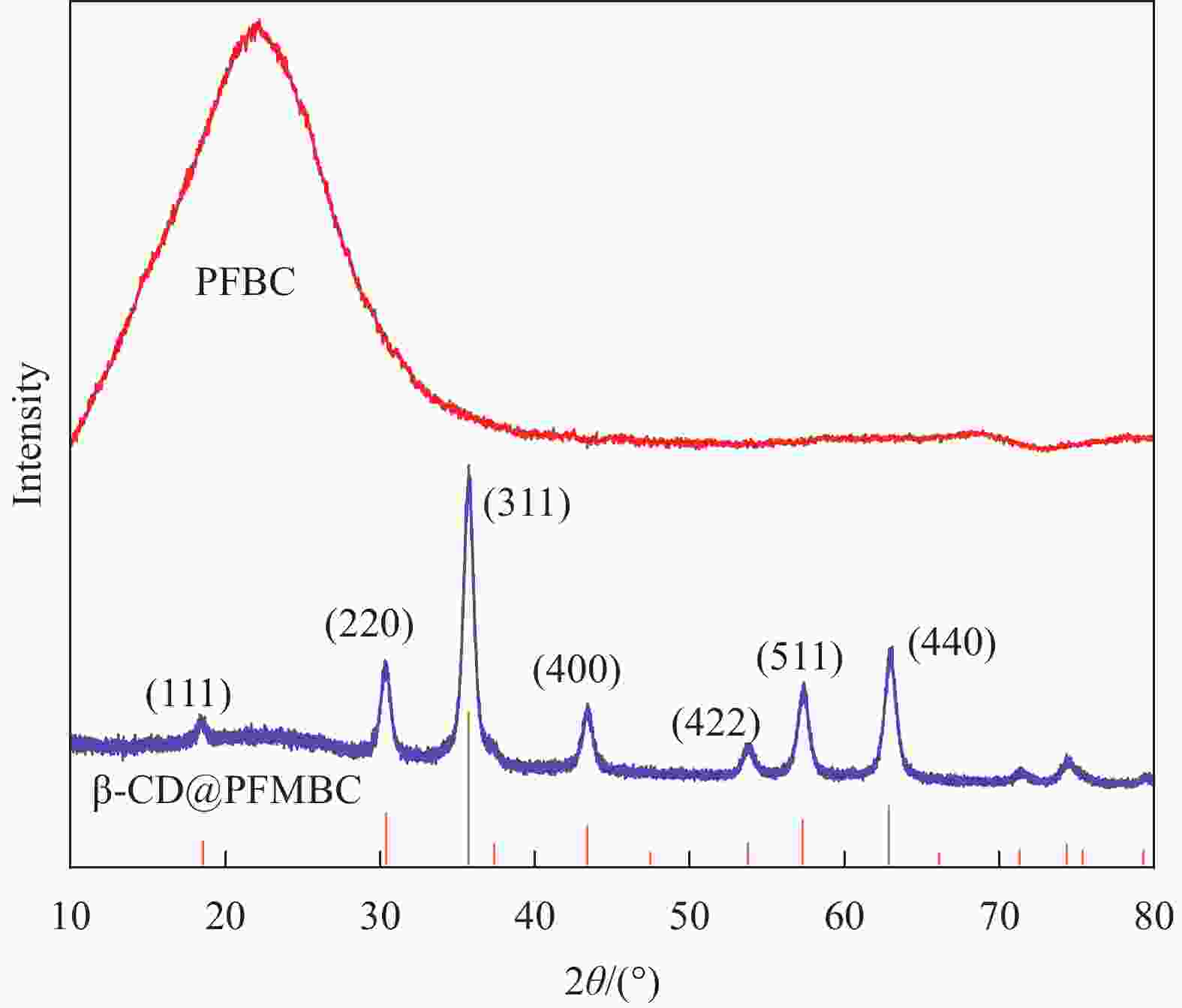
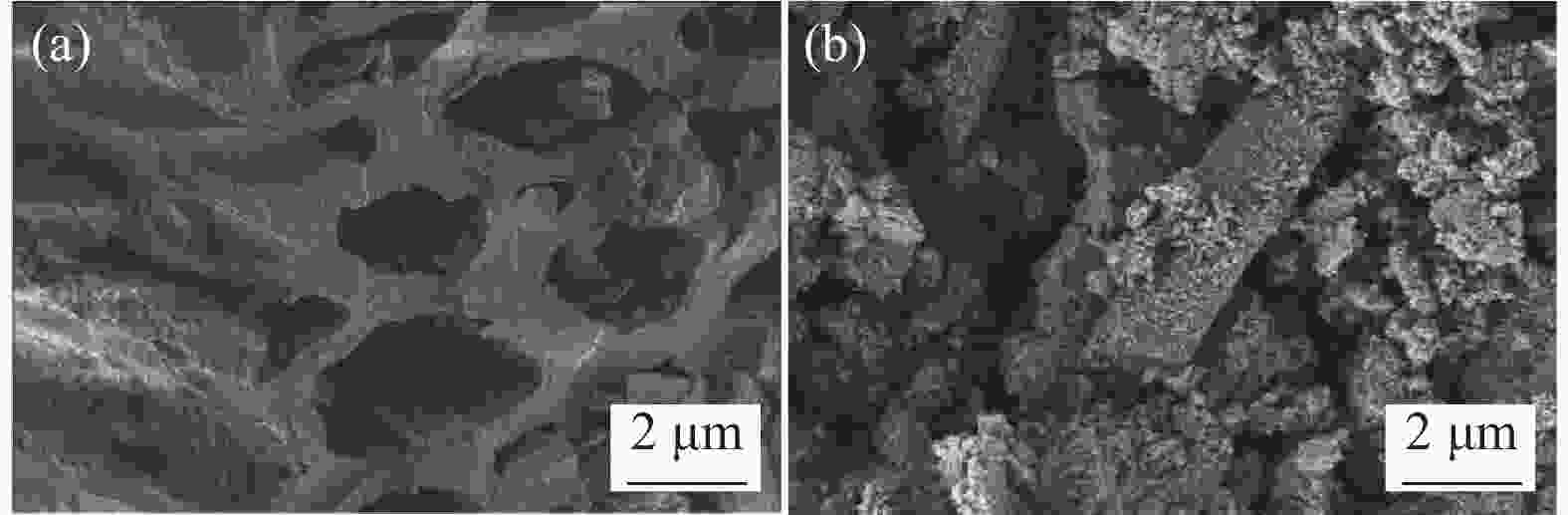
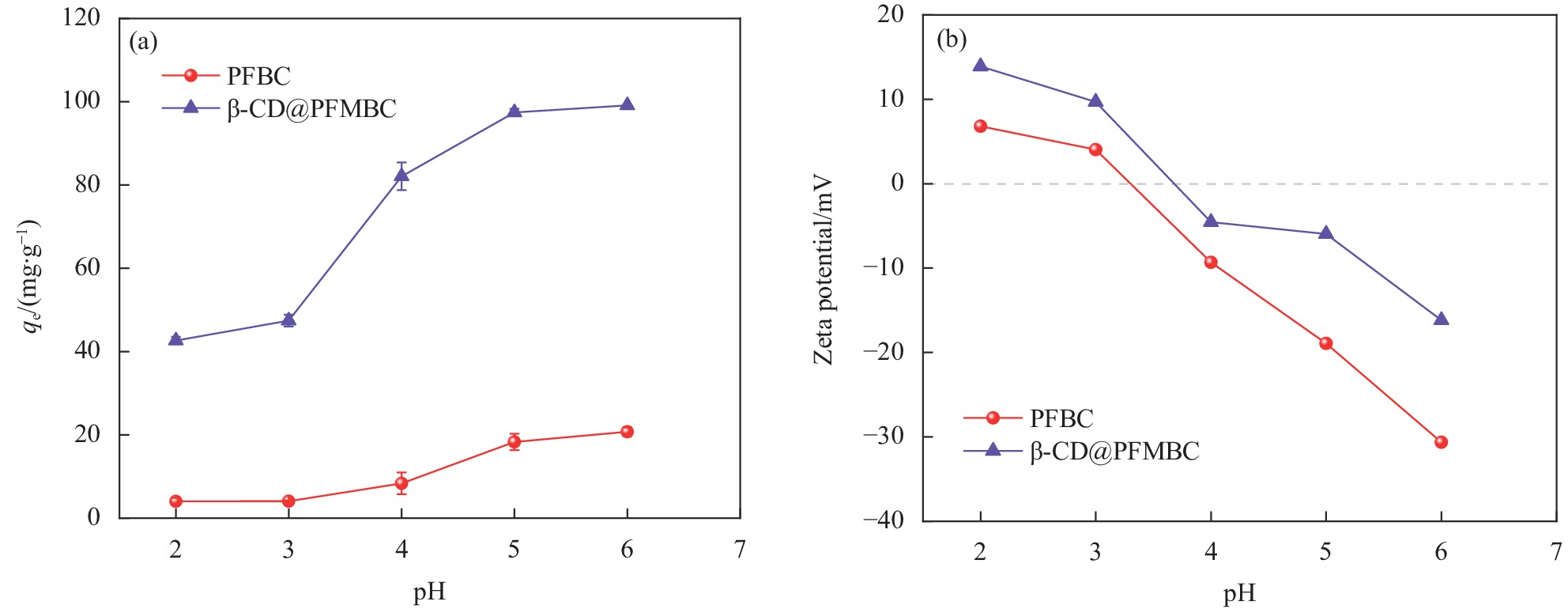
摘要: 为解决水体中重金属Pb(II)污染,本文以棕榈纤维为原材料,通过化学共沉淀法制备得到β-环糊精磁性棕榈纤维生物炭(β-CD@PFMBC)用于高效去除水溶液中的Pb(II)。通过FTIR、XRD、BET、SEM、Raman和VSM等手段对材料的结构和形貌进行了表征。通过单因素实验对Pb(II)的吸附性能进行了分析,探究了吸附剂对Pb(II)的吸附机制及回收利用性。结果表明:β-CD@PFMBC相比原始生物炭比表面积增加,表面官能团数量增多。拟二级动力学模型和Langmuir吸附等温线模型均能很好地描述对Pb(II)的吸附过程,表明吸附过程为化学吸附和单层吸附。由Langmuir吸附等温线模型拟合得知,β-CD@PFMBC在303 K时最大理论吸附量为625.49 mg∙g−1,明显高于原始生物炭。热力学研究表明吸附反应是自发吸热过程。β-CD@PFMBC表面的含氧基团与Pb(II)产生了表面络合和静电相互作用。5次循环解吸后,对Pb(II)去除率仍能达到79%以上。以上结果表明β-CD@PFMBC对水溶液中Pb(II)的去除具有一定的应用潜力。Abstract: In order to solve the water contamination arises from heavy metal Pb(II), β-cyclodextrin modified magnetic palm fiber biochar (β-CD@PFMBC) was prepared with palm fiber as raw material by chemical coprecipitation method for efficient removal of Pb(II) from aqueous solution. The structure and morphology of the material were characterized by FTIR, XRD, BET, SEM, Raman and VSM. The adsorption properties of Pb(II) were analyzed through single factor experiment. The adsorption mechanism and recycling of Pb(II) were also explored. The results show that the specific surface area and the number of surface functional groups of β-CD@PFMBC increased compared with the pristine biochar. The adsorption process of Pb(II) can be better described by both the pseudo-second-order kinetic model and the Langmuir adsorption isotherm model, which indicates that the adsorption process is chemical adsorption and monolayer adsorption. According to the Langmuir isotherm model, the maximum theoretical adsorption capacity of β-CD@PFMBC at 303 K is 625.49 mg∙g–1, which is significantly higher than that of the pristine biochar. Thermodynamic studies show that the adsorption is a spontaneous endothermic process. The oxygen-containing groups on the surface of β-CD@PFMBC produce surface complexation and electrostatic interaction with Pb(II). The removal ration remains above 79% after five cycles of adsorption-desorption. It can be expected that β-CD@PFMBC will be of potential application in removing of Pb(II) from aqueous solution.
-
Key words:
- biochar /
- magnetism /
- β-cyclodextrin /
- adsorption /
- Pb(II) /
- water contamination arises
-
表 1 PFBC和β-CD@PFMBC的多孔结构参数
Table 1. Porous structure parameters of PFBC and β-CD@PFMBC
Adsorbent Specific surface area/(m2∙g–1) Pore volume/(cm3∙g–1) Average pore diameter/nm PFBC 3.31 0.0082 13.6392 β-CD@PFMBC 23.69 0.0757 11.9255 表 2 PFBC与β-CD@PFMBC吸附Pb(II)的拟一级和拟二级动力学模型参数
Table 2. Kinetic adsorption parameters of pseudo-first-order and pseudo-second-order kinetic models for Pb(II) adsorption by PFBC and β-CD@PFMBC
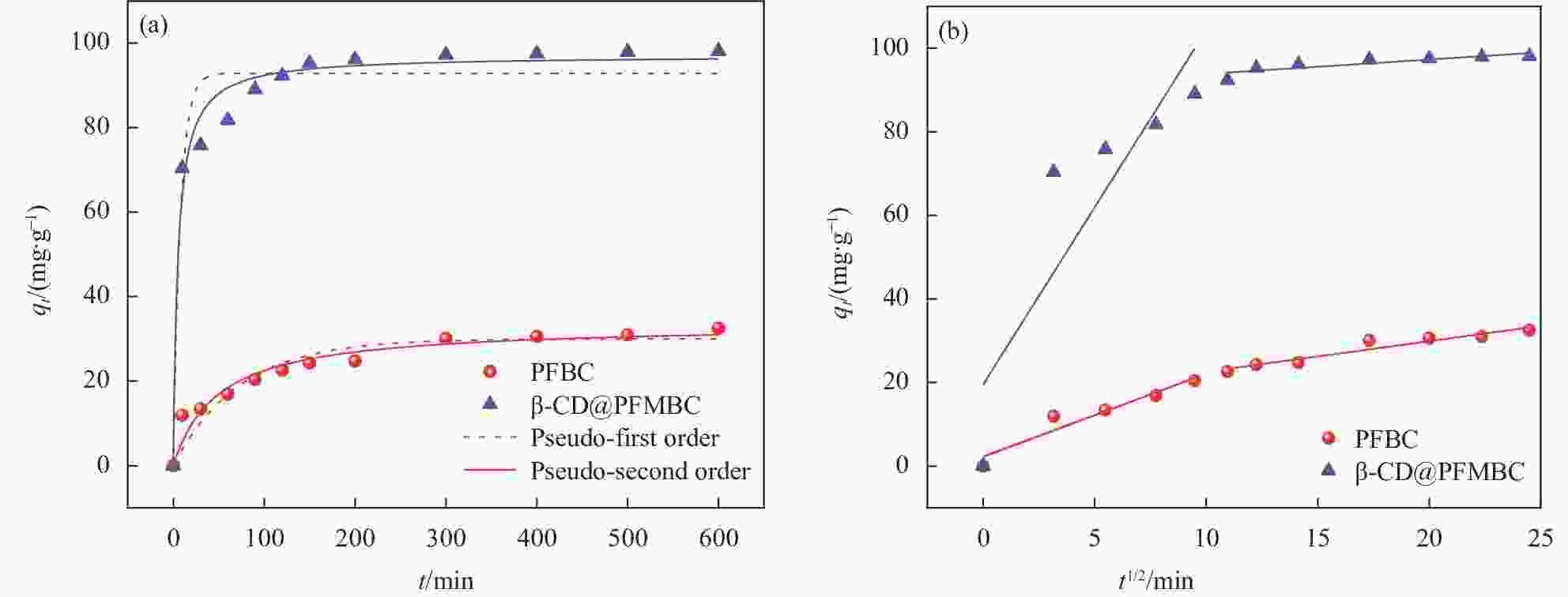
Adsorbent Pseudo-first-order model Pseudo-second-order model qe,cal/(mg∙g–1) k1/min–1 R12 qe,cal/(mg∙g–1) k2/(g∙mg–1∙min–1) R22 PFBC 30.0267 0.0138 0.9010 33.5026 0.0006 0.9465 β-CD@PFMBC 92.8959 0.1265 0.9400 97.1032 0.0020 0.9784 Notes: qe,cal—Equilibrium sorption capacity calculated by pseudo-first-order or pseudo-second-order kinetics; k—Rate constants; R2—Correlation coefficients. 表 3 PFBC与β-CD@PFMBC吸附Pb(II)的颗粒内扩散模型参数
Table 3. Intra-particle diffusion model parameters for Pb(II) adsorption by PFBC and β-CD@PFMBC
Adsorbent kid,1 kid,2 C1 C2 R12 R22 PFBC 1.987 0.732 2.246 15.2620 0.9263 0.9230 β-CD@PFMBC 8.476 0.345 19.544 90.3927 0.7727 0.7594 Notes: kid,1, kid,2—Rate constants at different stages of internal diffusion; C1, C2—Intercept of corresponding concentration. 表 4 PFBC与β-CD@PFMBC吸附Pb(II)的吸附等温线模型参数
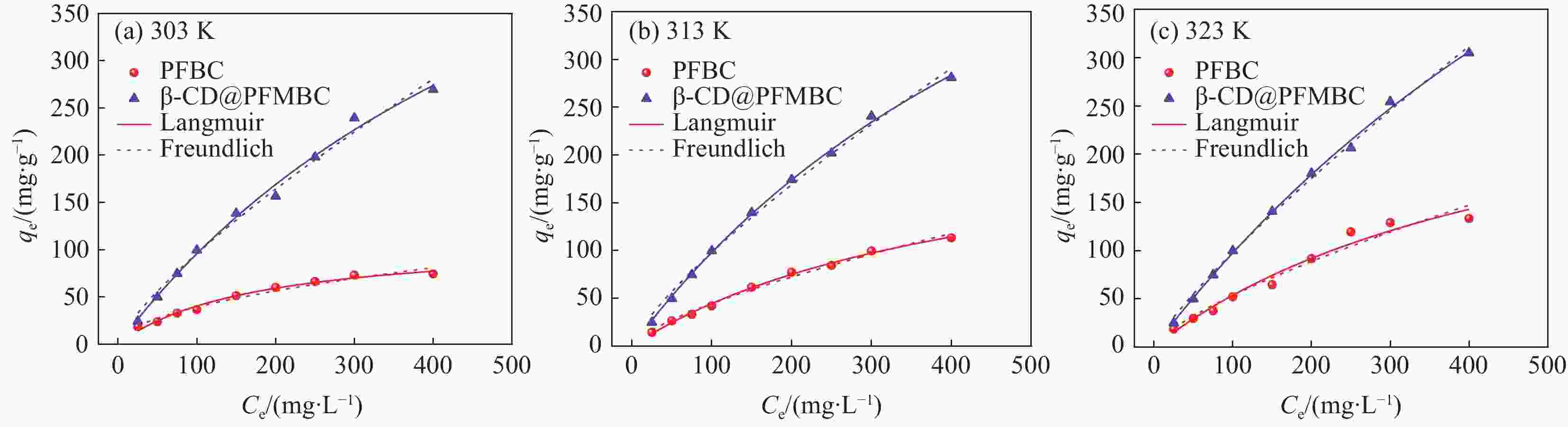
Table 4. Parameters of isotherm model for Pb(II) adsorption by PFBC and β-CD@PFMBC
Adsorbent Temperature/K Langmuir Freundlich qm/(mg∙g–1) KL/(L∙mg–1) R12 KF/(mg∙g–1) n R22 PFBC 303 110.90 0.0020 0.9845 1.086 1.304 0.9709 313 239.21 0.0023 0.9966 1.730 1.371 0.9913 323 319.65 0.0026 0.9725 1.859 1.408 0.9613 β-CD@PFMBC 303 625.49 0.0015 0.9936 2.119 1.200 0.9898 313 720.39 0.0014 0.9987 2.654 1.276 0.9943 323 957.73 0.0010 0.9983 2.705 1.291 0.9965 Notes: qm—Maximum adsorption capacity; KL—Adsorptive constant of the Langmuir model; KF—Adsorptive constant of the Freundlich model; n—Constants related to the adsorption intensity. 表 5 β-CD@PFMBC与其他吸附剂对Pb(II)吸附量对比
Table 5. Comparison of the adsorption capacity of Pb(II) by β-CD@PFMBC and other adsorbents
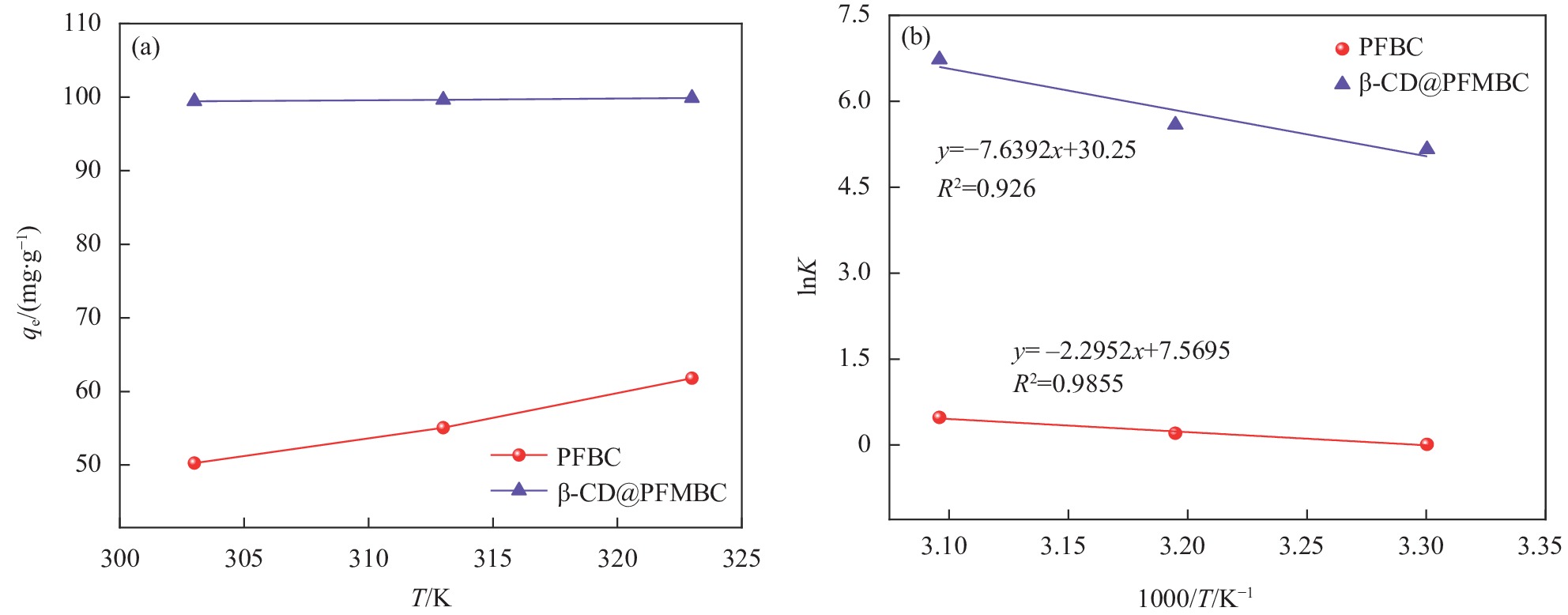
表 6 PFBC与β-CD@PFMBC吸附Pb(II)的热力学参数
Table 6. Thermodynamic parameters for Pb(II) adsorption by PFBC and β-CD@PFMBC
Adsorbent Temperature/K ∆H0/(kJ∙mol−1) ∆S0/(J∙mol−1·K−1) ∆G0/(kJ∙mol−1) PFBC 303 19.7823 63.2042 −0.0262 313 −0.5298 323 −1.2903 β-CD@PFMBC 303 79.3820 253.6170 −13.0040 313 −14.5450 323 −18.0760 Notes: ∆H0—Enthalpy change; ∆S0—Entropy change; ∆G0—Gibbs free energy change. -
[1] XU D M, FU R B, LIU H Q, et al. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review[J]. Journal of Cleaner Production,2021,286:124989. doi: 10.1016/j.jclepro.2020.124989 [2] DUAN Z Y, SONG M Y, LI T G, et al. Characterization and adsorption properties of cross-linked yeast/β-cyclodextrin polymers for Pb(II) and Cd(II) adsorption[J]. RSC Advances,2018,8(55):31542-31554. doi: 10.1039/C8RA06171H [3] WANG Y F, ZHANG X Y, LI R Z, et al. Competitive sorption of lead and methylene blue onto black soil and their interaction with dissolved organic matter using two-dimensional correlation analyses[J]. Ecotoxicology and Environmental Safety,2018,164:484-492. doi: 10.1016/j.ecoenv.2018.08.026 [4] GUL S, AHMAND Z, ASMA M, et al. Effective adsorption of cadmium and lead using SO3H-functionalized Zr-MOFs in aqueous medium[J]. Chemosphere,2022,307(1):135633. [5] BAI M, CHAI Y, CHEN A, et al. Enhancing cadmium removal efficiency through spinel ferrites modified biochar derived from agricultural waste straw[J]. Journal of Environmental Chemical Engineering,2023,11(1):109027. doi: 10.1016/j.jece.2022.109027 [6] LI J, CHEN M, YANG X, et al. Preparation of a novel hydrogel of sodium alginate using rural waste bone meal for efficient adsorption of heavy metals cadmium ion[J]. Science of the Total Environment,2023,863:160969. doi: 10.1016/j.scitotenv.2022.160969 [7] HU X J, HU Y, XU G Z, et al. Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater[J]. Environmental Research,2019,180(C):108796. [8] HUANG X X, LIU Y G, LIU S B, et al. Effective removal of Cr(VI) using β-cyclodextrin-chitosan modified biochars with adsorption/reduction bifuctional roles[J]. RSC Advances,2016,6(1):94-104. doi: 10.1039/C5RA22886G [9] ZOU C L, JIANG W, LIANG J Y, et al. Removal of Pb(II) from aqueous solutions by adsorption on magnetic bentonite[J]. Environmental Science and Pollution Research,2019,26(2):1315-1322. doi: 10.1007/s11356-018-3652-0 [10] MILONJIC S K. A consideration of the correct calculation of thermodynamic parameters of adsorption[J]. Journal of the Serbian Chemical Society,2007,72(12):1363-1367. doi: 10.2298/JSC0712363M [11] 王泽亚, 龚香宜, 任大军, 等. β-环糊精改性梧桐叶基生物炭对水中镉离子的去除研究[J]. 功能材料, 2022, 53(8):8092-8098.WANG Zeya, GONG Xiangyi, REN Dajun, et al. Preparation of β-cyclodextrin modified biochar from Chinese parasol leaves and removal of Cd2+ from waste water[J]. Journal of Functional Materials,2022,53(8):8092-8098(in Chinese). [12] BORAH H J, GOGOI M, DAS D B, et al. Cyclodextrine-glutaraldehyde cross-linked nanofiltration membrane for recovery of resveratrol from plant extract[J]. Journal of Environmental Chemical Engineering,2020,8(1):103620. doi: 10.1016/j.jece.2019.103620 [13] CHOU C M, LIEN H L. Dendrimer-conjugated magnetic nanoparticles for removal of zinc (II) from aqueous solutions[J]. Journal of Nanoparticle Research,2011,13(5):2099-2107. doi: 10.1007/s11051-010-9967-5 [14] 刘梅, 薛代惠美, 郭玉超, 等. 磁性生物炭材料在含油废水处理中的应用研究[J]. 现代化工, 2021, 41(3):149-153.LIU Mei, XUE Daihuimei, GUO Yuchao, et al. Study on application of magnetic biochar in treatment of oil-bearing wastewater[J]. Modern Chemical Industry,2021,41(3):149-153(in Chinese). [15] 邢敏, 雷西萍, 韩丁, 等. Fe3O4/高岭土磁性复合材料对Cu2+的吸附性能[J]. 复合材料学报, 2019, 36(9): 2204-2211.XING Min, LEI Xiping, HAN Ding, et al. Adsorption properties of Fe3O4/Kaolin magnetic composites for Cu2+[J]. Acta Materiae Compositae Sinica, 2019, 36(9): 2204-2211(in Chinese). [16] BAI B, GUAN W S, LI Z Y, et al. Bio-template route for facile fabrication of Cd(OH)2@yeast hybrid microspheres and their subsequent conversion to mesoporous CdO hollow microspheres[J]. Materials Research Bulletin,2011,46(1):26-31. doi: 10.1016/j.materresbull.2010.10.002 [17] 牛乙涛, 包国庆, 吴纯鑫, 等. 功能化纳米复合材料Fe3O4@SiO2-APTMS的制备及其对Pb(Ⅱ)的吸附[J]. 复合材料学报, 2023, 40(6):3350-3365.NIU Yitao, BAO Guoqing, WU Chunxin, et al. Preparation of functionalized nanocomposites Fe3O4@SiO2-APTMS and its adsorption to Pb(Ⅱ)[J]. Acta Materiae Compositae Sinica,2023,40(6):3350-3365(in Chinese). [18] QIU Q Y, ZHOU M Y, CAI W Z, et al. A comparative investigation on direct carbon solid oxide fuel cells operated with fuels of biochar derived from wheat straw, corncob, and bagasse[J]. Biomass and Bioenergy,2019,121:56-63. doi: 10.1016/j.biombioe.2018.12.016 [19] ZHOU Y, CAO S R, XI C X, et al. A novel Fe3O4/graphene oxide/citrus peel-derived bio-char based nanocomposite with enhanced adsorption affinity and sensitivity of ciprofloxacin and sparfloxacin[J]. Bioresource Technology,2019,292:121951. doi: 10.1016/j.biortech.2019.121951 [20] MA F F, ZHAO H, ZHENG X D, et al. Enhanced adsorption of cadmium from aqueous solution by amino modification biochar and its adsorption mechanism insight[J]. Journal of Environmental Chemical Engineering,2023,11(3):109747. doi: 10.1016/j.jece.2023.109747 [21] 包炳钦, 张军, 宋卫锋, 等. 磁性复合凝胶球对Pb(Ⅱ)的吸附特性与机制[J]. 复合材料学报, 2021, 38(6):1929-1938.BAO Bingqin, ZHANG Jun, SONG Weifeng, et al. Adsorption characteristics and mechanism of Pb(Ⅱ) on magnetic composite gel spheres[J]. Acta Materiae Compositae Sinica,2021,38(6):1929-1938(in Chinese). [22] 王申宛, 钟爽, 郑丽丽, 等. 共热解法制备方解石/生物炭复合材料及其吸附Pb(II)性能和机制[J]. 复合材料学报, 2021, 38(12):4282-4293.WANG Shenwan, ZHONG Shuang, ZHENG Lili, et al. Preparation of calcite/biochar composite by co-pyrolysis and its adsorption properties and mechanism for Pb(II)[J]. Acta Materiae Compositae Sinica,2021,38(12):4282-4293(in Chinese). [23] 毕景望, 单锐, 韩静, 等. 改性西瓜皮生物炭的制备及其对Pb(Ⅱ)的吸附特性[J]. 环境科学, 2020, 41(4):1770-1778.BI Jingwang, SHAN Rui, HAN Jing, et al. Preparation of modified watermelon biochar and its adsorption properties for Pb(Ⅱ)[J]. Environmental Science,2020,41(4):1770-1778(in Chinese). [24] 王志凯, 张胜利, 陈豪宇, 等. 磁性PEI功能化秸秆的制备及对Pb(Ⅱ)的吸附[J]. 环境科学研究, 2017, 30(8):1316-1324.WANG Zhikai, ZHANG Shengli, CHEN Haoyu, et al. Preparation of magnetic polyethyleneimine functionalized rice straw and its adsorption properties for Pb(II) ions[J]. Research of Environmental Sciences,2017,30(8):1316-1324(in Chinese). [25] 崔灿, 牛姣姣, 谢雅典, 等. 支化聚乙烯亚胺功能化磁性纳米吸附剂的制备及对Cu2+的吸附研究[J]. 地球与环境, 2022, 50(4):593-600.CUI Can, NIU Jiaojiao, XIE Yadian, et al. Preparation of branched polyethyleneimine functionalized magnetic nano-adsorbents for Cu2+ and it's adsorption properties[J]. Earth and Environment,2022,50(4):593-600(in Chinese). [26] HU R, WANG X K, DAI S Y, et al. Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions[J]. Chemical Engineering Journal,2015,260:469-477. doi: 10.1016/j.cej.2014.09.013 [27] 包国庆, 吴纯鑫, 赵德明. 磁性Fe3O4纳米复合材料的制备及其对Pb(II)的吸附[J]. 复合材料学报, 2023, 40(1):219-231.BAO Guoqing, WU chunxin, ZHAO Deming. Preparation of magnetic Fe3O4 nanocomposites and their adsorption to Pb(II)[J]. Acta Materiae Compositae Sinica,2023,40(1):219-231(in Chinese). [28] BAI L J, SU X J, FENG J P, et al. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr(VI) adsorption in water[J]. Journal of Cleaner Production,2021,320:128723. doi: 10.1016/j.jclepro.2021.128723 [29] QU J H, MENG Q J, LIN X F, et al. Microwave-assisted synthesis of β-cyclodextrin functionalized celluloses for enhanced removal of Pb(II) from water: Adsorptive performance and mechanism exploration[J]. Science of the Total Environment,2020,752(14):141854. [30] HE J Y, LI Y L, WANG C M, et al. Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers[J]. Applied Surface Science,2017,426:29-39. doi: 10.1016/j.apsusc.2017.07.103 [31] ZHAO H T, MA S, ZHENG S Y, et al. β-cyclodextrin functionalized biochars as novel sorbents for high-performance of Pb2+ removal[J]. Journal of Hazardous Materials,2019,362:206-213. doi: 10.1016/j.jhazmat.2018.09.027 [32] USMAN M, AHMED A, JI Z J, et al. Environmentally friendly fabrication of new β-cyclodextrin/ZrO2 nanocomposite for simultaneous removal of Pb(II) and BPA from water[J]. Science of the Total Environment,2021,784:147207. doi: 10.1016/j.scitotenv.2021.147207 [33] GE X Y, TIAN F, WU Z L, et al. Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects[J]. Chemical Engineering and Processing,2015,91:67-77. doi: 10.1016/j.cep.2015.03.019 [34] CHEN W Q, LU Z H, XIAO B H, et al. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping[J]. Journal of Cleaner Production,2019,211(C):1250-1258. [35] FEN Y W, YUNUS W M M, TALIB Z A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy[J]. Optik,2013,124(2):126-133. doi: 10.1016/j.ijleo.2011.11.035 [36] HE J, LU Y C, LUO G S. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions[J]. Chemical Engineering Journal,2014,244:202-208. doi: 10.1016/j.cej.2014.01.096 -






 下载:
下载: