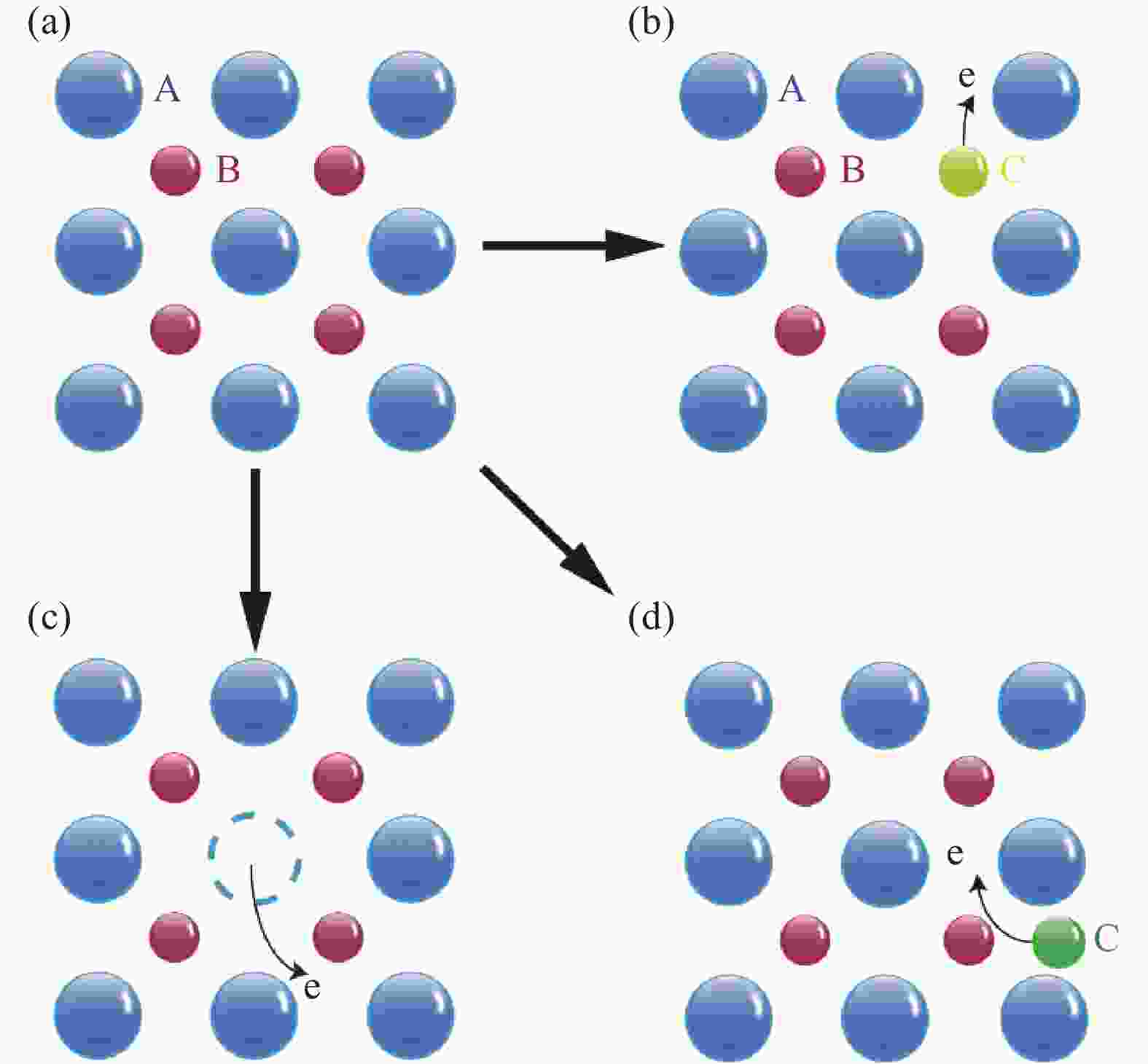
A review of electrochromic materials based on metal oxide nanocrystals
-
摘要: 电致变色材料是一类光学特性随电压可逆调控的材料,在智能窗、显示器、汽车变色天幕、智能热管理、军事伪装等领域应用广泛。近年来基于金属氧化物纳米晶的电致变色材料,由于其优异的性能和成本优势,引起了研究者的广泛关注。本文首先介绍了电化学调控纳米晶局域表面等离子体共振(LSPR)的基本原理。然后,结合国内外研究现状,综述了金属氧化物纳米晶及其复合材料在传统电致变色和基于电化学调控纳米晶LSPR的新型电致变色领域中的最新研究进展。最后提出了基于金属氧化物纳米晶电致变色材料存在的问题和解决的途径,并对其发展前景进行了展望。
-
关键词:
- 电致变色材料 /
- 金属氧化物纳米晶 /
- 局域表面等离子体共振 /
- 纳米晶复合材料 /
- 智能窗
Abstract: Electrochromic materials are a kind of materials whose optical characteristics can be regulated with voltage reversibly. It is widely used in smart windows, displays, electrochromic sunroof, smart thermal management, military camouflage, and other fields. In recent years, electrochromic materials based on metal oxide nanocrystals have attracted extensive attention of researchers due to their excellent performance and cost advantages. In this paper, we first introduce the principle of electrochemically controlled localized surface plasmon resonance (LSPR) of nanocrystals. Then, we review the latest research progress of metal oxide nanocrystals and their compo-sites in traditional electrochromic and novel electrochromic fields based on electrochemically regulated LSPR. Finally, we put forward the existing problems and solutions of electrochromic materials based on metal oxide nanocrystals and their development prospects are prospected. -
图 3 (a) 不同金属氧化物体系复介电函数的实部ε1和虚部ε2[43-47];(b) 由(a)中复介电函数推导出的相应折射率和吸收系数;(c) In∶CdO和Sn∶In2O3的ωLSPR和LSPR吸收峰的半峰宽(FWHMLSPR)[25]
Figure 3. (a) Real ε1 and imaginary ε2 parts of complex dielectric functions for different metal oxide systems[43-47]; (b) Corresponding complex refractive index and absorption coefficient derived from the complex dielectric functions shown in panel (a); (c) ωLSPR and full width at half-maximum (FWHMLSPR) versus doping for both In∶CdO and Sn∶In2O3[25]
ωLSPR—Frequency of LSPR; LSPR—Localized surface plasmon resonance
图 6 (a) WO3纳米棒的SEM和TEM图像[54];(b) 不同电压下WO3纳米棒薄膜的UV-VIS光谱和在±3 V循环下WO3纳米棒薄膜在着色和漂白状态之间的开关响应速度[54];(c) 在不同电压下WO3纳米棒的颜色变化[54];(d) {100}晶面和{
$ {\bar 1} 20$ }晶面为主的WO3纳米棒的着色效率[55]Figure 6. (a) SEM and TEM images of the WO3 nanorod film[54]; (b) UV-VIS spectra of WO3 nanorod films at different voltages and on/off response time of WO3 nanorod films between coloring and bleaching states at ± 3 V[54]; (c) Color changes of the WO3 nanorod film at different voltages[54]; (d) Coloration efficiency of WO3 nanorods with {100} crystal facets and {
$ {\bar 1}20 $ } crystal facets dominant[55]图 7 (a) Zn2+嵌入/脱出TiO2晶格的示意图和着色和褪色状态的光学照片[32];(b) TiO2纳米晶的TEM图像和尺寸分布[32];(c) 基于TiO2纳米晶的电致变色器件在550 nm波段、1.3~0 V循环下的实时透射光谱[32];(d) 在完全着色和褪色状态下,1000次循环前后,基于TiO2纳米晶的电致变色器件光学透过谱[32];(e) Nb12O29纳米片的STEM图像[59];(f) Nb12O29纳米片薄膜的在不同电压下的光谱图和光学照片[59]
Figure 7. (a) Schematic diagram of Zn2+ (de-)insertion of TiO2 lattice and photographs at fully bleached and colored state[32]; (b) TEM images and size distributions (inserts) of TiO2 nanocrystals[32]; (c) Real-time transmittance spectra of electrochromic devices based on TiO2 nanocrystals at 550 nm at 1.3-0 V[32]; (d) Optical transmittance spectra before and after 1000 cycles at fully bleached and colored states[32]; (e) STEM imaging of Nb12O29 nanoplatelets[59]; (f) Spectrum and photographs of Nb12O29 nanoplatelet film at different voltages[59]
GXU—Guangxi Univiersity; OCP—Open circuit potential; NIR—Night-time ozone profile; τc—Coloring time; τb—Fading time
图 8 (a) WO3-x纳米晶的TEM图像,插图为WO3-x纳米晶分散液的光学照片[73];(b) 不同电压下WO3-x纳米晶制备的电致变色器件的透过率变化光谱[73];(c) 不同电压下WO3-x晶体结构变化及相应的光学照片变化[73];(d) Cs:WO3纳米晶的晶体结构和形状各向异性对电致变色调制的着色效率和电荷容量的影响[79];(e) WO3-x纳米晶介孔膜的SEM图像[75];(f) WO3-x纳米晶介孔膜的在不同充电状态下LSPR吸收率的变化和致密WO3-x纳米晶薄膜在1.5 V饱和状态下的吸收率[75];(g) WO2.72纳米棒介孔膜的SEM图像[76];(h) WO2.72-NbO电致变色纳米复合膜的在不同电压下的透过率变化曲线和光学照片变化[76];(i) Na+和Li+电解质嵌入WO2.72纳米晶空隙位置的示意图[70]
Figure 8. (a) TEM diagram of WO3-x nanocrystals. The inset is optical photo of WO3-x nanocrystal dispersion[73]; (b) Transmission change spectra of electrochromic devices prepared by WO3-x nanocrystals at different voltages[73]; (c) WO3-x crystal structure changes and corresponding optical photo changes under different voltages[73]; (d) Effect of crystal structure and shape anisotropy of Cs: WO3 nanocrystals on the coloring efficiency and charge capacity of electrochromic modulation[79]; (e) SEM diagram of WO3-x nanocrystal mesoporous film[75]; (f) Change of LSPR absorbance of WO3-x nanocrystal mesoporous film under different charging states, and absorbance of compact WO3-x nanocrystal film under 1.5 V saturation[75]; (g) SEM diagram of WO2.72 nanorod mesoporous membrane[76]; (h) Transmittance change and optical photo change of WO2.72-NbO electrochromic nanocomposite film under different voltages[76];(i) Schematic Diagram of the Position of Na+ and Li+ Electrolytes Embedded in the Voids of WO2.72 Nanocrystals[70]
图 9 AZO (a)和ITO (c)纳米晶膜的充电容量在多次充电和放电过程中的变化曲线;AZO (b)和ITO (d) 纳米晶膜的循环20000次前后的透过率变化曲线[23]
Figure 9. Change curves of the charging capacity of AZO (a) and ITO (c) nanocrystal films during multiple charging and discharging; Transmission curves of AZO (b) and ITO (d) nanocrystal films before and after 20000 cycles[23]
图 10 (a) 不同尺寸的ITO纳米晶的LSPR峰位随电压变化的调制量[82];(b)不同掺杂量的ITO纳米晶的LSPR峰位随电压变化的调制量[82];(c) ITO纳米晶薄膜在0.1 mol/L LiClO4和0.1 mol/L 四正丁基高氯酸铵(TBAP)电解质中的透过率光谱曲线[22];(d) ITO纳米晶薄膜在0.1 mol/L LiClO4和0.1 mol/L TBAP电解质中的循环伏安曲线[22]
Figure 10. (a) Modulation amount of LSPR peak position of ITO nanocrystals with different sizes as a function of voltage[82]; (b) Modulation amount of the LSPR peak position of ITO nanocrystals with different doping concentrations as a function of voltage[82]; (c) Transmittance spectra of ITO nanocrystal film in 0.1 mol/L LiClO4 and 0.1 mol/L tetrabutylammonium perchlorate (TBAP) electrolyte[22]; (d) Cyclic voltammograms of ITO nanocrystal film in 0.1 mol/L LiClO4 and 0.1 mol/L TBAP electrolyte[22]
图 11 (a) 在1.5 V和4 V下,不同薄膜厚度(150、310和460 nm)的ITO纳米晶的透过率光谱曲线[22];(b) ITO纳米晶结合至NbO玻璃中的SEM图像[86];(c) ITO-NbOx复合材料中在不同电压下的透过率光谱曲线[86];(d) 介孔ITO纳米晶膜的SEM图像[84];(e) 介孔ITO纳米晶膜和随机填充的致密的纳米晶薄膜在多次循环过程中的比电容[84]
Figure 11. (a) Transmission spectra at 1.5 V and 4 V for various film thickness (150, 310, and 460 nm ) of ITO nanocrystals[22]; (b) SEM images of ITO nanocrystals combined into glass[86]; (c) Optical switching response under applied electrochemical voltage of a ITO-in-NbOx composite[86]; (d) SEM images of mesoporous ITO nanocrystal films[84]; (e) Specific capacity of mesoporous ITO nanocrystal film and randomly filled dense nanocrystal film during multiple cycles[84]
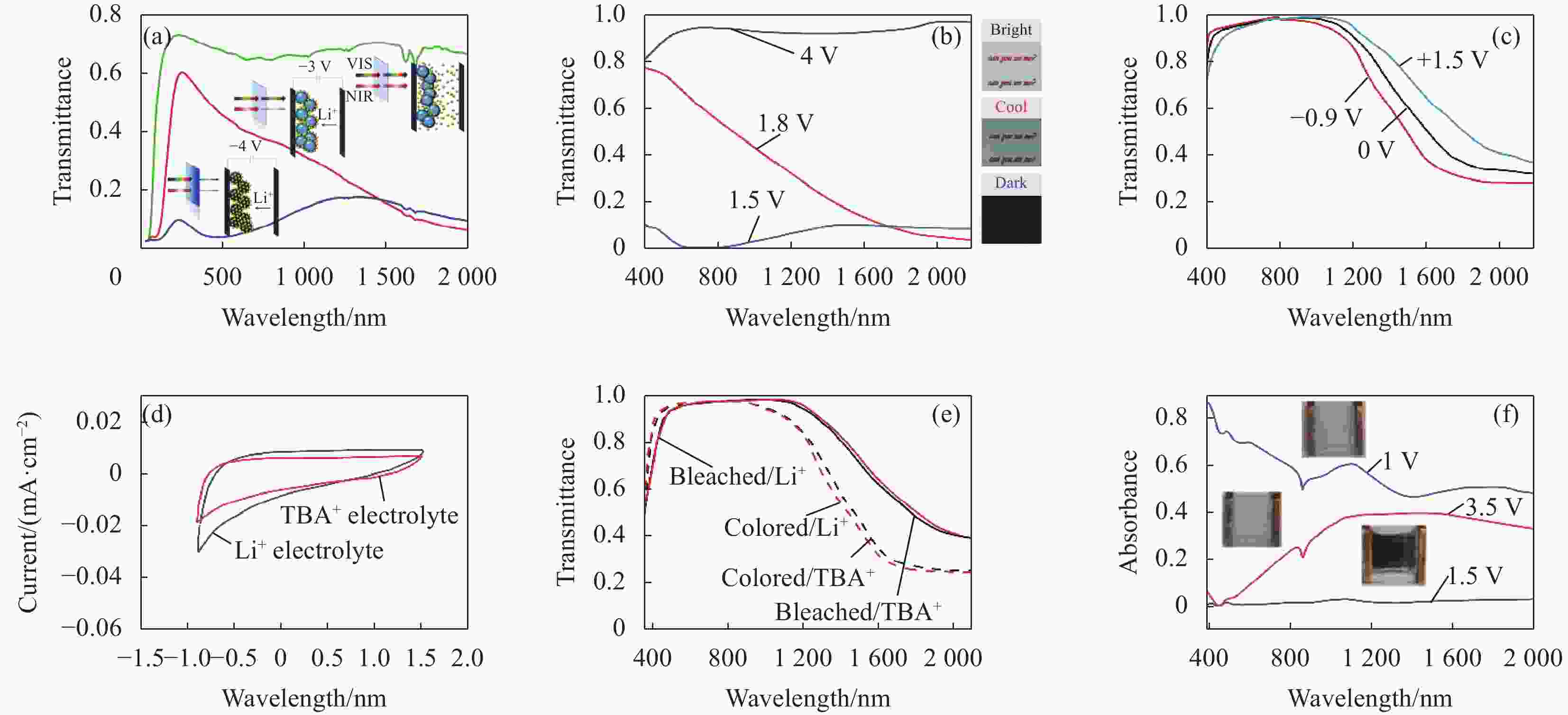
图 12 (a) Nb∶TiO2纳米晶电致变色器件在不同电压下的透过率光谱曲线[92];(b) Ta∶TiO2纳米晶薄膜在不同电压下的透过率光谱曲线和相应的光学图[93];(c) F-In∶CdO纳米晶薄膜在不同电压下的透过率光谱曲线[94];(d) F-In∶CdO纳米晶薄膜在Li+基电解质和硫代巴比妥酸(TBA+)基电解质中的循环伏安曲线[94];(e) F-In∶CdO纳米晶薄膜在Li+基电解质和TBA+基电解质中的透过率光谱曲线[94];(f) 基于MoO3-x纳米线的电致变色器件在不同电压下的吸收率曲线[95]
Figure 12. (a) Transmittance spectra of Nb∶TiO2 nanocrystal films at different voltages[92]; (b) Transmittance spectra and corresponding optical diagram of Ta∶TiO2 nanocrystal film at different voltages[93]; (c) Transmittance spectra of F-In∶CdO nanocrystal film under different voltage[94]; (d) Cyclic voltammograms of F-In∶CdO nanocrystal films in Li+-based electrolyte and thiobarbituric acid (TBA+)-based electrolyte[94]; (e) Transmittance spectra of F-In∶CdO nanocrystal film in Li+-based electrolyte and TBA+-based electrolyte[94]; (f) Absorption curves of electrochromic devices based on MoO3-x nanowires at different voltages[95]
TBA—Thiobarbituric acid
表 1 基于传统金属氧化物纳米晶的电致变色薄膜及器件的性能
Table 1. Performance of electrochromic films and devices based on traditional metal oxide nanocrystals
Materials Electrolyte On/Off response
time/sColoration efficiency /
(cm2·C−1)Maximum optical
modulationStability/Cycles Ref. WO3 nanorods and nanospheroids 1 mol/L H2SO4 — 42 (670 nm) — 3000 [60] WO3 nanorods 1 mol/L LiClO4 8 (632.8 nm) — ~66% (632.8 nm) 3000 [54] WO3 nanorods 0.5 mol/L H2SO4 <25 (632.8 nm) 37.6 (632.8 nm) 54.9% (800 nm) — [61] WO3 nanorods 1 mol/L LiClO4 <20 (940 nm) 80 (940 nm) — 500 [55] TiO2 Nanocrystals 1 mol/L ZnSO4 <9 (550 nm) 37.3 (550 nm) 66% (550 nm) 1000 [32] WO3/TiO2 nanowires LiClO4 <8 (630 nm) 85.7 (630 nm) 49% (630 nm) 100 [62] NbO nanorods 1 mol/L LiTFSI — 75 (1500 nm) ~10% (800 nm) 500 [63] Nb12O29 nanoplatelets Li-based — 77.3 (550 nm) 75% (600 nm) 500 [59] Note: LiTFSI—Lithium bistrifluoromethane sulfonimide. 表 2 基于掺杂WO3纳米晶的电致变色薄膜及器件的性能
Table 2. Performance of electrochromic films and devices based on doped WO3 nanocrystals
Materials Electrolyte On/Off response
time/sColoration efficiency/
(cm2·C−1)Maximum optical
modulationStability/Cycles Ref. WO3-x nanowires 0.5 mol/L Li-TFSI <8 — 91.7% (633 nm); 87.3% (1600 nm) 1000 [73] WO3-x nanoparticles 1 mol/L LiClO4 <1.5 50-60 (550 nm);
317 (1200 nm)71.1% (550 nm); 84.6% (1200 nm) 1000 [72] W18O49 nanorods 1 mol/L H2SO4 — — 34.3% (750 nm) — [74] WO3-x nanocrystal 0.1 mol/L Li-TFSI — — 71% (VIS);
84% (NIR)2000 [75] WO2.72-NbOx 1 mol/L Li-TFSI — — 78% (550 nm);
63% (1200 nm)2000 [76] WO2.72 nanocrystal 0.5 mol/L NaClO4; 0.5 mol/L LiClO4 — 81 (Na+); 49 (Li+) — — [70] WO3-x nanowires 1 mol/L Al(ClO4)3 <8 121 (633 nm);
254 (1200 nm)93.2% (633 nm); 86.8% (1600 nm) 2000 [77] Cs:WO3 0.1 mol/L XPF6, where X is Li+, Na+, K+, or TBA+ — 74.3 (TBA+); 70.1 (K+);
72.7 (Na+); 103 (Li+)47% (800 nm);
70% (1600 nm)— [78] 表 3 基于掺杂In2O3纳米晶和掺杂ZnO纳米晶的电致变色薄膜及器件的性能
Table 3. Performance of electrochromic films and devices based on doped In2O3 nanocrystals and doped ZnO nanocrystals
Materials Electrolyte On/Off response time/s Coloration efficiency/
(cm2·C−1)Maximum optical modulation Stability/cycles Ref. ITO nanocrystals 0.1 mol/L LiClO4;
1 mol/L LiClO4;
0.1 mol/L TBAP;
1 mol/L TBAP<3.4 (0.1 mol/L LiClO4);
<0.3 (1 mol/L LiClO4);
<0.24 (0.1 mol/L TBAP);
<0.06 (1 mol/L TBAP)400 (1800 nm) 25% (NIR) 5000-20000 [22-23] AZO nanocrystals 0.1 mol/L LiClO4;
1 mol/L LiClO4;
0.1 mol/L TBAP;
1 mol/L TBAP<0.9 (0.1 mol/L LiClO4);
<0.19 (1 mol/L LiClO4);
<0.11 (0.1 mol/L TBAP);
<0.07 (1 mol/L TBAP)450 (2000 nm) 39% (NIR) 20000 [23] ITO nanocrystals into NbOx glass 0.1 mol/L LiClO4 0.01 (NIR); Several minutes (VIS) 30 — 2000 [86] ITO nanocrystals 0.1 mol/L LiClO4 <2.22 493 (1750 nm) 56% (1750 nm) – [84] ITO nanocrystals 1 mol/L LiClO4 <82 1270 83% (2000) 100 [88] ITO nanocrystals 1 mol/L LiTFSI – 802 (1900 nm) 39% (1900 nm) – [89] ITO nanocrystals 1 mol/L LiClO4 – – 42% (2000 nm) – [90] -
[1] 张翔, 李文杰, 李森然, 等. 自供能电致变色器件研究进展[J]. 复合材料学报, 2021, 38(6):1724-1733. doi: 10.13801/j.cnki.fhclxb.20210210.007ZHANG Xiang, LI Wenjie, LI Senran, et al. Research process in self-powered electrochromic devices[J]. Acta Materiae Compositae Sinica,2021,38(6):1724-1733(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210210.007 [2] 汤凯, 管康威, 刘淑婧, 等. Ti掺杂核壳结构晶态@非晶WO3纳米线复合薄膜的制备与电致变色性能[J]. 复合材料学报, 2022, 40(6):3539-3552.TANG Kai, GUAN Kangwei, LIU Shujing, et al. Synthesis and electrochromic properties of Ti-doped core-shell crystalline@amorphous WO3 nanowire composite films[J]. Acta Materiae Compositae Sinica,2022,40(6):3539-3552(in Chinese). [3] WANG J L, SHENG S Z, HE Z, et al. Self-powered flexible electrochromic smart window[J]. Nano Letters,2021,21(23):9976-9982. doi: 10.1021/acs.nanolett.1c03438 [4] PHAN G T, VAN PHAM D, PATIL R A, et al. Fast-switching electrochromic smart windows based on NiO-nanorods counter electrode[J]. Solar Energy Materials Solar Cells,2021,231:111306. doi: 10.1016/j.solmat.2021.111306 [5] ERGOKTAS M S, BAKAN G, KOVALSKA E, et al. Multispectral graphene-based electro-optical surfaces with reversible tunability from visible to microwave wavelengths[J]. Nature Photonics,2021,15(7):493-498. doi: 10.1038/s41566-021-00791-1 [6] ZHANG Q, YUAN L, GUAN F, et al. Substituent-adjusted electrochromic behavior of symmetric viologens[J]. Materials,2021,14(7):1702. doi: 10.3390/ma14071702 [7] HU C, LI L, ZHOU J, et al. Enhanced contrast of WO3-based smart windows by continuous Li-ion insertion and metal electroplating[J]. ACS Applied Materials Interfaces,2022,14(28):32253-32260. doi: 10.1021/acsami.2c07546 [8] GONG H, AI J, LI W, et al. Self-driven Infrared electrochromic device with tunable optical and thermal management[J]. ACS Applied Materials Interfaces,2021,13(42):50319-50328. doi: 10.1021/acsami.1c14123 [9] TAO X, LIU D, YU J, et al. Reversible metal electrodeposition devices: An emerging approach to effective light modulation and thermal management[J]. Advanced Optical Materials,2021,9(8):2001847. doi: 10.1002/adom.202001847 [10] LI M, LIU D, CHENG H, et al. Manipulating metals for adaptive thermal camouflage[J]. Science Advances,2020,6(22):eaba3494. doi: 10.1126/sciadv.aba3494 [11] ZHANG Q, LI X, QIN M, et al. Preparation of a PB@SiO2 photonic crystal composite with enhanced electrochromic performance[J]. ACS Applied Electronic Materials,2021,3(10):4441-4447. doi: 10.1021/acsaelm.1c00600 [12] PLATT J R. Electrochromism, a possible change of color producible in dyes by an electric field[J]. The Journal of Chemical Physics,1961,34(3):862-863. doi: 10.1063/1.1731686 [13] DEB S. A novel electrophotographic system[J]. Applied Optics,1969,8(101):192-195. [14] JEONG C Y, WATANABE H, TAJIMA K. Adhesive electrochromic WO3 thin films fabricated using a WO3 nanoparticle-based ink[J]. Electrochimica Acta,2021,389:138764. doi: 10.1016/j.electacta.2021.138764 [15] ZHAO Y, ZHANG X, LI W, et al. High-performance electrochromic WO3 film driven by controllable crystalline structure and its all-solid-state device[J]. Solar Energy Materials Solar Cells,2022,237:111564. doi: 10.1016/j.solmat.2021.111564 [16] HUANG S, ZHANG R, SHAO P, et al. Electrochromic performance fading and restoration in amorphous TiO2 thin films[J]. Advanced Optical Materials,2022,10(16):2200903. doi: 10.1002/adom.202200903 [17] 张作胜, 张勇, 宋艳斌, 等. 低维氧化镍/聚苯胺核壳纳米结构的控制生长与电致变色性能研究[J]. 真空科学与技术学报, 2021, 41(7):632-639. doi: 10.13922/j.cnki.cjvst.202010022ZHANG Zuosheng, ZHANG Yong, SONG Yanbin, et al. Controlled growth and electrochromic properties of low dimensional nickel oxide/polyaniline core-shell nanostructures[J]. Chinese Journal of Vacuum Science and Technology,2021,41(7):632-639(in Chinese). doi: 10.13922/j.cnki.cjvst.202010022 [18] 王金敏, 后丽君, 马董云. 氧化钼电致变色材料与器件[J]. 无机材料学报, 2021, 36(5):461-470. doi: 10.15541/jim20200416WANG Jinmin, HOU Lijun, MA Dongyun. Molybdenum oxide electrochromic materials and devices[J]. Journal of Inorganic Materials,2021,36(5):461-470(in Chinese). doi: 10.15541/jim20200416 [19] KUMAR K N, SHAIK H, PAWAR A, et al. Effect of annealing and oxygen partial pressure on the RF sputtered WO3 thin films for electrochromic applications[J]. Materials Today: Proceedings,2022,59:339-344. doi: 10.1016/j.matpr.2021.11.185 [20] CHEN H C, CHEN Y R, LIU T F. Photoelectrochemical performance of a UV-cured all-solid-state complementary ITO/WO3/Ta2O5/electrolyte/NiO/ITO electrochromic device deposited by ion-beam assisted electron-beam evaporation[J]. Electrochimica Acta,2021,382:138355. doi: 10.1016/j.electacta.2021.138355 [21] RUNNERSTROM E L, LLORDES A, LOUNIS S D, et al. Nanostructured electrochromic smart windows: Traditional materials and NIR-selective plasmonic nanocrystals[J]. Chemical Communications,2014,50(73):10555-10572. doi: 10.1039/C4CC03109A [22] GARCIA G, BUONSANTI R, RUNNERSTROM E L, et al. Dynamically modulating the surface plasmon resonance of doped semiconductor nanocrystals[J]. Nano Letters,2011,11(10):4415-4420. doi: 10.1021/nl202597n [23] GARCIA G, BUONSANTI R, LLORDES A, et al. Near-infrared spectrally selective plasmonic electrochromic thin films[J]. Advanced Optical Materials,2013,1(3):215-220. doi: 10.1002/adom.201200051 [24] AGRAWAL A, CHO S H, ZANDI O, et al. Localized surface plasmon resonance in semiconductor nanocrystals[J]. Chemical Reviews,2018,118(6):3121-3207. doi: 10.1021/acs.chemrev.7b00613 [25] AGRAWAL A, JOHNS R W, MILLIRON D J. Control of localized surface plasmon resonances in metal oxide nanocrystals[J]. Annual Review of Materials Research,2017,47(1):1-31. doi: 10.1146/annurev-matsci-070616-124259 [26] WANG K, MENG Q, WANG Q, et al. Advances in energy-efficient plasmonic electrochromic smart windows based on metal oxide nanocrystals[J]. Advanced Energy and Sustainability Research, 2021, 2(12): 2170033. [27] 毛雯菲, 王敏敏. 局域表面等离子共振的影响因素及其研究进展[J]. 分析化学进展, 2021, 11(3):182-199. doi: 10.12677/AAC.2021.113021MAO Wenfei, WANG Minmin. Influencing factors and research progress of local surface plasmon resonance[J]. Advances in Analytical Chemistry,2021,11(3):182-199(in Chinese). doi: 10.12677/AAC.2021.113021 [28] JIANG N, ZHUO X, WANG J. Active plasmonics: principles, structures, and applications[J]. Chemical Reviews,2018,118(6):3054-3099. doi: 10.1021/acs.chemrev.7b00252 [29] CONTI III C R, QUIROZ-DELFI G, SCHWARCK J S, et al. Carrier density, effective mass, and nuclear relaxation pathways in plasmonic Sn: In2O3 nanocrystals[J]. The Journal of Physical Chemistry C,2020,124(51):28220-28229. doi: 10.1021/acs.jpcc.0c09448 [30] ZHANG Z, ZHANG R, XU L, et al. Visible and infrared optical modulation of PSLC smart films doped with ATO nanoparticles[J]. Dalton Transactions,2021,50(29):10033-10040. doi: 10.1039/D1DT01575C [31] WAINER P, KENDALL O, LAMB A, et al. Continuous growth synthesis of zinc oxide nanocrystals with tunable size and doping[J]. Chemistry of Materials,2019,31(23):9604-9613. doi: 10.1021/acs.chemmater.9b02655 [32] LIANG Y, CAO S, WEI Q, et al. Reversible Zn2+ insertion in tungsten ion-activated titanium dioxide nanocrystals for electrochromic windows[J]. Nano-Micro Letters,2021,13(12):120-131. doi: 10.1007/s40820-021-00719-y [33] CAO S, ZHANG S, ZHANG T, et al. A visible light-near-infrared dual-band smart window with internal energy storage[J]. Joule,2019,3(4):1152-1162. doi: 10.1016/j.joule.2018.12.010 [34] GUO C, YAN P, ZHU C, et al. Amorphous MoO3−x nanosheets prepared by the reduction of crystalline MoO3 by Mo metal for LSPR and photothermal conversion[J]. Chemical Communications,2019,55(83):12527-12530. doi: 10.1039/C9CC06704C [35] ZHANG S, CAO S, ZHANG T, et al. Plasmonic oxygen-deficient TiO2-x nanocrystals for dual-band electrochromic smart windows with efficient energy recycling[J]. Advanced Materials,2020,32(43):2004686. doi: 10.1002/adma.202004686 [36] LEE J T, DAS D, DAVIS G A, et al. Inorganic-organic interfacial electronic effects in ligand-passivated WO3–x nanoplatelets induce tunable plasmonic properties for smart windows[J]. ACS Applied Nano Materials,2022,5(7):9970-9980. doi: 10.1021/acsanm.2c02218 [37] MANDAL J, DU S, DONTIGNY M, et al. Li4Ti5O12: A visible-to-infrared broadband electrochromic material for optical and thermal management[J]. Advanced Functional Materials,2018,28(36):1802180. doi: 10.1002/adfm.201802180 [38] WU C, SHAO Z, ZHAI W, et al. Niobium tungsten oxides for electrochromic devices with long-term stability[J]. ACS Nano,2022,16(2):2621-2628. doi: 10.1021/acsnano.1c09234 [39] KIM K, REIMNITZ L C, CHO S H, et al. Effect of nonincorporative cations on the size and shape of indium oxide nanocrystals[J]. Chemistry of Materials,2020,32(21):9347-9354. doi: 10.1021/acs.chemmater.0c03281 [40] MEHRA S, BERGERUD A, MILLIRON D J, et al. Core/shell approach to dopant incorporation and shape control in colloidal zinc oxide nanorods[J]. Chemistry of Materials,2016,28(10):3454-3461. doi: 10.1021/acs.chemmater.6b00981 [41] SAEZ CABEZAS C A, SHERMAN Z M, HOWARD M P, et al. Universal gelation of metal oxide nanocrystals via depletion attractions[J]. Nano Letters,2020,20(5):4007-4013. doi: 10.1021/acs.nanolett.0c01311 [42] LOUNIS S D, RUNNERSTROM E L, LLORDÉS A, et al. Defect chemistry and plasmon physics of colloidal metal oxide nanocrystals[J]. The Journal of Physical Chemistry Letters,2014,5(9):1564-1574. doi: 10.1021/jz500440e [43] RUNNERSTROM E L, BERGERUD A, AGRAWAL A, et al. Defect engineering in plasmonic metal oxide nanocrystals[J]. Nano Letters,2016,16(5):3390-3398. doi: 10.1021/acs.nanolett.6b01171 [44] SACHET E, LOSEGO M D, GUSKE J, et al. Mid-infrared surface plasmon resonance in zinc oxide semiconductor thin films[J]. Applied Physics Letters,2013,102(5):051111. doi: 10.1063/1.4791700 [45] ALI H E, GANESH V, HARITHA L, et al. Kramers-Kronig analysis of the optical linearity and nonlinearity of nanostructured Ga-doped ZnO thin films[J]. Optics Laser Technology,2021,135:106691. doi: 10.1016/j.optlastec.2020.106691 [46] MENDELSBERG R J, ZHU Y, ANDERS A. Determining the nonparabolicity factor of the CdO conduction band using indium doping and the Drude theory[J]. Journal of Physics D: Applied Physics,2012,45(42):425302. doi: 10.1088/0022-3727/45/42/425302 [47] SYGLETOU M, MARANGI F, VARAS S, et al. Effective medium optical modelling of indium tin oxide nanocrystal films[J]. Physical Chemistry Chemical Physics,2022,24(9):5317-5322. doi: 10.1039/D1CP05897E [48] WANG J, SUN X W, JIAO Z. Application of nanostructures in electrochromic materials and devices: Recent progress[J]. Materials,2010,3(12):5029-5053. doi: 10.3390/ma3125029 [49] GUTPA J, SHAIK H, KUMAR K N, et al. PVD techniques proffering avenues for fabrication of porous tungsten oxide (WO3) thin films: A review[J]. Materials Science in Semiconductor Processing,2022,143:106534. doi: 10.1016/j.mssp.2022.106534 [50] MA D, WANG J. Inorganic electrochromic materials based on tungsten oxide and nickel oxide nanostructures[J]. Science China Chemistry,2017,60(1):54-62. doi: 10.1007/s11426-016-0307-x [51] WANG M, THIMONT Y, PRESMANES L, et al. The effect of the oxygen ratio control of DC reactive magnetron sputtering on as-deposited non stoichiometric NiO thin films[J]. Applied Surface Science,2017,419:795-801. doi: 10.1016/j.apsusc.2017.05.095 [52] DIXIT D, MADHURI K V. Effect of oxygen partial pressure on the growth of molybdenum trioxide thin films[J]. Materials Today: Proceedings,2019,19:2688-2692. doi: 10.1016/j.matpr.2019.10.140 [53] HAN Q, WANG R, ZHU H, et al. The preparation and investigation of all thin film electrochromic devices based on reactively sputtered MoO3 thin films[J]. Materials Science in Semiconductor Processing,2021,126:105686. doi: 10.1016/j.mssp.2021.105686 [54] WANG J, KHOO E, LEE P S, et al. Synthesis, assembly, and electrochromic properties of uniform crystalline WO3 nanorods[J]. The Journal of Physical Chemistry C,2008,112(37):14306-14312. doi: 10.1021/jp804035r [55] EVANS R C, AUSTIN R, MILLER R C, et al. Surface-facet-dependent electrochromic properties of WO3 nanorod thin films: Implications for smart windows[J]. ACS Applied Nano Materials,2021,4(4):3750-3759. doi: 10.1021/acsanm.1c00215 [56] YANG Z, CHOI D, KERISIT S, et al. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review[J]. Journal of Power Sources,2009,192(2):588-598. doi: 10.1016/j.jpowsour.2009.02.038 [57] WEN R T, NIKLASSON G A, GRANQVIST C G. Eliminating electrochromic degradation in amorphous TiO2 through Li-ion detrapping[J]. ACS Applied Materials & Interfaces,2016,8(9):5777-5782. [58] DHANDAYUTHAPANI T, SIVAKUMAR R, ILANGOVAN R, et al. High coloration efficiency, high reversibility and fast switching response of nebulized spray deposited anatase TiO2 thin films for electrochromic applications[J]. Electrochimica Acta,2017,255:358-368. doi: 10.1016/j.electacta.2017.09.187 [59] LU H C, GHOSH S, KATYAL N, et al. Synthesis and dual-mode electrochromism of anisotropic monoclinic Nb12O29 colloidal nanoplatelets[J]. ACS Nano,2020,14(8):10068-10082. doi: 10.1021/acsnano.0c03283 [60] LEE S H, DESHPANDE R, PARILLA P A, et al. Crystalline WO3 nanoparticles for highly improved electrochromic applications[J]. Advanced Materials,2006,18(6):763-766. doi: 10.1002/adma.200501953 [61] WANG J, KHOO E, LEE P S, et al. Controlled synthesis of WO3 nanorods and their electrochromic properties in H2SO4 electrolyte[J]. The Journal of Physical Chemistry C,2009,113(22):9655-9658. doi: 10.1021/jp901650v [62] 成明, 杨继凯, 杨馥瑜, 等. WO3/TiO2复合薄膜的制备及其电致变色性能[J]. 复合材料学报, 2019, 36(4):914-920.CHENG Ming, YANG Jikai, YANG Fuyu, et al. Preparation and electrochromic properties of WO3/TiO2 composite films[J]. Acta Materiae Compositae Sinica,2019,36(4):914-920(in Chinese). [63] ONG G K, SAEZ CABEZAS C A, DOMINGUEZ M N, et al. Electrochromic niobium oxide nanorods[J]. Chemistry of Materials,2020,32(1):468-475. doi: 10.1021/acs.chemmater.9b04061 [64] TANDON B, LU H C, MILLIRON D J. Dual-band electrochromism: Plasmonic and polaronic mechanisms[J]. The Journal of Physical Chemistry C,2022,126(22):9228-9238. doi: 10.1021/acs.jpcc.2c02155 [65] GUO C, YIN S, HUANG L, et al. Synthesis of one-dimensional potassium tungsten bronze with excellent near-infrared absorption property[J]. ACS Applied Materials & Interfaces,2011,3(7):2794-2799. [66] WU C M, NASEEM S, CHOU M H, et al. Recent advances in tungsten-oxide-based materials and their applications[J]. Frontiers in Materials, 2019, 6: 49. [67] YANG C, CHEN J F, ZENG X, et al. Enhanced near-infrared shielding ability of (Li, K)-codoped WO3 for smart windows: DFT prediction validated by experiment[J]. Nanotechnology,2016,27(7):075203. doi: 10.1088/0957-4484/27/7/075203 [68] KIM J, AGRAWAL A, KRIEG F, et al. The interplay of shape and crystalline anisotropies in plasmonic semiconductor nanocrystals[J]. Nano Letters,2016,16(6):3879-3884. doi: 10.1021/acs.nanolett.6b01390 [69] NAKAKURA S, ARIF A F, MACHIDA K, et al. Cationic defect engineering for controlling the infrared absorption of hexagonal cesium tungsten bronze nanoparticles[J]. Inorganic Chemistry,2019,58(14):9101-9107. doi: 10.1021/acs.inorgchem.9b00642 [70] HEO S, DAHLMAN C J, STALLER C M, et al. Enhanced coloration efficiency of electrochromic tungsten oxide nanorods by site selective occupation of sodium ions[J]. Nano Letters,2020,20(3):2072-2079. doi: 10.1021/acs.nanolett.0c00052 [71] MANTHIRAM K, ALIVISATOS A P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals[J]. Journal of the American Chemical Society,2012,134(9):3995-3998. doi: 10.1021/ja211363w [72] PARK S, PARK H S, DAO T T, et al. Solvothermal synthesis of oxygen deficient tungsten oxide nano-particle for dual band electrochromic devices[J]. Solar Energy Materials and Solar Cells,2022,242:111759. doi: 10.1016/j.solmat.2022.111759 [73] ZHANG S, CAO S, ZHANG T, et al. Monoclinic oxygen-deficient tungsten oxide nanowires for dynamic and independent control of near-infrared and visible light transmittance[J]. Materials Horizons,2018,5(2):291-297. doi: 10.1039/C7MH01128H [74] 丁举宣, 许争杰, 陈章贤. W18O49纳米棒的制备及电致变色性能研究[J]. 化工新型材料, 2022, 50(2):90-94.DING Juxuan, XU Zhengjie, CHEN Zhangxian. Preparation and electrochromic property of W18O49 nanorod[J]. New Chemical Materials,2022,50(2):90-94(in Chinese). [75] KIM J, ONG G K, WANG Y, et al. Nanocomposite architecture for rapid, spectrally-selective electrochromic modulation of solar transmittance[J]. Nano Letters,2015,15(8):5574-5579. doi: 10.1021/acs.nanolett.5b02197 [76] HEO S, KIM J, ONG G K, et al. Template-free mesoporous electrochromic films on flexible substrates from tungsten oxide nanorods[J]. Nano Letters,2017,17(9):5756-5761. doi: 10.1021/acs.nanolett.7b02730 [77] ZHANG S, CAO S, ZHANG T, et al. Al3+ intercalation/de-intercalation-enabled dual-band electrochromic smart windows with a high optical modulation, quick response and long cycle life[J]. Energy & Environmental Science,2018,11(10):2884-2892. [78] ZYDLEWSKI B Z, LU H C, CELIO H, et al. Site-selective ion intercalation controls spectral response in electrochromic hexagonal tungsten oxide nanocrystals[J]. The Journal of Physical Chemistry C,2022,126(34):14537-14546. doi: 10.1021/acs.jpcc.2c02865 [79] HEO S, CHO S H, DAHLMAN C J, et al. Influence of crystalline and shape anisotropy on electrochromic modulation in doped semiconductor nanocrystals[J]. ACS Energy Letters,2020,5(8):2662-2670. doi: 10.1021/acsenergylett.0c01236 [80] PASQUARELLI R M, GINLEY D S, O'HAYRE R. Solution processing of transparent conductors: From flask to film[J]. Chemical Society Reviews,2011,40(11):5406-5441. doi: 10.1039/c1cs15065k [81] JIA Y, LIU D Q, JIN Y Z, et al. Transparent dynamic infrared emissivity regulators[J]. Nature Communications, 2023, 14(1): 1-9. [82] ZANDI O, AGRAWAL A, SHEARER A B, et al. Impacts of surface depletion on the plasmonic properties of doped semiconductor nanocrystals[J]. Nature Materials,2018,17(8):710-717. doi: 10.1038/s41563-018-0130-5 [83] LU H C, ZYDLEWSKI B Z, TANDON B, et al. Understanding the role of charge storage mechanisms in the electrochromic switching kinetics of metal oxide nanocrystals[J]. Chemistry of Materials,2022,34(12):5621-5633. doi: 10.1021/acs.chemmater.2c00930 [84] WILLIAMS T E, CHANG C M, ROSEN E L, et al. NIR-selective electrochromic heteromaterial frameworks: A platform to understand mesoscale transport phenomena in solid-state electrochemical devices[J]. Journal of Materials Chemistry C,2014,2(17):3328-3335. doi: 10.1039/c3tc32247e [85] 贾汉祥, 曹逊, 金平实. 无机全固态电致变色材料与器件研究进展[J]. 无机材料学报, 2020, 35(5):511-524. doi: 10.15541/jim20190305JIA Hanxiang, CAO Xun, JIN Pingshi. Advances in inorganic all-solid-state electrochromic materials and devices[J]. Journal of Inorganic Materials,2020,35(5):511-524(in Chinese). doi: 10.15541/jim20190305 [86] LLORDES A, GARCIA G, GAZQUEZ J, et al. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites[J]. Nature,2013,500(7462):323-326. doi: 10.1038/nature12398 [87] 许可俊, 汪刘应, 刘顾, 等. 无机复合纳米材料电致变色薄膜研究进展[J]. 稀有金属材料与工程, 2021, 50(5):1840-1852.XU Kejun, WANG Liuying, LIU Gu, et al. Progress in inorganic composite nanomaterial electrochromic film[J]. Rare Metal Materials and Engineering,2021,50(5):1840-1852(in Chinese). [88] PATTATHIL P, GIANNUZZI R, MANCA M. Self-powered NIR-selective dynamic windows based on broad tuning of the localized surface plasmon resonance in mesoporous ITO electrodes[J]. Nano Energy,2016,30:242-251. doi: 10.1016/j.nanoen.2016.10.013 [89] MAHO A, SAEZ CABEZAS C A, MEYERTONS K A, et al. Aqueous processing and spray deposition of polymer-wrapped tin-doped indium oxide nanocrystals as electrochromic thin films[J]. Chemistry of Materials,2020,32(19):8401-8411. doi: 10.1021/acs.chemmater.0c02399 [90] REN Y, ZHOU X, WANG Q, et al. Novel preparation of ITO nanocrystalline films with plasmon electrochromic properties by the sol-gel method using benzoylacetone as a chemical modifier[J]. Ceramics International,2018,44(3):3394-3399. doi: 10.1016/j.ceramint.2017.11.130 [91] LIU R, REN Y, WANG J, et al. Preparation of Nb-doped TiO2 films by sol-gel method and their dual-band electrochromic properties[J]. Ceramics International,2021,47(22):31834-31842. doi: 10.1016/j.ceramint.2021.08.067 [92] BARAWI M, DE TRIZIO L, GIANNUZZI R, et al. Dual band electrochromic devices based on Nb-Doped TiO2 nanocrystalline electrodes[J]. ACS Nano,2017,11(4):3576-3584. doi: 10.1021/acsnano.6b06664 [93] CAO S, ZHANG S, ZHANG T, et al. Fluoride-assisted synthesis of plasmonic colloidal Ta-Doped TiO2 nanocrystals for near-infrared and visible-light selective electrochromic modulation[J]. Chemistry of Materials,2018,30(14):4838-4846. doi: 10.1021/acs.chemmater.8b02196 [94] GIANNUZZI R, DE DONATO F, DE TRIZIO L, et al. Tunable near-infrared localized surface plasmon resonance of F, In-codoped CdO nanocrystals[J]. ACS Applied Materials & Interfaces,2019,11(43):39921-39929. [95] RAO T, ZHOU Y, JIANG J, et al. Fluoride-assisted preparation of plasmonic oxygen-deficient MoO3−x nanowires for dual-band electrochromic smart windows[J]. Journal of The Electrochemical Society,2022,169(6):066506. doi: 10.1149/1945-7111/ac741e -






 下载:
下载: