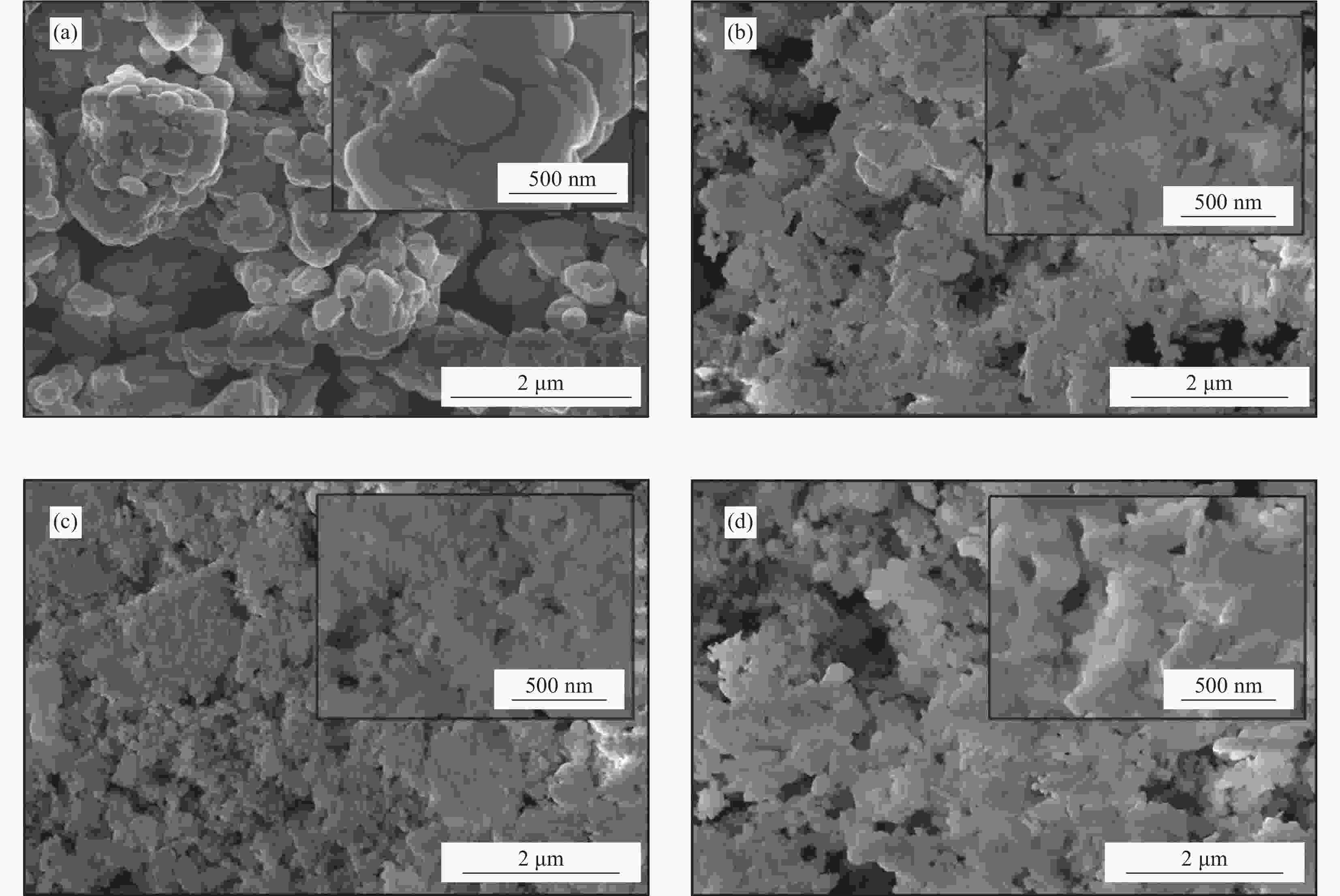
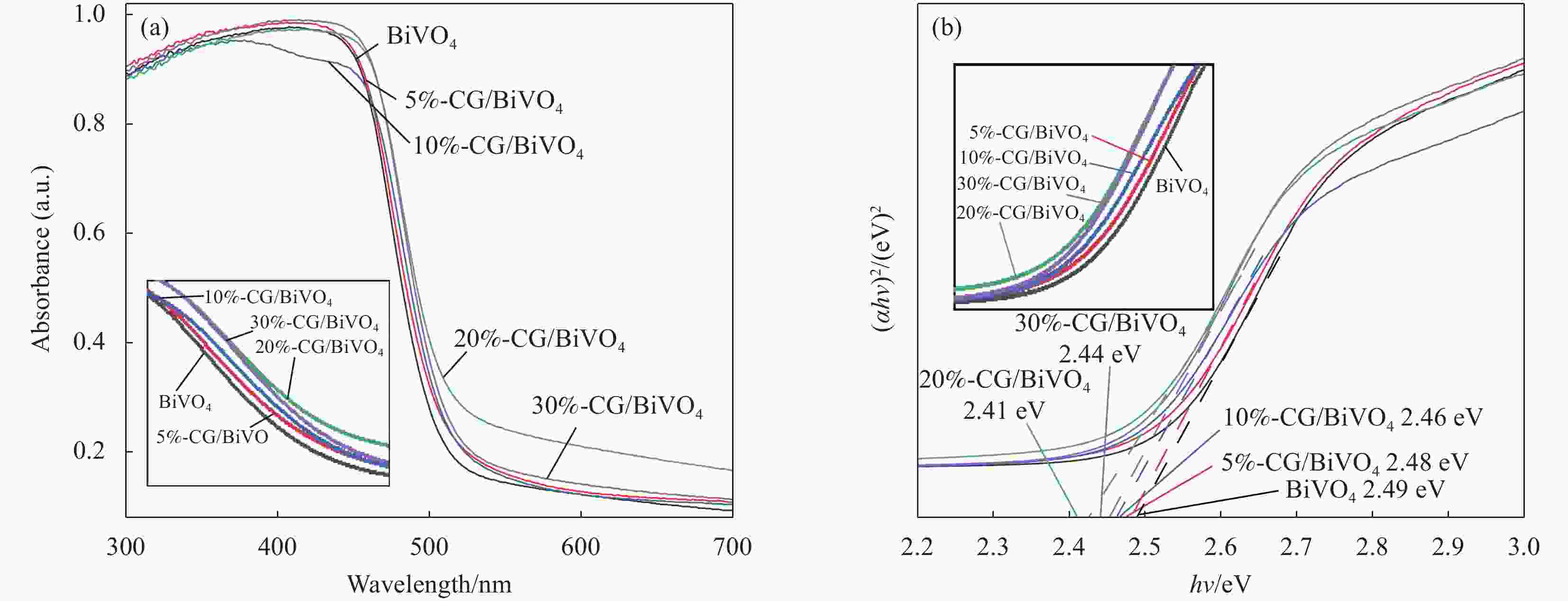
Preparation of coal gangue/BiVO4 composite photocatalyst and its degradation of xanthate wastewater
-
摘要: 选矿废水中的黄药会严重危害环境,钒酸铋能在可见光条件下实现黄药的降解,但其严重的电子-空穴复合影响其实用性,鉴于煤矸石丰富的孔隙结构及成分组成,本文采用水热法合成了煤矸石负载型光催化剂(CG/BiVO4),运用XRD、FTIR、SEM、UV-Vis DRS、PL等手段对催化剂进行表征,以黄药为目标污染物,在可见光作用下考察黄药的光催化降解性能及煤矸石改性钒酸铋的作用机制。结果表明,与纯BiVO4相比,负载型CG/BiVO4的光催化性能显著提高,在煤矸石负载量20wt%,pH=7、催化剂投加量为1.5 g/L、黄药初始浓度C0=10 mg/L的条件下,CG/BiVO4在540 min时对黄药的降解率达到最大,为93%,黄药的光降解过程符合一级动力学模型,处理后化学需氧量浓度CCOD为11.47 mg/L,符合排放标准要求。响应面分析得到的最优反应条件下的预测值与实际降解率仅相差0.96%,可见该模型可较好地预测20%-CG/BiVO4对黄药废水的降解率。结合各项表征分析可知,CG的负载可显著增加催化剂的比表面积,提高其对可见光的响应,改善光催化体系中电子和空穴的分离效率的同时降低光生电子-空穴对复合几率,这是复合光催化剂性能提高的重要原因;机制分析得知,黄药光降解的主要自由基为h+,•O2− 和•OH次之,黄药中的烷基、C=S在自由基作用下首先发生断裂,形成中间产物过黄药(ROCSSO−),随后矿化生成SO42−等小分子,光反应7 h,S的转化率与黄药降解率接近,且循环5次,降解率不低于90%,可见该催化剂具有较好的光催化性能,这为煤矸石在光催化领域的资源化利用奠定了理论基础。Abstract: Xanthate in mineral processing wastewater can do serious harm to environment. Bismuth vanadate can degrade xanthate under visible light, but its serious electron-hole complex affects its practicability, in view of the abundant pore structure and composition of coal gangue, the coal gangue supported photocatalyst (CG/BiVO4) was synthesized by hydrothermal method. The catalysts were characterized by XRD, FTIR, SEM, UV-Vis DRS, PL, the photocatalytic degradation of xanthate and the mechanism of coal gangue modified bismuth vanadate were investigated under visible light. The results showed that the photocatalytic activity of the supported CG/BiVO4 is significantly higher than that of the pure BiVO4. Under the conditions of 20wt% coal gangue loading, pH=7, catalyst dosage is 1.5 g/L, initial concentration of xanthate C0=10 mg/L, the degradation rate of xanthate over CG/BiVO4 reached the maximum at 540 min, which was 93% . The photodegradation process of xanthate complied with the first-order kinetic model. After treatment, the concentration of chemical oxygen demand CCOD was 11.47 mg/L, which met the emission standard. The predicted degradation rate of 20%-CG/BiVO4 was only 0.96% different from the actual degradation rate, which indicated that the model could predict the degradation rate of 20%-CG/BiVO4. The results show that the loading of CG can significantly increase the specific surface area of the catalyst, improve its response to visible light, increase the separation efficiency of electrons and holes in the photocatalytic system, and decrease the recombination probability of photogenerated electrons and holes, this is an important reason for the improvement of the performance of the composite photocatalyst, the mechanism analysis showed that h+ was the main free radical in xanthate photodegradation, while •O2 − and •OH were secondary, under the action of free radical, the alkyl group and C=S in the xanthate were firstly broken, and formed the intermediate product, peroxy xanthate (ROCSSO−) , then mineralized to form small molecules such as SO42−. After 7 h of photoreaction, the conversion of Sulfur was close to the degradation rate of xanthate, and the degradation rate was not less than 90% after 5 cycles, it can be seen that the catalyst has good photocatalytic performance, which lays a theoretical foundation for the resource utilization of coal gangue in the photocatalytic field.
-
Key words:
- coal gangue /
- BiVO4 /
- photocatalysis /
- xanthate /
- visible light /
- degradation /
- wastewater
-
图 8 (a) CG、BiVO4与CG/BiVO4对黄药的吸附平衡图;(b) CG、BiVO4与CG/BiVO4对黄药的降解效果图;(c) 一级动力学模型
Figure 8. (a) Adsorption equilibrium diagram of CG, BiVO4 and CG/BiVO4 composite on xanthate; (b) Degradation effect of CG, BiVO4 and CG/BiVO4 composite on xanthate; (c) The first-order kinetic model
k—First-order kinetics constant; C0—Initial concentration of xanthate solution (25 mg/L); C—Xanthate concentration in solution
表 1 BiVO4与煤矸石(CG)/BiVO4的命名与比表面积
Table 1. Naming and specific surface area of BiVO4 and coal gangue (CG)/BiVO4
Sample Specific surface area/(m2∙g−1) Mass fraction of CG/wt% BiVO4 2.5 — 5%-CG/BiVO4 7.5 5 10%-CG/BiVO4 8.0 10 20%-CG/BiVO4 8.2 20 30%-CG/BiVO4 8.7 30 表 2 影响CG/BiVO4光降解黄药的因素及水平
Table 2. Factors and levels affecting CG/BiVO4 photodegradation of xanthate
Factor Lever −1 0 +1 pH 7 9 11 Dosage of catalyst/(g∙L−1) 3 3.5 4 C0/(mg∙L−1) 10 25 50 Note: C0—Xanthate initial concentration. 表 3 优化CG/BiVO4光降解黄药实验中回归模型的方差分析1
Table 3. Variance analysis of regression model 1 in optimizing CG/BiVO4 photodegradation of xanthate
Source SS DF Mean square F value p value prob>F Coefficient Model 118.69 9 13.19 49.93 <0.0001 Significant A 43.88 1 43.88 166.11 <0.0001 −2.38 B 0.26 1 0.26 1.00 0.3505 −0.18 C 32.36 1 32.36 122.52 <0.0001 −2.01 AB 0.56 1 0.56 2.13 0.1878 0.37 AC 14.63 1 14.63 55.37 0.0001 1.88 BC 0.36 1 0.36 1.35 0.2830 0.29 A2 12.23 1 12.23 46.30 0.0003 1.70 B2 2.77 1 2.77 10.50 0.0142 0.81 C2 1.19 1 1.19 4.49 0.0718 0.58 Residual 1.85 7 0.26 Lack of fit 1.30 3 0.43 3.19 0.1460 Not significant Pure error 0.55 4 0.14 Cor total 120.54 16 Notes: A—Initial pH of the reaction; B—Catalyst dosage; C—Initial concentration of xanthate; SS—Sum of squares; DF—Degree of freedom. 表 4 优化CG/BiVO4光降解黄药实验中回归模型的方差分析2
Table 4. Variance analysis of regression model 2 in optimizing CG/BiVO4 photodegradation of xanthate
Project Value Project Value Std.Dev 0.51 R2 0.9847 Mean 87.49 Adj R-Squared 0.9649 CV/% 0.59 Pred R-Squared 0.8166 PRESS 22.10 Adeq precisior 23.555 Notes: Std.Dev—Standard deviation; CV—Coefficient of variation; PRESS—Predicted residual error sum of square; R2—Coefficient of determination; Adj—Adjusted multiple correlation cofficient; Pred—Predictive correlation coefficient; Adeq—Adeq precision. 表 5 CG/BiVO4光降解黄药过程中的SO42−浓度与S转化率
Table 5. SO42− concentration and sulfur conversion in photodegradation of xanthate by CG/BiVO4
Light time/h Concentration of
SO42−/(mg·L−1)Conversion of
sulfur/%1 12.86 25.72 2 22.25 44.50 3 27.48 54.96 4 28.75 57.50 5 31.29 62.58 6 38.24 76.48 7 45.34 90.68 -
[1] AMROLLAHI A, MASSINAEI M, ZERAATKAR MOGHADDAM A. Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles[J]. Minerals Engineering,2019,134:142-155. doi: 10.1016/j.mineng.2019.01.031 [2] 尚锦燕. 选矿药剂中的环保问题[J]. 现代矿业, 2020, 36(2):212-215.SHANG Jinyan. Discussion on environmental protection in mineral processing reagents[J]. Modern Mining,2020,36(2):212-215(in Chinese). [3] MOHAMED R M, IBRAHIM F M. Visible light photocatalytic reduction of nitrobenzene using Ag/Bi2MoO6 nanocomposite[J]. Journal of Industrial and Engineering Chemistry,2015,22:28-33. doi: 10.1016/j.jiec.2014.06.021 [4] SHEN Y B, ZHOU P F, ZHAO S K, et al. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate[J]. Minerals Engineering,2020,159:106640. doi: 10.1016/j.mineng.2020.106640 [5] ZHOU P F, SHEN Y B, ZHAO S K, et al. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates[J]. Applied Surface Science,2021,542:148578. doi: 10.1016/j.apsusc.2020.148578 [6] BIAN Z Z, FENG Y L, LI H R, et al. Fabrication of Ag3PO4/TiO2@molecular sieve(MS) ternary composites with remarkably enhanced visible light-responded photocatalytic activity and mechanism insight[J]. Environmental Research,2020,190:109984. doi: 10.1016/j.envres.2020.109984 [7] 梁锐, 李明阳, 高翔鹏, 等. 选矿废水中残留黄药光催化处理及降解效率改进方式研究进展[J]. 过程工程学报, 2022, 22(1):1-13.LIANG Rui, LI Mingyang, GAO Xiangpeng, et al. Research progress on photocatalytic treatment of residual xanthatein mineral processing waste water and improvement of degradation efficiency[J]. The Chinese Journal of Process Engineering,2022,22(1):1-13(in Chinese). [8] ZHOU L, WANG W Z, LIU S W, et al. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst[J]. Journal of Molecular Catalysis A: Che-mical,2006,252(1-2):120-124. doi: 10.1016/j.molcata.2006.01.052 [9] GE L. Novel visible-light-driven Pt/BiVO4 photocatalyst for efficient degradation of methyl orange[J]. Journal of Molecular Catalysis A: Chemical,2008,282(1-2):62-66. doi: 10.1016/j.molcata.2007.11.017 [10] 李欣, 王铁成, 屈广周, 等. 碳纳米管/钒酸铋光催化降解盐酸四环素[J]. 环境工程学报, 2017, 11(5):2738-2742.LI Xin, WANG Tiecheng, QU Guangzhou, et al. Degradation of tetracycline hydrochloride by CNTs/BiVO4 photocatalytic[J]. Chinese Journal of Environmental Engineering,2017,11(5):2738-2742(in Chinese). [11] ZHOU S X. Study on the reaction degree of calcined coal gangue powder in blended cement by selective solution method[J]. Procedia Earth and Planetary Science,2009,1(1):634-639. doi: 10.1016/j.proeps.2009.09.100 [12] 高平强, 魏建雄, 陈嘉, 等. TiO2/改性煤矸石复合光催化材料的制备及其去除水体中苯酚[J]. 矿产综合利用, 2021, 42(6):73-80.GAO Pingqiang, WEI Jianxiong, CHEN Jia, et al. Preparation of TiO2/coal gangue composite photocatalyst and its application in phenol removal from water[J]. Multipurpose Utilization of Mineral Resources,2021,42(6):73-80(in Chinese). [13] UMAPATHY V, MANIKANDAN A, ARUL ANTONY S, et al. Structure, morphology and opto-magnetic properties of Bi2MoO6 nano-photocatalyst synthesized by sol-gel method[J]. Transactions of Nonferrous Metals Society of China,2015,25(10):3271-3278. doi: 10.1016/S1003-6326(15)63948-6 [14] 索静, 柳丽芬, 杨凤林. 负载型Cu-BiVO4复合光催化剂的制备及可见光降解气相甲苯[J]. 催化学报, 2009, 30(4):323-327.SUO Jing, LIU Lifen, YANG Fenglin. Preparation of supported Cu-BiVO4 photocatalyst and its application in oxidative removal of toluene in air[J]. Chinese Journal of Catalysis,2009,30(4):323-327(in Chinese). [15] 徐晶威, 李政, 王泽普, 等. 交错能带结构钕掺杂钒酸铋形貌与光催化性能调控[J]. 无机材料学报, 2020, 35(7):789-795.XU Jingwei, LI Zheng, WANG Zepu, et al. Morphology and photocatalytic performance regulation of Nd3+-doped BiVO4 with staggered band structure[J]. Journal of Inorganic Materials,2020,35(7):789-795(in Chinese). [16] SHEN Q, ZHANG Y H, FAN Y J, et al. On-line in situ ATR-FTIR study on the adsorption behavior of heptyl xanthate on ZnO and Cu (II) activated ZnO surface[J]. Transactions of Nonferrous Metals Society of China,2022,32(7):2370-2378. doi: 10.1016/S1003-6326(22)65953-3 [17] SAMSUDIN M F R, BASHIRI R, MOHAMED N M, et al. Tailoring the morphological structure of BiVO4 photocatalyst for enhanced photoelectrochemical solar hydrogen production from natural lake water[J]. Applied Surface Science,2020,504:144417. doi: 10.1016/j.apsusc.2019.144417 [18] CHEN Y S, LIN L Y. Novel synthesis of highly ordered BiVO4 nanorod array for photoelectrochemical water oxidation using a facile solution process[J]. Journal of Power Sources,2019,436:226842. doi: 10.1016/j.jpowsour.2019.226842 [19] WANG Y, TAN G Q, LIU T, et al. Photocatalytic properties of the g-C3N4/{010} facets BiVO4, interface Z-scheme photocatalysts induced by BiVO4, surface heterojunction[J]. Applied Catalysis B: Environmental,2018,234:37-49. doi: 10.1016/j.apcatb.2018.04.026 [20] 李秀玲, 邓丽霞, 黄秀枫, 等. 纳米TiO2/改性煤矸石复合材料的制备及性能研究[J]. 化工新型材料, 2021, 49(4):142-147.LI Xiuling, DENG Lixia, HUANG Xiufeng, et al. Synthesis and property of nano-TiO2/modified coal gangue compo-site[J]. New Chemical Materials,2021,49(4):142-147(in Chinese). [21] 刘景景, 张泽兰, 赵伟. Tb改性BiVO4/BiOCl复合光催化剂的制备及性能研究[J]. 钢铁钒钛, 2021, 42(4):39-46.LIU Jingjing, ZHANG Zelan, ZHAO Wei. Synthesis and properties of Tb modified BiVO4/BiOCl composite photocatalysts[J]. Iron Steel Vanadium Titanium,2021,42(4):39-46(in Chinese). [22] RESSNIG D, KONTIC R, PATZKE G R. Morphology control of BiVO4 photocatalysts: pH optimization vs self-organization[J]. Materials Chemistry and Physics,2012,135(2-3):457-466. [23] 高善民, 乔青安, 赵培培, 等. 沉淀法制备不同形貌和结构的纳米BiVO4[J]. 无机化学学报, 2007, 23(7):1153-1158.GAO Shanmin, QIAO Qing'an, ZHAO Peipei, et al. Nano-BiVO4 with different morphologies and structures was prepared by precipitation method[J]. Chinese Journal of Inorganic Chemistry,2007,23(7):1153-1158(in Chinese). [24] HONG T T, LIU Z F, ZHANG J, et al. Flower-like Cu2In2ZnS5 nanosheets: A novel promising photoelectrode for water splitting[J]. Chemcatchem,2016,8(7):1288-1292. doi: 10.1002/cctc.201600066 [25] MA D M, ZHONG J B, PENG R F, et al. Effective photoinduced charge separation and photocatalytic activity of hierarchical microsphere-like C60/BiOCl[J]. Applied Surface Science,2019,465:249-258. doi: 10.1016/j.apsusc.2018.09.192 [26] CHEN F, YANG Q, SUN J, et al. Enhanced photocatalytic degradation of tetracycline by Agl/BiVO4 heterojunction under visible-light irradiation: Mineralization efficiency and mechanism[J]. ACS Applied Materials & Interfaces,2016,8(48):32887-32900. [27] ZHANG S, ZHANG N, ZHAO W, et al. Green preparation of hierarchical porous C/SiOx composites from coal gangue as anodes for Li-ion batteries[J]. Solid State Ionics,2021,371:115772. doi: 10.1016/j.ssi.2021.115772 [28] LI H, ZHENG F, WANG J, et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance[J]. Chemical Engineering Journal,2020,390:124513. doi: 10.1016/j.cej.2020.124513 [29] 单爽, 杨占旭. 四角星形BiVO4/Bi2O3催化剂的制备及性能[J]. 无机化学学报, 2016, 32(4):649-654.SHAN Shuang, YANG Zhanxu. Preparation and performance of four anglestar-like BiVO4/Bi2O3 catalyst[J]. Chinese Journal of Inorganic Chemistry,2016,32(4):649-654(in Chinese). [30] 宋卫军, 谢妤, 胡家朋, 等. BiVO4/RGO复合光催化剂的制备与光催化活性分析[J]. 武夷学院学报, 2022, 41(12):7-13.SONG Weijun, XIE Yu, HU Jiapeng, et al. Preparation and photocatalytic activity analysis of BiVO4/RGO composite photocatalyst[J]. Journal of Wuyi College,2022,41(12):7-13(in Chinese). [31] 国家环境保护总局. 污水综合排放标准: GB 8978—1996[S]. 北京: 中国标准出版社, 1996.Ministry of Environmental Protection of the People's Republic of China. Comprehensive sewage discharge standard: GB 8978—1996[S]. Beijing: China Standard Press, 1996(in Chinese). [32] 付保军, 陈建华. TiO2光催化降解黄药试验研究[J]. 矿产保护与利用, 2005, 25(2):43-47.FU Baojun, CHEN Jianhua. Research on the photocatalytic degradation of xanthate in TiO2 suspension[J]. Mineral Protection and Utilization,2005,25(2):43-47(in Chinese). [33] 宋小霞, 朱静, 张承屏, 等. 黄药的研制、应用和水污染处理研究进展[J]. 贵州化工, 2012, 37(3): 19-22, 25.SONG Xiaoxia, ZHU Jing, ZHANG Chengping, et al. Advance in research of production, application and treatment process of xanthate in dressing wastewater[J]. Guizhou Chemical Industry, 2012, 37(3): 19-22, 25(in Chinese). [34] WANG T, LI W W, XUD D, et al. Strong visible absorption and excellent photocatalytic performance of brown TiO2 nanoparticles synthesized using one-step low-temperature process[J]. Chinese Journal of Catalysis,2017,38(7):1184-1195. doi: 10.1016/S1872-2067(17)62855-9 [35] 刘嘉友, 聂倩倩, 俞和胜, 等. 卷心菜状Bi2WO6光催化降解黄药废水[J]. 金属矿山, 2020, 524(2):122-128.LIU Jiayu, NIE Qianqian, YU Hesheng, et al. Photocatalytic degradation of xanthate in wastewater using cabbage-like Bi2WO6[J]. Metal Mine,2020,524(2):122-128(in Chinese). [36] VAHUR S, TEEARU A, PEETS P, et al. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm−1[J]. Analytical and Bioanalytical Chemistry,2016,408(13):3373-3379. doi: 10.1007/s00216-016-9411-5 [37] GUO Y J, CUI K X, HU M Y, et al. Fe (III) ions enhanced catalytic properties of (BiO)2CO3 nanowires and mechanism study for complete degradation of xanthate[J]. Chemosphere,2017,181:190-196. doi: 10.1016/j.chemosphere.2017.04.069 [38] HAO F P, SILVESTER E, SENIOR G D. Spectroscopic characterization of ethyl xanthate oxidation products and analysis by ion interaction chromatography[J]. Analytical Chemistry,2000,72(20):4836-4845. doi: 10.1021/ac991277o [39] 李官超, 祝瑄, 滕青, 等. TiO2@芽孢杆菌光催化性能研究[J]. 金属矿山, 2021(8):186-195.LI Guanchao, ZHU Xuan, TENG Qing, et al. Study on photocatalytic performance of TiO2@Bacillus[J]. Metal Mine,2021(8):186-195(in Chinese). [40] 陈运双, 马瑞雪, 蒋潇宇, 等. TiO2/蒙脱土复合材料光催化降解丁基黄药性能研究[J]. 金属矿山, 2022(5):212-220.CHEN Yunshuang, MA Ruixue, JIANG Xiaoyu, et al. Study on photocatalytic degradation properties of butyl xanthate by TiO2/montmorillonite composites[J]. Metal Mine,2022(5):212-220(in Chinese). -






 下载:
下载: