Preparation and biological application of high strength hydrogels based on silk fibroin
-
摘要: 水凝胶具有亲水三维网络结构,因其结构和功能与生物组织相似的特性,使其在生物医学等领域中得以广泛应用。蚕丝蛋白(Silk fibroin,SF)由于其资源丰富,具有良好的生物降解性和生物相容性,成为一种极具潜力的水凝胶基材。然而,由于在蚕丝蛋白制备过程中对蚕丝纤维天然层级结构的溶解和破坏,丧失了蚕丝纤维机械强度高的天然优势,导致力学性能差成为限制蚕丝蛋白基水凝胶广泛应用的主要原因之一,因此,研究者不断寻求策略制备蚕丝蛋白基高强度水凝胶(SF-high strength hydrogels,SF-HSHs)。本文首先介绍了SF的基本结构;然后阐述了SF水凝胶的制备方法和凝胶化机制;进而详细讨论了物理交联、双交联、双网络和复合SF-HSHs;最后简要分析了SF-HSHs的生物应用及其前景与挑战。Abstract: Hydrogels have hydrophilic three-dimensional network structure, which are widely used in biomedicine and other fields because of its similar structure and function to biological tissue. Silk fibroin (SF) has become one of potential hydrogel substrates due to its abundant resources, good biodegradability and biocompatibility. However, due to the dissolution and destruction of the natural hierarchical structure of silk fibers in the process of preparing silk fibroin, the natural advantage of high mechanical strength of silk fibers is lost, and the poor mechanical properties become one of the main reasons limiting the wide application of silk fibroin-based hydrogels. Therefore, researchers are constantly seeking strategies to prepare silk fibroin-based high strength hydrogels (SF-HSHs). This review first introduced the basic structure of SF. Then the preparation methods and gelation mechanisms of SF hydrogel were described. Furthermore, physical cross-linking, dual cross-linking, dual network and composite SF-HSHs were discussed in detail. Finally, the biological applications, prospects and challenges of SF-HSHs were briefly analyzed.
-
Key words:
- silk fibroin /
- hydrogel /
- high strength /
- gelation mechanism /
- biological applications
-
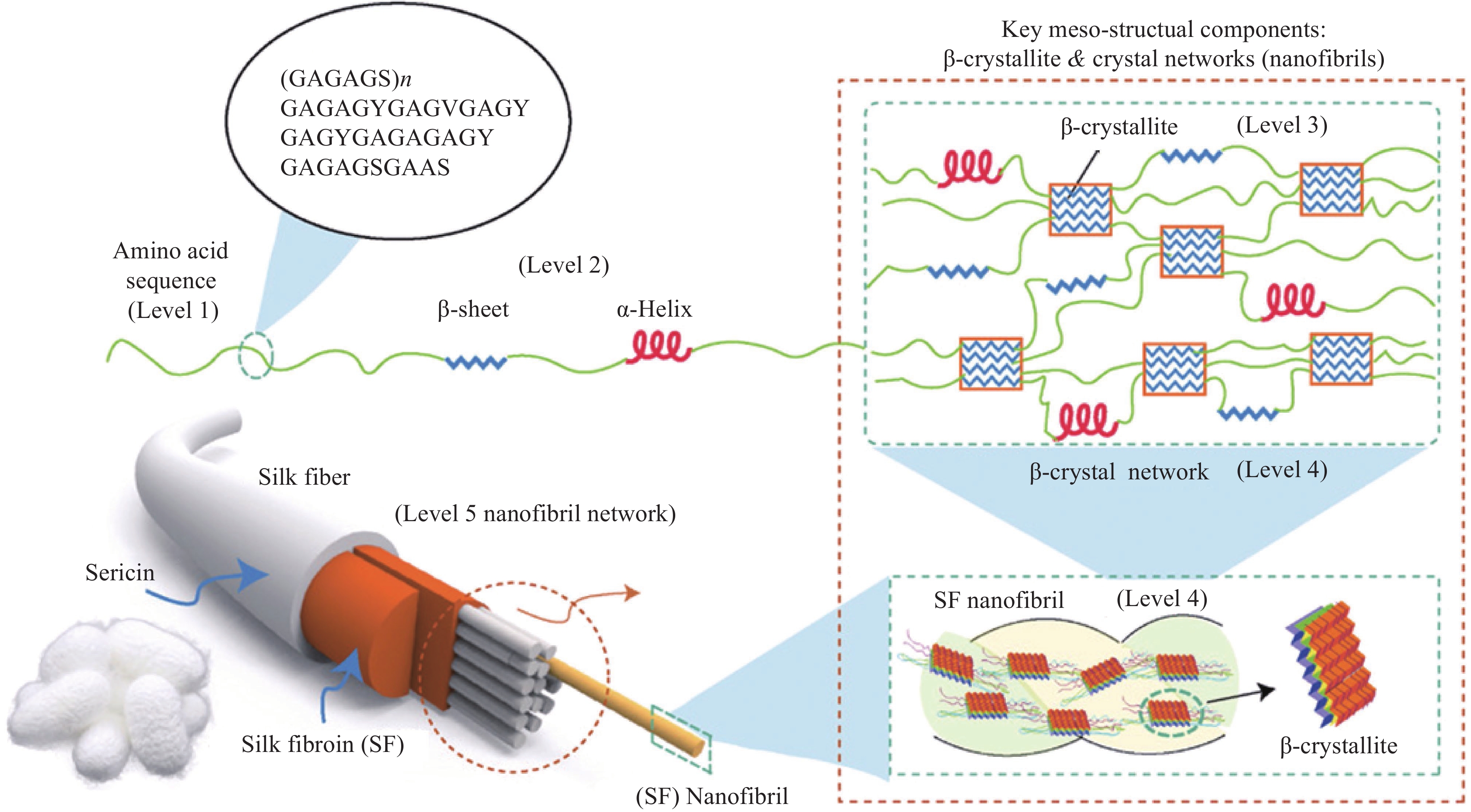
图 3 蚕丝蛋白纳米纤维分散液的制备:(a) 氯化钙/乙醇/水三元体系[13];(b) 硫酸水解体系[28];(c) 碱处理体系[29];(d) 低共熔溶剂(DES)体系预处理[30]
Figure 3. Preparation of silk nanofiber dispersion: (a) Ternary system of CaCl2/ethanol/water[13]; (b) Sulfuric acid hydrolysis treatment[28]; (c) Alkali treatment system[29]; (d) Pretreatment of deep eutectic solvents (DES) system[30]
SMFs—Silk millimeter/microfibers
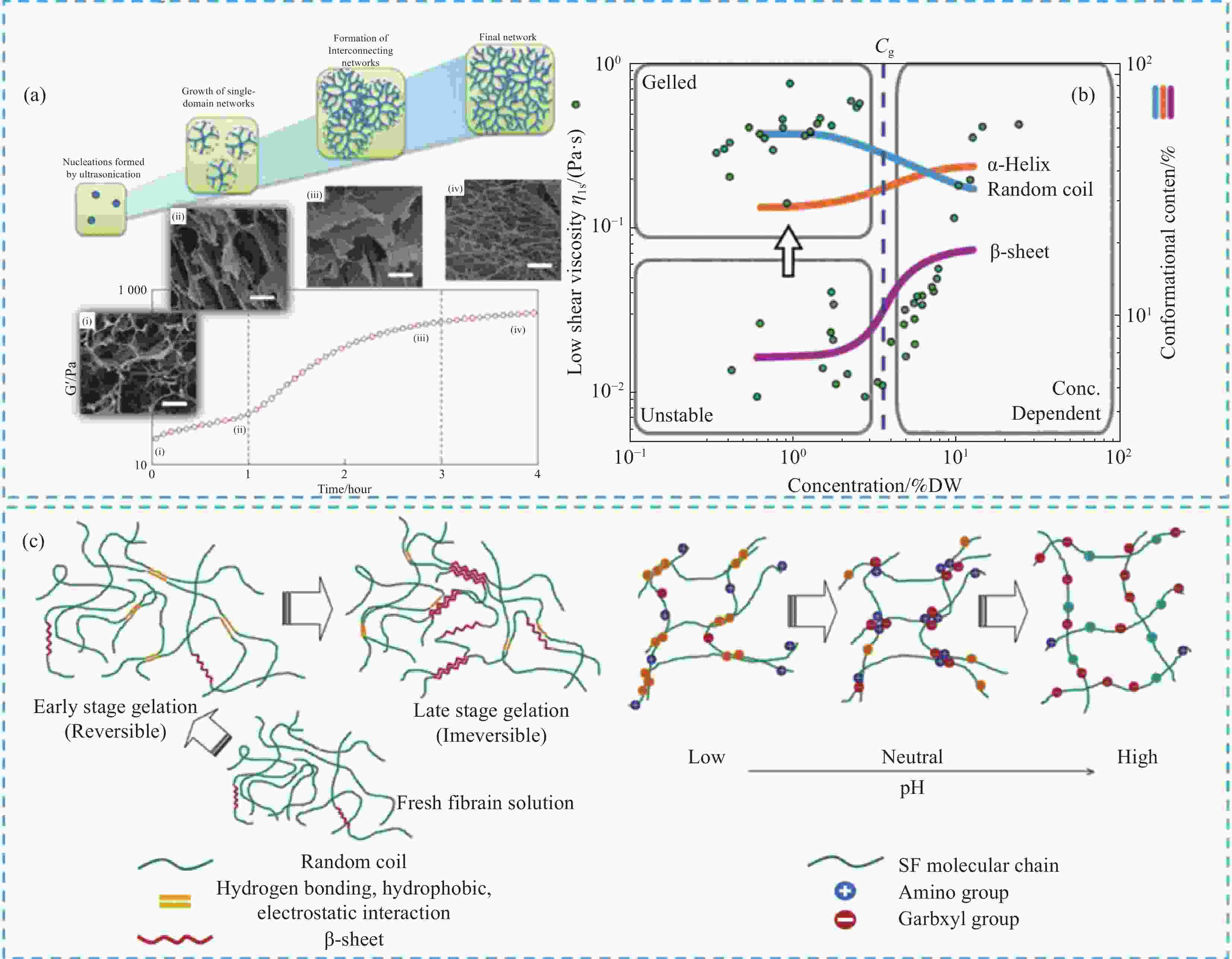
图 4 (a) 不同凝胶化阶段纳米纤维网络的形成图解[33];(b) 丝蛋白浓度对二级结构和凝胶化的影响[34];(c) SF凝胶化阶段与pH对凝胶化的调控[35]
Figure 4. (a) Illustration of the formation of nanofibrillar network at different gelation stages[33]; (b) Effect of silk protein concentration on secondary structure and gelation[34];(c) The gelation stage of SF and the regulation of pH on gelation[35]
Cg—Critical concentration; DW—Drained mass
图 7 (a) 二元溶剂诱导构象转变(BSICT)策略制备具有高力学性能SF水凝胶[61];(b) SDS调节SF物理凝胶化[62]
Figure 7. (a) Preparation of SF hydrogels with high mechanical properties by binary solvent Induced conformational transformation (BSICT) strategy[61]; (b) SDS regulates the physical gelation of SF[62]
DI—Deionized water; DS—Negatively charged ionic groups ionized from SDS in water
图 8 (a) 双交联SF-HSHs双交联凝胶化示意图[70];(b) 辣根过氧化物酶(HRP)/H2O2+乙醇交联[63];(c) γ射线+乙醇诱导双交联[55]
Figure 8. (a) Double-crosslinked SF-HSHs schematic diagram of double-crosslinked gelation[70]; (b) Horse radish peroxidase (HRP)/H2O2+ ethanol crosslinking[63]; (c) Double cross-linking induced by γ-ray and ethanol[55]
RSF—Regenerated silk fibroin; EE—Enzyme-electric field; N—Naturally formed physical crosslinking; EE—Enzyme-electric field-alcohol; EA—Enzyme and Alcohol-treated; E—Enzymatic; SF-E—Silk fibroin-ethanol crosslinking; SF-S—Silk fibroin-γ-ray radiation crosslinking; SF-D—Silk fibroin- γ-ray radiation and ethanol crosslinking
图 9 (a) 制备复合SF水凝胶的反向透析示意图;(b) SF水凝胶和其他基于SF的水凝胶的断裂应力与杨氏模量对比图;(c) 断裂应变与杨氏模量的关系[65]
Figure 9. (a) Scheme of reverse dialysis procedure to fabricate composite SF hydrogels; (b) Ashby plot of fracture stress versus Young’s modulus; (c) Fracture strain versus Young’s modulus of SF hydrogels and other reported SF-based hydrogels[65]
PAA—Polyacrylic acid; PVA—Polyvinyl alcohol; PAMPS—Poly(2-acrylamido-2-methylpropanesulfonic acid)
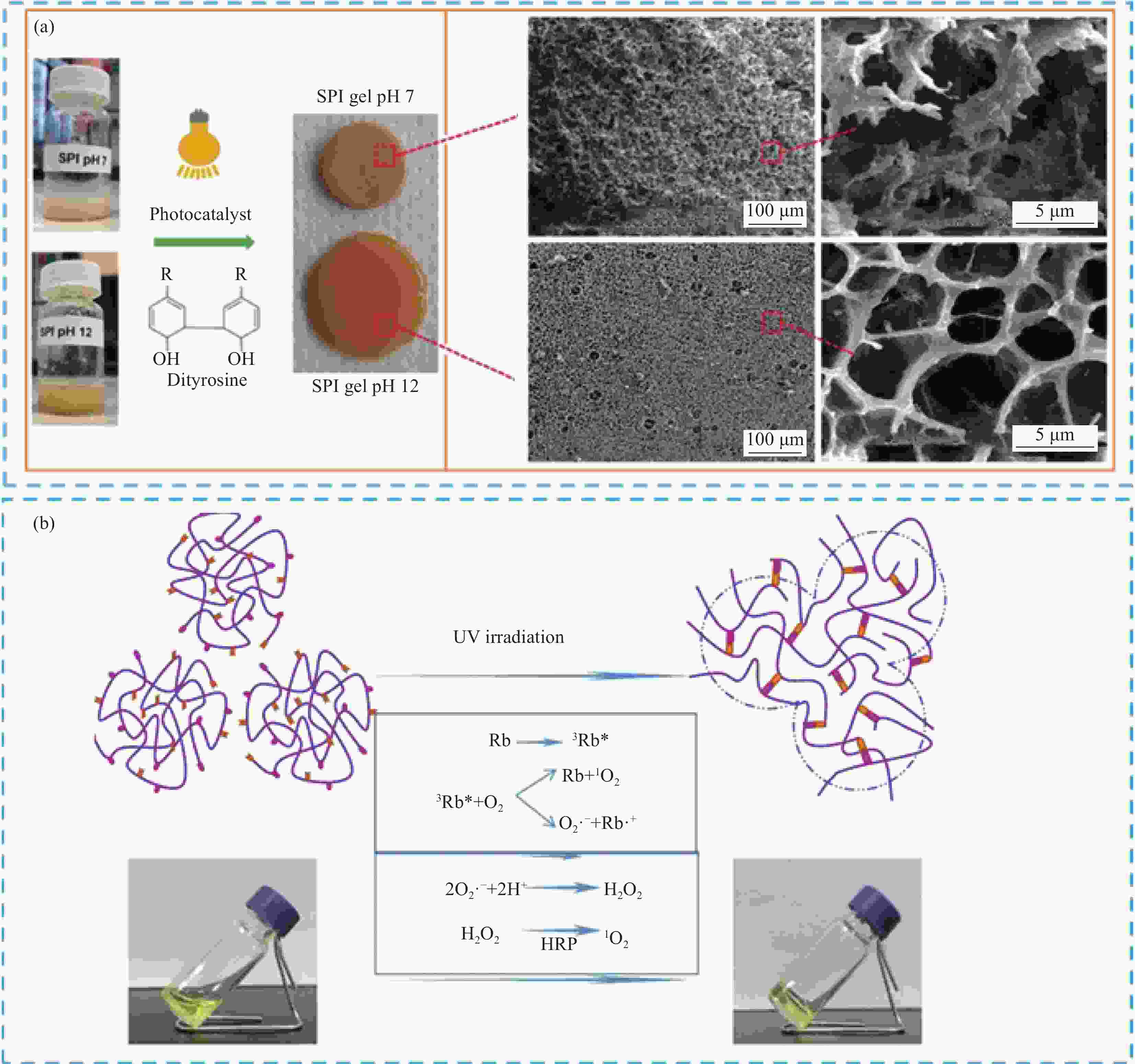
图 11 SF-HSHs应用于组织工程:(a) 细菌纤维素与明胶-丝蛋白水凝胶混合,增强3D打印水凝胶支架力学性能[78];(b) 内源性骨髓干细胞(BMSC)与明胶/丝蛋白结合,用于关节软骨修复[79];(c) 两种丝纳米纤维与HRP和电场诱导交联增强水凝胶,用于骨组织再生[80];(d) 光化学交联SPI/SF水凝胶,提高力学性能和细胞附着效果[81]
Figure 11. Application of SF-HSHs in tissue engineering: (a) Mixing bacterial cellulose with gelatin-silk hydrogel to enhance the mechanical properties of 3D printed hydrogel scaffolds[78]; (b) Endogenous bone marrow stem cells (BMSC) bound to gelatin/silk protein scaffold for articular cartilage repair[79]; (c) Two kinds of silk nanofibers were crosslinked with HRP and electric field induced hydrogel for bone regeneration[80]; (d) Photochemically cross-linked SPI/SF hydrogels to improve mechanical properties and cell attachment[81]
SFG—Silk fibroin gelatin; MF—Microfracture; E7—EPLQLKM molecular; SANS—Small angle neutron scattering
图 12 SF-HSHs用作黏合剂:(a) 丝素蛋白-聚丙烯酰胺双网络水凝胶在汗湿条件下对人体皮肤有强附着力[83];(b) SF@单宁酸(TA)@羟基磷灰石(HA)提高了水凝胶的韧性和黏附强度,可作为骨黏合剂[84]
Figure 12. SF-HSHs used as adhesive: (a) SF-PAAm DN hydrogel has strong adhesion to human skin under the condition of sweat[83]; (b) SF@tannic acid (TA)@hydroxyapatite (HA) improves the toughness and adhesion strength of hydrogel and can be used as bionic bone adhesive[84]
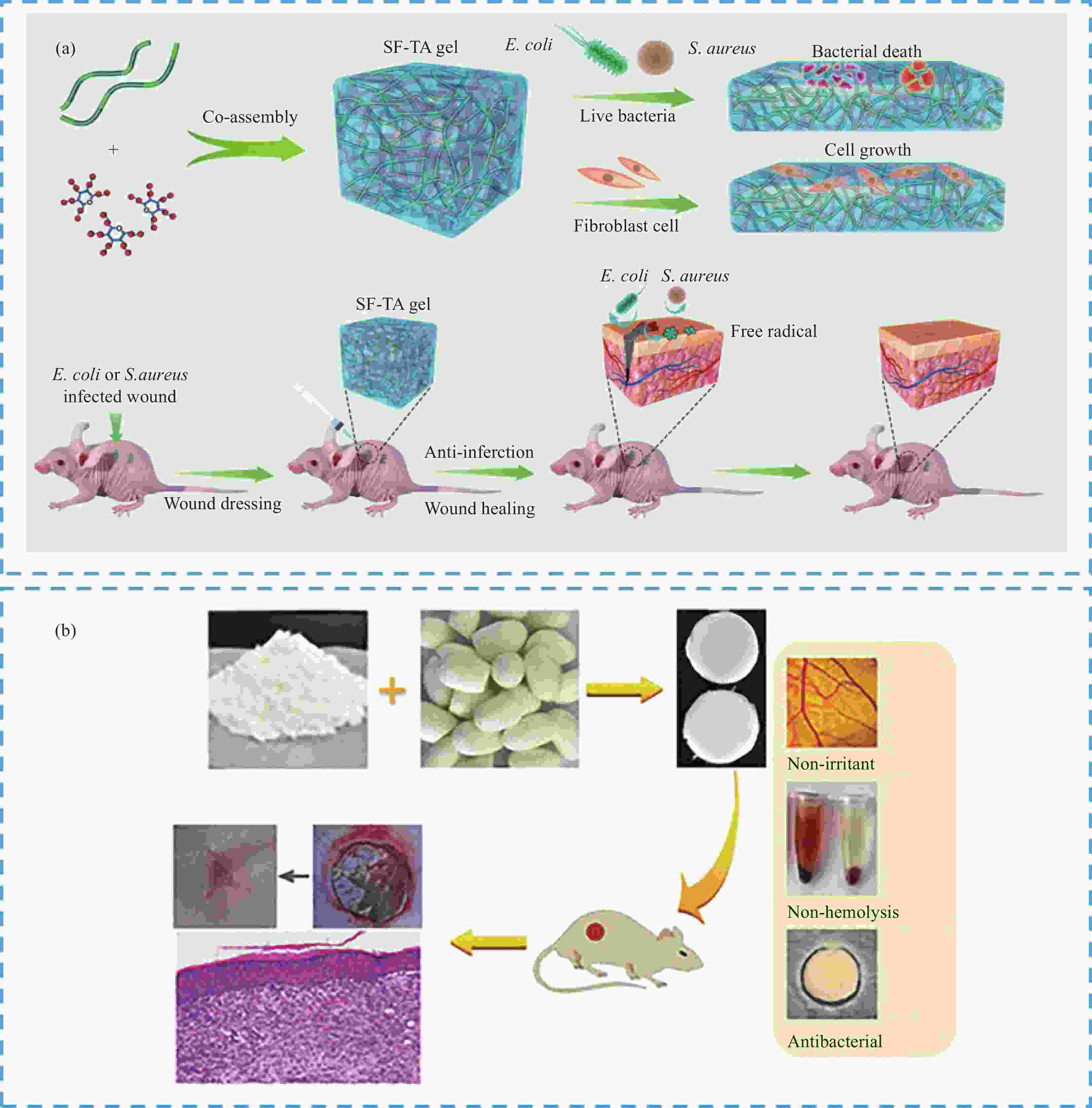
图 13 SF-HSHs用作伤口愈合:(a) SF-TA水凝胶作为伤口敷料促进伤口愈合并预防细菌感染[87];(b) SF-Ag-甘草酸(GA)水凝胶用于抗菌和伤口愈合[88]
Figure 13. SF-HSHs used for wound healing: (a) Use of SF-TA hydrogel as a wound dressing to promote wound healing and prevent bacterial infection[87]; (b) SF-Ag-glycyrrhizic acid (GA) hydrogels are used for antibacterial and wound healing[88]
图 14 SF-HSHs用作药物缓释:(a) 丝纳米纤维与去铁胺共混形成持续的药物递送系统[89];(b) 京尼平交联热敏水凝胶修饰的丝质医用敷料,用于对乙酰氨基酚的缓释[90]
Figure 14. SF-HSHs is used for sustained drug release: (a) A continuous drug delivery system formed by blending silk nanofibers with desferrioxamine[89]; (b) Genipin cross-linked thermosensitive hydrogel modified silk medical dressing for sustained release of paracetamol[90]
DFO—Desferrioxamine; CGG—Chitosan/glycerol-phosphate disodium salt/genipin; VEGF—vascular endothelial growth factor; SDF-1α—Stromal cell-derived factor-1alpha
Amino acid content/mol% Native silk Regenerated silk Amino acid content/mol% Native silk Regenerated silk Aspartic acid 2.4 1.5 Methionine 0.1 0.1 Threonine 1.6 0.8 Isoleucine 0.6 0.6 Serine 12.3 10.8 Leucine 0.5 0.4 Glutamic acid 1.2 0.9 Tyrosine 5 4.9 Proline 0.7 0.5 Phenylalanine 0.7 0.6 Glycine 43.5 46.2 Histidine 0.2 0.2 Alanine 28 29.7 Lysine 0.5 0.3 Cysteine 0.1 0 Arginine 0.6 0.4 Valine 2.3 2.1 表 2 SF基高强度水凝胶(SF-HSHs)交联方式、力学性能对比
Table 2. Comparison of crosslinking methods and mechanical properties of SF-high strength hydrogels (SF-HSHs)
Type of gelation Hydrogel systems Mechanical properties Applications Ref. Physical crosslinked SF/HFIP/water Water content: 85%-90%, tensile strength: (0.7±0.04) MPa, Young's modulus:
(6.5±0.2) MPaCell culture [60-61,62] SF/SDS Tensile modulus: 3.0 MPa, tensile strength: (0.7±0.12) MPa, elongation: (134±21)% Tissue engineering SF/EMImAc/EtOH Water content: 90%, tensile modulus: 0.5-3.68 MPa, compressive modulus: 0.59-4.6 MPa Conductor material Dual crosslinked SF/HRP/H2O2/EtOH Water content: 90%, tensile modulus: 2.5-3.0 MPa, compressive strength: 0.14-0.7 MPa Tissue engineering [55,63] SF/γ-ray /EtOH Water content: 85%, compressive modulus: 1.2-2.41 MPa, compressive strength: (1.37±0.1) MPa Tissue engineering SF composite gel SF/PEG/HPMC Water content: 51%-58%, Young's modulus:
30-91 MPa, tensile strength: 3.42-4.1 MPaBiomedical science [64-65] SF/cellulose Young's modulus: 9.45-14 MPa, ultimate stress: 0.94-1.1 MPa, toughness: 84.73-108.3 KJ·m−3 Cell adhesion Dual/IPN network SF/SDS/HPAAm Water content: 67%-81%, tensile strength:
0.3-1.17 MPa, toughness: 1.98-11.25 MJ·m−3Biosensor [66-67] SF/Gelatin/EtOH Water content: 50%-75%, compressive modulus: 0.85-11.6 MPa Tissue regeneration Notes: HFIP—Hexafluoroisopropanol; SDS—Sodium Dodecyl Sulfonate; EMImAc—1-ethyl-3-methylimidazole acetate; EtOH—Ethyl alcohol; PEG—Polyethylene glycol; HPMC—Hydroxyl propyl methyl cellulose; HPAAm—Hydrophobically associated acrylamide; HRP—Horse radish peroxidase. -
[1] LING S J, KAPLAN D L, BUEHLER M J. Nanofibrils in nature and materials engineering[J]. Nature Reviews Materials,2018,3(4):18016. doi: 10.1038/natrevmats.2018.16 [2] ISHIKAWA S, IIJIMA K, MATSUKUMA D, et al. Interpenetrating polymer network hydrogels via a one-pot and in situ gelation system based on peptide self-assembly and orthogonal cross-linking for tissue regeneration[J]. Chemistry of Materials,2020,32(6):2353-2364. doi: 10.1021/acs.chemmater.9b04725 [3] VOGA M, DRNOVSEK N, NOVAK S, et al. Silk fibroin induces chondrogenic differentiation of canine adipose-derived multipotent mesenchymal stromal cells/mesenchymal stem cells[J]. Journal of Tissue Engineering, 2019, 10: 20417314. [4] BAO Z T, XIAN C H, YUAN Q J, et al. Natural polymer-based hydrogels with enhanced mechanical performances: Preparation, structure, and property[J]. Advanced Healthcare Materials,2019,8(17):1900670. doi: 10.1002/adhm.201900670 [5] SHI L Y, WANG F L, ZHU W, et al. Self-healing silk fibroin-based hydrogel for bone regeneration: Dynamic metal-ligand self-assembly approach[J]. Advanced Functional Materials,2017,27(37):1700591. doi: 10.1002/adfm.201700591 [6] HUANG L, LI C, YUAN W J, et al. Strong composite films with layered structures prepared by casting silk fibroin-graphene oxide hydrogels[J]. Nanoscale,2013,5(9):3780-3786. doi: 10.1039/c3nr00196b [7] WANG C, DU Y L, CHEN B Y, et al. A novel highly stretchable, adhesive and self-healing silk fibroin powder-based hydrogel containing dual-network structure[J]. Materials Letters,2019,252:126-129. doi: 10.1016/j.matlet.2019.05.129 [8] WU J J, LIU J Y, SHI Y M, et al. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels[J]. Journal of the Mechanical Behavior of Biomedical Materials,2016,64:161-172. doi: 10.1016/j.jmbbm.2016.07.007 [9] MA L Y, LIU Q A, WU R H, et al. From molecular reconstruction of mesoscopic functional conductive silk fibrous materials to remote respiration monitoring[J]. Small,2020,16(26):2000203. doi: 10.1002/smll.202000203 [10] CHEN Z W, ZHANG H H, LIN Z F, et al. Programing performance of silk fibroin materials by controlled nucleation[J]. Advanced Functional Materials,2016,26(48):8978-8990. doi: 10.1002/adfm.201602908 [11] LIU R C, DENG Q Q, YANG Z, et al. “Nano-Fishnet” structure making silk fibers tougher[J]. Advanced Functional Materials,2016,16(30):5534-5541. [12] QIU W, PATIL A, HU F, et al. Hierarchical structure of silk materials versus mechanical performance and mesoscopic engineering principles[J]. Small,2019,15(51):201903948. [13] HU Z N, YAN S Q, LI X F, et al. Natural silk nanofibril aerogels with distinctive filtration capacity and heat-retention performance[J]. ACS Nano,2021,15(5):8171-8183. doi: 10.1021/acsnano.1c00346 [14] GONG Z G, HUANG L, YANG Y H, et al. Two distinct β-sheet fibrils from silk protein[J]. Chemical Communications,2009(48):7506. doi: 10.1039/b914218e [15] TSUKADA M, FREDDI G, GOTOH Y, et al. Physical and chemical properties of tussah silk fibroin films[J]. Journal of Polymer Science Part B: Polymer Physics,1994,32(8):1407-1412. doi: 10.1002/polb.1994.090320812 [16] MENG L, SHAO C Y, CUI C, et al. Autonomous self-healing silk fibroin injectable hydrogels formed via surfactant-free hydrophobic association[J]. ACS Applied Materials & Interfaces,2020,12(1):1628-1639. [17] LI S C, CHEN C Y, ZHANG D, et al. Microwave-assisted fast and efficient dissolution of silkworm silk for constructing fibroin-based biomaterials[J]. Chemical Engineering Science,2018,189:286-295. doi: 10.1016/j.ces.2018.06.003 [18] LUO J, ZHANG L L, PENG Q F, et al. Tough silk fibers prepared in air using a biomimetic microfluidic chip[J]. International Journal of Biological Macromolecules,2014,66:319-324. doi: 10.1016/j.ijbiomac.2014.02.049 [19] BAI S, LIU S, ZHANG C, et al. Controllable transition of silk fibroin nanostructures: An insight into in vitro silk self-assembly process[J]. Acta Biomaterialia,2013,9(8):7806-7813. doi: 10.1016/j.actbio.2013.04.033 [20] YAO J M, MASUDA H, ZHAO C H, et al. Artificial spinning and characterization of silk fiber from Bombyx mori silk fibroin in hexafluoroacetone hydrate[J]. Macromolecules,2002,35(1):6-9. doi: 10.1021/ma011335j [21] HA S W, TONELLI A E, HUDSON S M. Structural studies of Bombyx mori silk fibroin during regeneration from solutions and wet fiber spinning[J]. Biomacromolecules,2005,6(3):1722-1731. doi: 10.1021/bm050010y [22] PHILLIPS D M, DRUMMY L F, CONRADY D G, et al. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids[J]. Journal of the American Chemical Society,2004,126(44):14350-14351. doi: 10.1021/ja046079f [23] ROCKWOOD D N, PREDA R C, YÜCEL T, et al. Materials fabrication from Bombyx mori silk fibroin[J]. Nature Protocols,2011,6(10):1612-1631. doi: 10.1038/nprot.2011.379 [24] GREVING I, CAI M Z, VOLLRATH F, et al. Shear-induced self-assembly of native silk proteins into fibrils studied by atomic force microscopy[J]. Biomacromolecules,2012,13(3):676-682. doi: 10.1021/bm201509b [25] ZHONG J A, MA M J, LI W Y, et al. Self-assembly of regenerated silk fibroin from random coil nanostructures to antiparallel β-sheet nanostructures[J]. Biopolymers,2014,101(12):1181-1192. doi: 10.1002/bip.22532 [26] LING S J, CHEN W S, FAN Y M, et al. Biopolymer nanofibrils: Structure, modeling, preparation, and applications[J]. Progress in Polymer Science,2018,85:1-56. doi: 10.1016/j.progpolymsci.2018.06.004 [27] ZHANG F, LU Q, MING J F, et al. Silk dissolution and regeneration at the nanofibril scale[J]. Journal of Materials Chemistry B,2014,2(24):3879-3885. doi: 10.1039/c3tb21582b [28] HU Y L, YU J, LIU L, et al. Preparation of natural amphoteric silk nanofibers by acid hydrolysis[J]. Journal of Materials Chemistry B,2019,7(9):1450-1459. [29] SHI M Y, HU Y L, LUO X, et al. Tiny NaOH assisted facile preparation of silk nanofibers and their nanotube-compositing strong, flexible, and conductive films[J]. ACS Biomaterials Science & Engineering,2022,8(9):4014-4023. [30] HU Y L, LIU L A, YU J A, et al. Preparation of natural multicompatible silk nanofibers by green deep eutectic solvent treatment[J]. ACS Sustainable Chemistry and Engineering,2020,8(11):4499-4510. doi: 10.1021/acssuschemeng.9b07668 [31] KIM S H, YEON Y K, LEE J M, et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing[J]. Nature Communications,2018,9(1):1620. doi: 10.1038/s41467-018-03759-y [32] WANG W, ZHANG Y Y, LIU W G. Bioinspired fabrication of high strength hydrogels from non-covalent interactions[J]. Progress in Polymer Science,2017,71:1-25. doi: 10.1016/j.progpolymsci.2017.04.001 [33] LIN N B, LIU X Y. Correlation between hierarchical structure of crystal networks and macroscopic performance of mesoscopic soft materials and engineering principles[J]. Chemical Society Reviews,2015,44(21):7881-7915. doi: 10.1039/C5CS00074B [34] MO C L, HOLLAND C, PORTER D, et al. Concentration state dependence of the rheological and structural properties of reconstituted silk[J]. Biomacromolecules,2009,10(10):2724-2728. doi: 10.1021/bm900452u [35] MATSUMOTO A, CHEN J S, COLLETTE A L, et al. Mechanisms of silk fibroin sol-gel transitions[J]. Journal of Physical Chemistry B,2006,110(43):21630-21638. doi: 10.1021/jp056350v [36] GUO X, ZUO B. Research progress of silk fibroin-based composite hydrogels[J]. Journal of Silk,2020,57(6):45-51. [37] LU Q, HUANG Y L, LI M Z, et al. Silk fibroin electrogelation mechanisms[J]. Acta Biomaterialia,2011,7(6):2394-2400. doi: 10.1016/j.actbio.2011.02.032 [38] LEFÈVRE T, BOUDREAULT S, CLOUTIER C, et al. Conformational and orientational transformation of silk proteins in the major ampullate gland of Nephila clavipes spiders[J]. Biomacromolecules,2008,9(9):2399-2407. doi: 10.1021/bm800390j [39] BAI S M, ZHANG X L, LU Q, et al. Reversible hydrogel-solution system of silk with high beta-sheet content[J]. Biomacromolecules,2014,15(8):3044-3051. doi: 10.1021/bm500662z [40] SRISAWASDI T, PETCHAROEN K, SIRIVAT A, et al. Electromechanical response of silk fibroin hydrogel and conductive polycarbazole/silk fibroin hydrogel composites as actuator material[J]. Materials Science and Engineering: C,2015,56:1-8. doi: 10.1016/j.msec.2015.06.005 [41] ZHOU H H, WANG Z G, CAO H Q, et al. Genipin-crosslinked polyvinyl alcohol/silk fibroin/nano-hydroxyapatite hydrogel for fabrication of artificial cornea scaffolds—A novel approach to corneal tissue engineering[J]. Journal of Biomaterials Science,2019,30(17):1604-1619. doi: 10.1080/09205063.2019.1652418 [42] WANG X, DING Z Z, WANG C, et al. Bioactive silk hydrogels with tunable mechanical properties[J]. Journal of Materials Chemistry B,2018,6(18):2739-2746. doi: 10.1039/C8TB00607E [43] FERRERO F, PERIOLATTO M, BURELLI S, et al. Silk grafting with chitosan and crosslinking agents[J]. Fibers and Polymers,2010,11(2):185-192. doi: 10.1007/s12221-010-0185-7 [44] ELLIOTT W H, BONANI W, MANIGLIO D, et al. Silk hydrogels of tunable structure and viscoelastic properties using different chronological orders of genipin and physical cross-linking[J]. ACS Applied Materials & Interfaces,2015,7(22):12099-12108. [45] HENNINK W E, VAN NOSTRUM C F. Novel crosslinking methods to design hydrogels[J]. Advanced Drug Delivery Reviews, 2012, 64: 223-236. [46] WANG L, XU B, NONG Y L, et al. Laccase-mediated construction of flexible double-network hydrogels based on silk fibroin and tyramine-modified hyaluronic acid[J]. International Journal of Biological Macromolecules,2020,160:795-805. doi: 10.1016/j.ijbiomac.2020.05.258 [47] HENNINK W E, VAN NOSTRUM C F. Novel crosslinking methods to design hydrogels[J]. Advanced Drug Delivery Reviews,2012,64:223-236. doi: 10.1016/j.addr.2012.09.009 [48] MCGILL M, GRANT J M, KAPLAN D L. Enzyme-mediated conjugation of peptides to silk fibroin for facile hydrogel functionalization[J]. Annals of Biomedical Engineering,2020,48(7):1905-1915. doi: 10.1007/s10439-020-02503-2 [49] KANG G D, LEE K H, KI C S, et al. Silk fibroin/chitosan conjugate crosslinked by tyrosinase[J]. Macromolecular Research,2004,12(5):534-539. doi: 10.1007/BF03218439 [50] ZHOU Q, CUI L, REN L M, et al. Preparation of a multifunctional fibroin-based biomaterial via laccase-assisted grafting of chitooligosaccharide[J]. International Journal of Biological Macromolecules,2018,113:1062-1072. doi: 10.1016/j.ijbiomac.2018.03.042 [51] ZHENG H Y, ZUO B Q. Functional silk fibroin hydrogels: preparation, properties and applications[J]. Journal of Materials Chemistry B,2021,9(5):1238-1258. doi: 10.1039/D0TB02099K [52] DORISHETTY P, BALU R, SREEKUMAR A, et al. Robust and tunable hybrid hydrogels from photo-cross-linked soy protein isolate and regenerated silk fibroin[J]. ACS Sustainable Chemistry& Engineering,2019,7(10):9257-9271. [53] KUANG D J, JIANG F J, WU F, et al. Highly elastomeric photocurable silk hydrogels[J]. International Journal of Biological Macromolecules,2019,134:838-845. doi: 10.1016/j.ijbiomac.2019.05.068 [54] AHMED E M. Hydrogel: Preparation, characterization, and applications: A review[J]. Journal of Advanced Research,2015,6(2):105-121. doi: 10.1016/j.jare.2013.07.006 [55] WU N E, YU H L, SUN M Y, et al. Investigation on the structure and mechanical properties of highly tunable elastomeric silk fibroin hydrogels cross-linked by γ-ray radiation[J]. ACS Applied Bio Materials,2020,3(1):721-734. doi: 10.1021/acsabm.9b01062 [56] KIM M H, KIM B S, LEE J, et al. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering[J]. Biomaterials Research, 2017, 21(1): 1-9. [57] WHITTAKER J L, DUTTA N K, ZANNETTINO A, et al. Engineering DN hydrogels from regenerated silk fibroin and poly(N-vinylcaprolactam)[J]. Journal of Materials Chemistry B,2016,4(33):5519-5533. doi: 10.1039/C6TB01055E [58] ZHANG H J, SUN T L, ZHANG A K, et al. Tough physical double-network hydrogels based on amphiphilic triblock copolymers[J]. Advanced Materials,2016,28(24):4884-4890. doi: 10.1002/adma.201600466 [59] ZHAO Y, GUAN J A, WU S J. Highly stretchable and tough physical silk fibroin–based double network hydrogels[J]. Macromolecular Rapid Communications,2019,40(23):1900389. doi: 10.1002/marc.201900389 [60] MCGILL M, COBURN J M, PARTLOW B P, et al. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design[J]. Acta Biomaterialia,2017,63:76-84. [61] ZHU Z H, LING S J, YEO J, et al. High-strength, durable all-silk fibroin hydrogels with versatile processability toward multifunctional applications[J]. Advanced Functional Materials,2018,28(10):1704757. doi: 10.1002/adfm.201704757 [62] WU X L, HOU J, LI M Z, et al. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin[J]. Acta Biomaterialia,2012,8(6):2185-2192. doi: 10.1016/j.actbio.2012.03.007 [63] SU D H, YAO M, LIU J E, et al. Enhancing mechanical properties of silk fibroin hydrogel through restricting the growth of β-sheet domains[J]. ACS Applied Materials & Interfaces,2017,9(20):17489-17498. [64] GONG Z G, YANG Y H, REN Q G, et al. Injectable thixotropic hydrogel comprising regenerated silk fibroin and hydroxypropylcellulose[J]. Soft Matter,2012,8(10):2875-2883. doi: 10.1039/c2sm06984a [65] CHANG H L, MENG L, SHAO C Y, et al. Physically cross-linked silk hydrogels with high solid content and excellent mechanical properties via a reverse dialysis concentrated procedure[J]. ACS Sustainable Chemistry & Engineering,2019,7(15):13324-13332. [66] CHEN F, LU S P, ZHU L, et al. Conductive regenerated silk-fibroin-based hydrogels with integrated high mechanical performances[J]. Journal of Materials Chemistry B,2019,7(10):1708-1715. doi: 10.1039/C8TB02445F [67] PARK S, EDWARDS S, HOU S J, et al. A multi-interpenetrating network (IPN) hydrogel with gelatin and silk fibroin[J]. Biomaterials Science,2019,7(4):1276-1280. doi: 10.1039/C8BM01532E [68] PARK J H, KIM M H, JEONG L, et al. Effect of surfactants on sol-gel transition of silk fibroin[J]. Journal of Sol-Gel Science and Technology,2014,71(2):364-371. doi: 10.1007/s10971-014-3379-4 [69] LI Z, ZHENG Z K, YANG Y H, et al. Robust protein hydrogels from silkworm silk[J]. ACS Sustainable Chemistry & Engineering,2016,4(3):1500-1506. [70] ZHAO Y, ZHU Z S, GUAN J, et al. Processing, mechanical properties and bio-applications of silk fibroin-based high-strength hydrogels[J]. Acta Biomaterialia,2021,125:57-71. doi: 10.1016/j.actbio.2021.02.018 [71] GONG J P, KATSUYAMA Y, KUROKAWA T, et al. Double-network hydrogels with extremely high mechanical strength[J]. Advanced Materials,2003,15(14):1155-1158. doi: 10.1002/adma.200304907 [72] YANG Y Y, WANG X, YANG F, et al. A universal soaking strategy to convert composite hydrogels into extremely tough and rapidly recoverable double-network hydrogels[J]. Advanced Materials,2016,28(33):7178-7184. doi: 10.1002/adma.201601742 [73] TAYLOR D L, IN HET PANHUIS M. Self-healing hydrogels[J]. Advanced Materials,2016,28(41):9060-9093. doi: 10.1002/adma.201601613 [74] GAO G R, DU G L, SUN Y N, et al. Self-healable, tough, and ultrastretchable nanocomposite hydrogels based on reversible polyacrylamide/montmorillonite adsorption[J]. ACS Applied Materials and Interfaces,2015,7(8):5029-5037. doi: 10.1021/acsami.5b00704 [75] DENG Z X, WANG H, MA P X, et al. Self-healing conductive hydrogels: Preparation, properties and applications[J]. Nanoscale,2020,12(3):1224-1246. doi: 10.1039/C9NR09283H [76] FANG X, LI Y X, LI X A, et al. Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly(vinyl alcohol)-based hydrogels with excellent self-healing ability[J]. ACS Materials Letters,2020,2(7):764-770. doi: 10.1021/acsmaterialslett.0c00075 [77] DAI X Y, ZHANG Y Y, GAO L N, et al. A mechanically strong, highly stable, thermoplastic, and self-healable supramolecular polymer hydrogel[J]. Advanced Materials,2015,27(23):3566-3571. doi: 10.1002/adma.201500534 [78] HUANG L, DU X Y, FAN S N, et al. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores[J]. Carbohydrate Polymers,2019,221:146-156. doi: 10.1016/j.carbpol.2019.05.080 [79] SHI W L, SUN M Y, HU X Q, et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo[J]. Advanced Materials,2017,29(29):1701089. doi: 10.1002/adma.201701089 [80] DING Z Z, LU G Z, CHENG W N, et al. Tough anisotropic silk nanofiber hydrogels with osteoinductive capacity[J]. ACS Biomaterials Science and Engineering,2020,6(4):2357-2367. doi: 10.1021/acsbiomaterials.0c00143 [81] DORISHETTY P, BALU R, GELMI A, et al. 3D printable soy/silk hybrid hydrogels for tissue engineering applications[J]. Biomacromolecules,2021,22(9):3668-3678. doi: 10.1021/acs.biomac.1c00250 [82] YUAN M H, YAN S, LIU H, et al. Performance of water-immiscible silk fibroin based hydrogel as underwater biomedical adhesive[J]. Fibers and Polymers,2019,20(10):2032-2041. doi: 10.1007/s12221-019-1206-9 [83] WANG J L, ZHANG N, TAN Y R, et al. Sweat-resistant silk fibroin-based double network hydrogel adhesives[J]. ACS Applied Materials & Interfaces,2022,14(19):21945-21953. [84] BAI S M, ZHANG X L, LYU X L, et al. Bioinspired mineral–organic bone adhesives for stable fracture fixation and accelerated bone regeneration[J]. Advanced Functional Materials,2020,30(5):1908381. doi: 10.1002/adfm.201908381 [85] WANG Z J, HU W K, DU Y Y, et al. Green gas-mediated cross-linking generates biomolecular hydrogels with enhanced strength and excellent hemostasis for wound healing[J]. ACS Applied Materials & Interfaces,2020,12(12):13622-13633. doi: 10.1021/acsami.9b21325 [86] HEICHEL D L, BURKE K A. Dual-mode cross-linking enhances adhesion of silk fibroin hydrogels to intestinal tissue[J]. ACS Biomaterials Science and Engineering,2019,5(7):3246-3259. doi: 10.1021/acsbiomaterials.9b00786 [87] JING J, LIANG S F, YAN Y F, et al. Fabrication of hybrid hydrogels from silk fibroin and tannic acid with enhanced gelation and antibacterial activities[J]. ACS Biomaterials Science & Engineering,2019,5(9):4601-4611. [88] ZHANG F, YIN C, QI X H, et al. Silk fibroin crosslinked glycyrrhizic acid and silver hydrogels for accelerated bacteria-infected wound healing[J]. Macromolecular Bioscience,2022,22(4):2100407. doi: 10.1002/mabi.202100407 [89] DING Z Z, ZHOU M L, ZHOU Z Y, et al. Injectable silk nanofiber hydrogels for sustained release of small-molecule drugs and vascularization[J]. ACS Biomaterials Science and Engineering,2019,5(8):4077-4088. doi: 10.1021/acsbiomaterials.9b00621 [90] QIAO N, ZHANG Y F, FANG Y, et al. Silk fabric decorated with thermo-sensitive hydrogel for sustained release of paracetamol[J]. Macromolecular Bioscience,2022,22(8):2200029. doi: 10.1002/mabi.202200029 [91] YAN S Q, HAN G C, WANG Q S, et al. Directed assembly of robust and biocompatible silk fibroin/hyaluronic acid composite hydrogels[J]. Composites Part B: Engineering,2019,176:107204. doi: 10.1016/j.compositesb.2019.107204 [92] DORISHETTY P, DUTTA N K, CHOUDHURY N R. Silk fibroins in multiscale dimensions for diverse applications[J]. RSC Advances,2020,10(55):33227-33247. doi: 10.1039/D0RA03964K [93] DE CARVALHO B G, TAKETA T B, GARCIA B B M, et al. Hybrid microgels produced via droplet microfluidics for sustainable delivery of hydrophobic and hydrophilic model nanocarriers[J]. Materials Science and Engineering C,2021,118:111467. doi: 10.1016/j.msec.2020.111467 [94] LUO J, YANG J J, ZHENG X R, et al. A highly stretchable, real-time self-healable hydrogel adhesive matrix for tissue patches and flexible electronics[J]. Advanced Healthcare Materials,2020,9(4):1901423. doi: 10.1002/adhm.201901423 [95] OKUMURA Y, ITO K. The polyrotaxane gel: A topological gel by figure-of-eight cross-links[J]. Advanced Materials,2001,13(7):485-487. doi: 10.1002/1521-4095(200104)13:7<485::AID-ADMA485>3.0.CO;2-T [96] YANG N N, QI P, REN J, et al. Polyvinyl alcohol/silk fibroin/borax hydrogel ionotronics: A highly stretchable, self-healable, and biocompatible sensing platform[J]. ACS Applied Materials & Interfaces,2019,11(26):23632-23638. doi: 10.1021/acsami.9b06920 -






 下载:
下载: