Construction strategy of metal-organic frameworks derived single-atom catalysts and their application in hydrogen production
-
摘要: 与传统纳米催化剂相比,单原子催化剂(SACs)具有独特的结构、高活性和最大程度的原子利用率等特点,使SACs成为当前催化领域的研究热点。金属有机框架(Metal-organic frameworks,MOFs)中的金属离子节点是原子分散的、配位环境明确,且结构可调,是构筑单原子催化剂的理想前驱物。近年来,大量研究报道了通过热解MOFs制得性能优异的SACs。本文介绍了通过热解MOFs构建SACs的5种主要策略,包括直接热解MOFs策略、混合金属策略、混合配体策略、空间限域策略和其他策略及由热解MOFs制得的SACs在电解水、光解水和催化储氢小分子制氢中的应用。最后,指出了未来使用MOFs衍生物构建SACs的发展方向。Abstract: Compared to traditional nanocatalysts, single-atom catalysts (SACs) with advantages of unique structure, remarkable performance and maximum atom utilization efficiency have emerged as a new research focus in catalysis field. Metal-organic frameworks (MOFs) is recognized as one of ideal precursors for constructing SACs, due to the unique features of MOFs including atomically dispersed of metal ion nodes, clear coordination environment and tailorable structure. Recently, a large number of studies reported SACs with excellent performance derived from pyrolysis of MOFs. In this review, five major construction strategies of MOFs-derived SACs, including direct pyrolysis of MOFs, mixed-metal strategy, mixed-ligand strategy, spatial confinement strategy and other strategies, as well as the application of these SACs for hydrogen evolution through electrocatalysis, photocatalysis and hydrogen storage small molecule catalysis are summarized. Finally, the future development directions of MOFs-derived SACs are pointed out.
-
Key words:
- metal-organic frameworks /
- pyrolysis /
- single-atom catalysts /
- hydrogen production /
- review
-
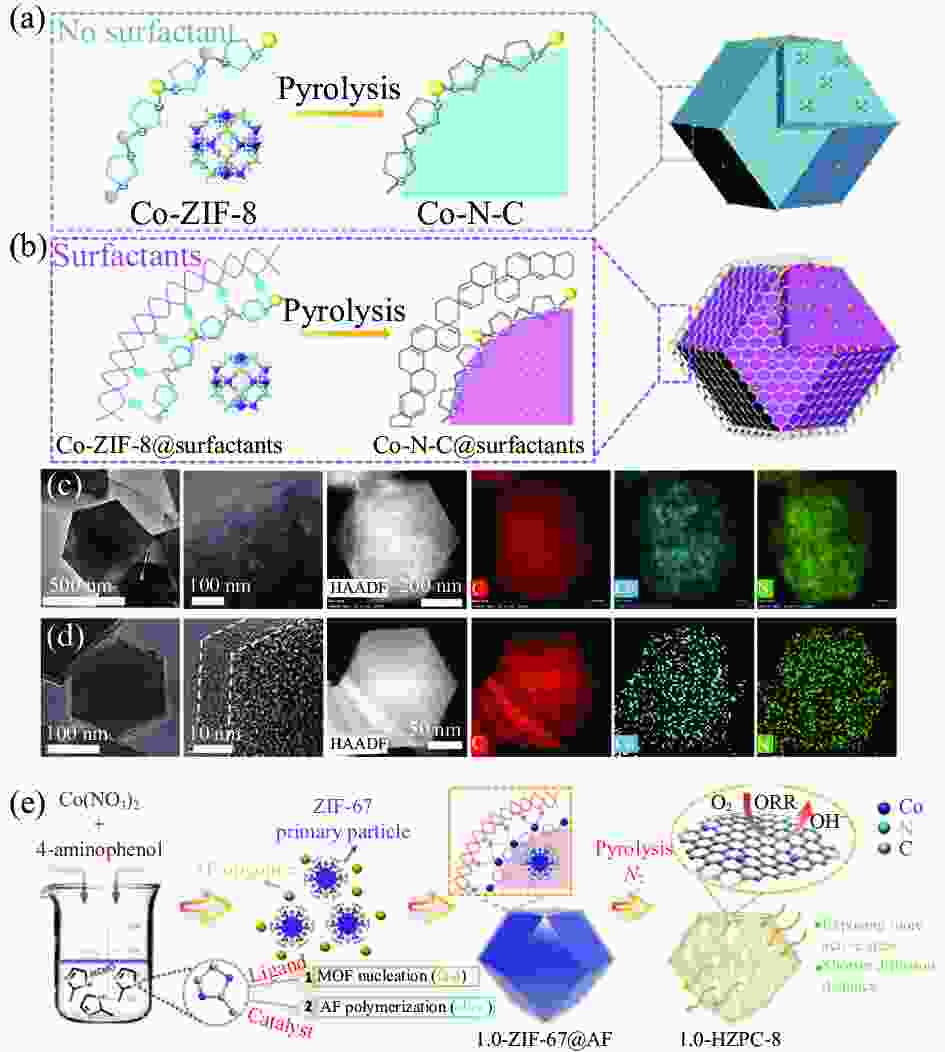
图 2 ((a), (b)) 有/无表面活性剂构筑Co单原子催化剂(SACs)示意图;((c), (d)) Co-N-C、Co-N-C@F127的HAADF-STEM图像和STEM-EDS图谱[38];(e) 酚醛树脂辅助MOFs构筑Co SACs示意图[39]
Figure 2. ((a), (b)) Schematic illustration of the synthetic process of Co single-atom catalysts (SACs) without/with surfactant; ((c), (d)) HAADF-STEM and STEM-EDS images of Co-N-C and Co-N-C@F127[38]; (e) Schematic illustration of the synthetic process of Co SACs through phenolic resin-assisted[39]
图 3 (a) 以ZnCo双金属ZIFs为前驱物构筑Co SCAs流程示意图;((b), (c)) Co SAs/N-C(900)的HAADF-STEM图像[45];(d) Co-Nx(x=2, 3和4)的X射线吸收精细结构光谱(EXAFS)和X射线吸收近边结构(XANES)图谱[46]
Figure 3. (a) Schematic illustration of the synthetic process of Co SCAs; ((b), (c)) HAADF-STEM images of Co SAs/N-C(900)[45]; (d) X-ray absorptionfine structure (EXAFS) and X-ray absorption near edge structure (XANES) spectra of Co atoms in Co-Nx (x=2, 3, 4)[46]
NPs—Nano particles
图 4 (a) 负压热解双金属前驱物构筑Co单原子催化剂流程示意图;(b) 形成三维石墨烯框架结构的Co单原子催化剂(Co SAs/3D GFs)的TEM图像;(c) MN4 (M=Co, Zn)与应变的示意图及MN4中单原子的形成能与应变计算结果;(d) Co SAs/3D GFs形成过程示意图[47]
Figure 4. (a) Schematic illustration of the synthetic process of Co SCAs through pyrolysis ZnCo-ZIFs under negative pressure; (b) In-situ TEM images of Co monatomic catalyst with 3D graphene frame structure (Co SAs/3D GFs) during pyrolysis; (c) Schematic illustration of MN4 (M=Co, Zn) with strain and the calculated dependence of the formation energy of catalyst on the biaxial strain in MN4; (d) Mechanism illustration of the formation of Co SAs/3D GFs[47]
ΔP—Dfferential pressure (inside vs. outside); P1—Pressure inside; P2—pressure outside
图 5 (a) ZIF-8掺杂Fe构筑Fe SCAs流程示意图;(b) 不同尺寸大小前驱物的SEM图像[49];(c) 酸辅助水系体系合成Mn SCAs流程示意图[51]
Figure 5. (a) Schematic illustration of the synthetic process of Fe SCAs through Fe doping with ZIF-8; (b) SEM images of precursors in different sizes[49]; (c) Schematic illustration of the synthetic process of Mn SCAs in acid-assisted aqueous solution system[51]
图 6 混合配体策略构筑Fe SACs[53](a) 和Ru SACs (b) 流程示意图[54];((c)~(h)) Ru单原子、Ru簇催化剂的TEM和HAADF-STEM图像[54]
Figure 6. Schematic illustration of the synthetic process of Fe SACs[53] (a) and Ru SACs[54] (b) through mixed-ligand strategy; ((c)-(h)) TEM and magnified HAADF-STEM images of Ru SACs and Ru nanoclusters[54]
Fe20—Molar percentage of Fe-TCPP is 20%; FeSA—Single-atom Fe; PCN-222—MOF-545; dFe-Fe—Distance of adjacent Fe-TCPP ligands
图 8 (a) Pd纳米粒子演变成Pd单原子过程示意图及各阶段HAADF-STEM图像;(b) 从Pd10团簇转变为Pd-N4的DFT[60]
Figure 8. Schematic illustration of the transformation of nanoparticles to single atoms and HAADF-STEM images of each stage; (b) Calculated energies along the stretching pathway of the Pd atom from the Pd10 cluster to Pd-N4[60]
ΔG—Difference Gibbs free energy beteween initial state and final state; Ea—Reaction energy and barrier; Ts—Transition states; CI-NEB—Climbing image nudged elastic band
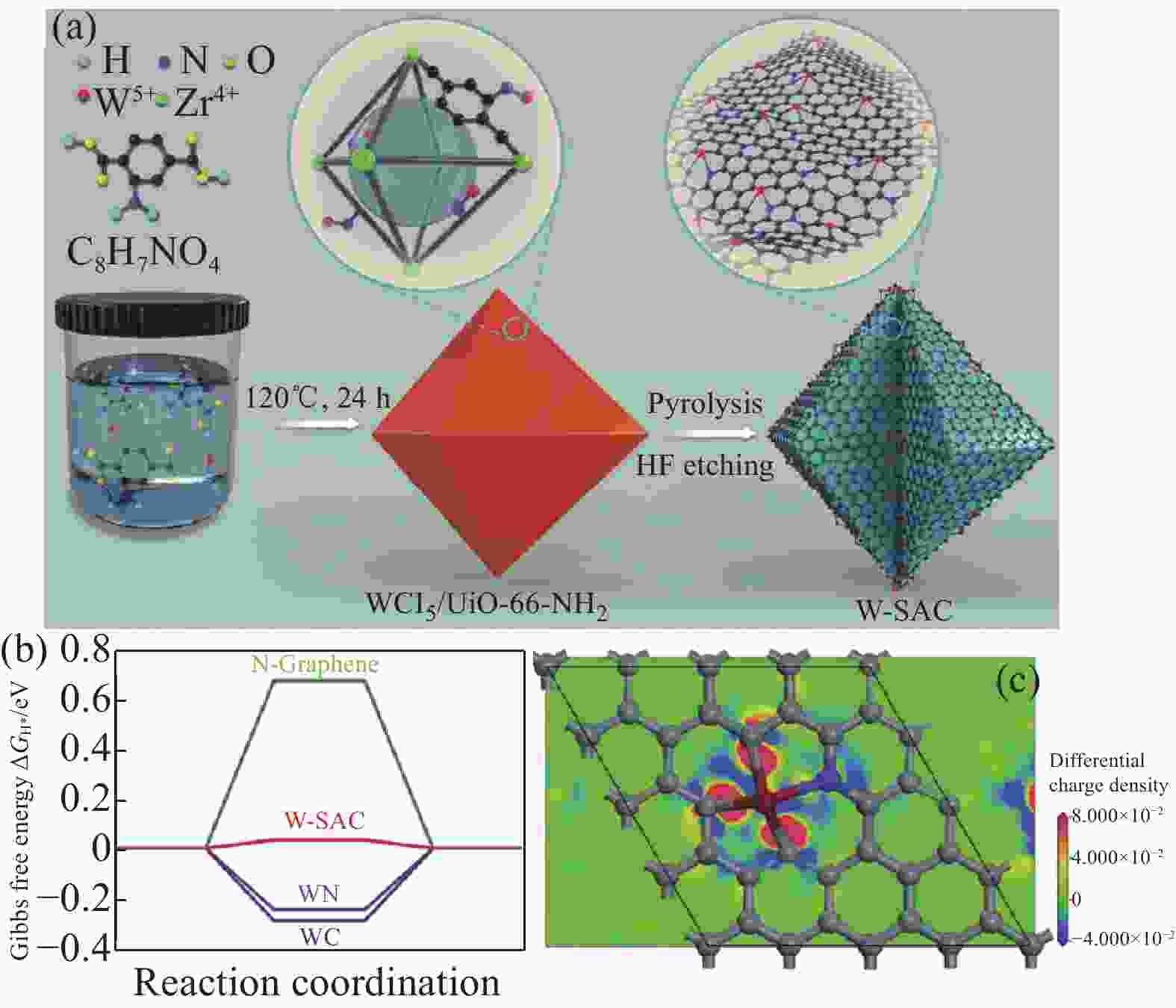
图 11 (a) 混合金属策略构筑W-SAC流程示意图;(b) 催化剂上吸附氢的吉布斯自由能∆GH*;(c) W-SAC的态密度[75]
Figure 11. (a) Schematic illustration of the synthetic process of W-SAC through mixed-metal strategy; (b) Gibbs free energy ΔGH* of hydrogen adsorption on catalysts; (c) A slice section of differential charge density paralleled to xγ plane for W-SAC[75]
图 12 (a) 钴单原子/原位磷(Co-SA/P-in situ)催化剂制备流程示意图;(b) Co-SA/P-in situ催化剂的析氢反应(HER)机制;(c) 不同电压下Co的平均氧化态值;(d) 催化剂上吸附氢的∆GH*;(e) HER过程中Co1P1N3电荷分布图[77]
Figure 12. (a) Schematic illustration of the synthetic process of cobalt single-atom/phosphorus-in situ (Co-SA/P-in situ); (b) Proposed hydrogen evolution reaction (HER) mechanism of Co-SA/P-in situ; (c) Average oxidation states of Co at different potentials; (d) ∆GH* of H adsorption on the surface of catalysts; (e) Charge transfer of Co1P1N3 configuration[77]
PPh3—Triphenylphosphine
图 13 (a) Ru单原子催化剂制备流程示意图;(b) 光催化制氢性能曲线;(c) 催化剂EPR图谱;(d) 催化剂PL图谱;(e) 催化剂瞬态光电流图谱[81]
Figure 13. (a) Schematic illustration of the synthetic process of Ru SACs; (b) Hydrogen production performance curves; (c) EPR spectra of catalysts; (d) PL spectra of catalysts; (e) Photocurrent response of catalysts[81]
Ov—Oxygen vacancy; g—g value; TC—TiO2/C; H2BDC-NH2—2-aminoterephthalic acid
图 14 (a) Co-N-C(SACs)和Co-N-C(NPs)制备流程示意图;(b) 催化甲酸制氢性能;((c), (d)) Co-N-C的EXAFS图谱[91]
Figure 14. (a) Schematic illustration of the synthetic process of Co-N-C (SACs) and Co-N-C (NPs); (b) Hydrogen production performance over catalysts; ((c), (d)) EXAFS spectra of Co-N-C[91]
FT—Fourier transform; R—Bond distance; k—K-edge
-
[1] WANG C, KIM J, TANG J, et al. New strategies for novel MOF-derived carbon materials based on nanoarchitectures[J]. Chem,2020,6(1):19-40. doi: 10.1016/j.chempr.2019.09.005 [2] ZHANG J, ZHANG Q, FENG X. Support and interface effects in water-splitting electrocatalysts[J]. Advanced Materials,2019,31(31):1808167. doi: 10.1002/adma.201808167 [3] OU H, WANG D, LI Y. How to select effective electrocatalysts: nano or single atom?[J]. Nano Select,2021,2(3):492-511. doi: 10.1002/nano.202000239 [4] 张宁强, 李玲聪, 黄星, 等. 单原子催化剂的研究进展[J]. 中国稀土学报, 2018, 36(5):513-532. doi: 10.1016/j.jre.2017.11.009ZHANG Ningqiang, LI Lingcong, HUANG Xing, et al. Research progress of single-atom catalysis[J]. Journal of the Chinese Society of Rare Earths,2018,36(5):513-532(in Chinese). doi: 10.1016/j.jre.2017.11.009 [5] YANG X F, WANG A, QIAO B, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research,2013,46(8):1740-1748. doi: 10.1021/ar300361m [6] LIU L, CORMA A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews,2018,118(10):4981-5079. doi: 10.1021/acs.chemrev.7b00776 [7] ZHANG T, WANG A, YANG X, et al. Single atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry,2011,3:634-641. doi: 10.1038/nchem.1095 [8] WANG Y, SU H, HE Y, et al. Advanced electrocatalysts with single-metal-atom active sites[J]. Chemical Reviews,2020,120(21):12217-12314. doi: 10.1021/acs.chemrev.0c00594 [9] WANG A, LI J, ZHANG T. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry,2018,2:65-81. doi: 10.1038/s41570-018-0010-1 [10] ZHOU Z, ZHANG J, MUKHERJEE S, et al. Porphyrinic MOF derived single-atom electrocatalyst enables methanol oxidation[J]. Chemical Engineering Journal,2022,449:137888-137897. doi: 10.1016/j.cej.2022.137888 [11] ZHANG W-D, DONG H, ZHOU L, et al. Fe single-atom catalysts with pre-organized coordination structure for efficient electrochemical nitrate reduction to ammonia[J]. Applied Catalysis B:Environmental,2022,317:121750-121759. doi: 10.1016/j.apcatb.2022.121750 [12] PAN Y, ZHANG C, LIU Z, et al. Structural regulation with atomic-level precision: from single-atomic site to diatomic and atomic interface catalysis[J]. Matter,2020,2(1):78-110. doi: 10.1016/j.matt.2019.11.014 [13] ZHAO D, ZHUANG Z, CAO X, et al. Atomic site electrocatalysts for water splitting, oxygen reduction and selective oxidation[J]. Chemical Society Reviews,2020,49(7):2215-2264. doi: 10.1039/C9CS00869A [14] VAJDA S, WHITE M G. Catalysis applications of size-selected cluster deposition[J]. ACS Catalysis,2015,5(12):7152-7178. doi: 10.1021/acscatal.5b01816 [15] SUN S, ZHANG G, GAUQUELIN N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports,2013,3:1775. doi: 10.1038/srep01775 [16] QIAO B, LIU J, WANG Y G, et al. Highly efficient catalysis of preferential oxidation of CO in H2-rich stream by gold single-atom catalysts[J]. ACS Catalysis,2015,5(11):6249-6254. doi: 10.1021/acscatal.5b01114 [17] LIN L, ZHOU W, GAO R, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature,2017,544:80-83. doi: 10.1038/nature21672 [18] WEI H, HUANG K, WANG D, et al. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth[J]. Nature Communications,2017,8:1490-1498. doi: 10.1038/s41467-017-01521-4 [19] WANG J, LI Z, WU Y, et al. Fabrication of single-atom catalysts with precise structure and high metal loading[J]. Advance Materials,2018,30(48):1801649. doi: 10.1002/adma.201801649 [20] QU Y, CHEN B, LI Z, et al. Thermal emitting strategy to synthesize atomically dispersed Pt metal sites from bulk Pt metal[J]. Journal of the American Chemical Society,2019,141(11):4505-4509. doi: 10.1021/jacs.8b09834 [21] HAN A, CHEN W, ZHANG S, et al. A polymer encapsulation strategy to synthesize porous nitrogen-doped carbon-nanosphere-supported metal isolated-single-atomic-site catalysts[J]. Advance Materials,2018,30(15):1706508. doi: 10.1002/adma.201706508 [22] HOWARTH A, LIU Y, LI P, et al. Chemical, thermal and mechanical stability of metal-organic frameworks[J]. Nature Reviews Materials,2016,1:15018. doi: 10.1038/natrevmats.2015.18 [23] LIU H, CHENG M, LIU Y, et al. Single atoms meet metal-organic frameworks: collaborative efforts for efficient photocatalysis[J]. Energy & Environmental Science,2022,15(9):3722-3749. [24] FANG X, SHANG Q, WANG Y, et al. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis[J]. Advanced Materials,2018,30(7):1705112. doi: 10.1002/adma.201705112 [25] ABDEL-MAGEED A, RUNGTAWEEVORANIT B, PARLINSKA-WOJTAN M, et al. Highly active and stable single-atom Cu catalysts supported by a metal-organic framework[J]. Journal of the American Chemical Society,2019,141(13):5201-5210. doi: 10.1021/jacs.8b11386 [26] ZHANG Z, ZHANG L, WOJTAS L, et al. Template-directed synthesis of nets based upon octahemioctahedral cages that encapsulate catalytically active metalloporphyrins[J]. Journal of the American Chemical Society,2012,134(2):928-933. doi: 10.1021/ja208256u [27] HOU C-C, WANG H-F, LI C, et al. From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications[J]. Energy & Environmental Science,2020,13(6):1658-1693. [28] LIU B, SHIOYAMA H, AKITA T, et al. Metal-organic framework as a template for porous carbon synthesis[J]. Journal of the American Chemical Society,2008,130(16):5390-5391. doi: 10.1021/ja7106146 [29] WANG H-F, CHEN L, PANG H, et al. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions[J]. Chemical Society Review,2020,49(5):1414-1448. doi: 10.1039/C9CS00906J [30] YANG Z, CAO S, LV T, et al. Recent progress in the synthesis of metal-organic-framework-derived carbon materials[J]. MRS Energy & Sustainability,2022,9(2):281-312. [31] XIA W, MAHMOOD A, ZOU R, et al. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion[J]. Energy & Environmental Science,2015,8(7):1837-1866. [32] JIAO L, JIANG H L. Metal-organic-framework-based single-atom catalysts for energy applications[J]. Chem,2019,5(4):786-804. doi: 10.1016/j.chempr.2018.12.011 [33] HU L, LI W, WANG L, et al. Turning metal-organic frameworks into efficient single-atom catalysts via pyrolysis with a focus on oxygen reduction reaction catalysts[J]. EnergyChem,2021,3(3):1-31. [34] HAN A, WANG B, KUMAR A, et al. Recent advances for MOF-derived carbon-supported single-atom catalysts[J]. Small Methods,2019,3(9):1800471-1800492. doi: 10.1002/smtd.201800471 [35] XU Q, FENG B, YE C, et al. Atomically dispersed vanadium sites anchored on N-doped porous carbon for the efficient oxidative coupling of amines to imines[J]. ACS Applied Materials Interfaces,2021,13(13):15168-15177. doi: 10.1021/acsami.0c22453 [36] YANG Y, MAO K, GAO S, et al. O-, N-atoms-coordinated Mn cofactors within a graphene framework as bioinspired oxygen reduction reaction electrocatalysts[J]. Advanced Materials,2018,30(28):1801732. doi: 10.1002/adma.201801732 [37] FAN L, LIU P F, YAN X, et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis[J]. Nature Communications,2016,7:10667. doi: 10.1038/ncomms10667 [38] ZHANG M, WANG C, LUO R, et al. A phenolic resin-assisted strategy for MOF-derived hierarchical Co/N-doped carbon rhombic dodecahedra for electrocatalysis[J]. Journal of Materials Chemistry A,2019,7:5173-5178. doi: 10.1039/C8TA10918D [39] HAN X, LING X, YU D, et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution[J]. Advanced Materials,2019,31(49):1905622. doi: 10.1002/adma.201905622 [40] HE Y, HWANG S, CULLEN D A, et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: carbon-shell confinement strategy[J]. Energy & Environmental Science,2019,12:250-260. [41] CHENG W, LU X F, LUAN D, et al. NiMn-based bimetal-organic framework nanosheets supported on multi-channel carbon fibers for efficient oxygen electrocatalysis[J]. Angewandte Chemie International Edition,2020,59(41):18234-18239. doi: 10.1002/anie.202008129 [42] HAN X, LING X, WANG Y, et al. Generation of nanoparticle, atomic-cluster, and single-atom cobalt catalysts from zeolitic imidazole frameworks by spatial isolation and their use in zinc-air batteries[J]. Angewandte Chemie International Edition,2019,58(16):5359-5364. doi: 10.1002/anie.201901109 [43] CZERW R, TERRONES M, CHARLIER J C, et al. Identification of electron donor states in N-doped carbon nano-tubes[J]. Nano Letters,2001,1(9):457-460. doi: 10.1021/nl015549q [44] CHEN Y Z, WANG C, WU Z Y, et al. From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis[J]. Advanced Materials,2015,27(34):5010. doi: 10.1002/adma.201502315 [45] YIN P, YAO T, WU Y, et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angewandte Chemie International Edition,2016,55(36):10800-10805. doi: 10.1002/anie.201604802 [46] WANG X, CHEN Z, ZHAO X, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2[J]. Angewandte Chemie International Edition,2018,57(7):1944-1948. doi: 10.1002/anie.201712451 [47] ZHOU H, YANG T, KOU Z, et al. Negative pressure pyrolysis induced highly accessible single sites dispersed on 3 D graphene frameworks for enhanced oxygen reduction[J]. Angewandte Chemie International Edition,2020,59(46):20465-20469. doi: 10.1002/anie.202009700 [48] XIAO M, ZHANG H, CHEN Y, et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 site[J]. Nano Energy,2018,46:396-403. doi: 10.1016/j.nanoen.2018.02.025 [49] ZHANG H, HWANG S, WANG M, et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation[J]. Journal of the American Chemical Society,2017,139(40):14143-14149. doi: 10.1021/jacs.7b06514 [50] LI J, CHEN M, CULLEN D A, et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells[J]. Nature Catalysis,2018,1:935-945. doi: 10.1038/s41929-018-0164-8 [51] CHEN M, LI X, YANG F, et al. Atomically dispersed MnN4 catalysts via environmentally benign aqueous synthesis for oxygen reduction: mechanistic understanding of activity and stability improvements[J]. ACS Catalysis,2020,10(18):10523-10534. doi: 10.1021/acscatal.0c02490 [52] DAI X, CHEN Z, YAO T, et al. Single Ni sites distributing on N-doped carbon for selective hydrogenation of acetylene[J]. Chemical Communications,2017,53(84):11568-11571. doi: 10.1039/C7CC04820C [53] JIAO L, WAN G, ZHANG R, et al. From metal-organic frameworks to single-atom Fe implanted N-doped porous carbons: efficient oxygen reduction in both alkaline and acidic media[J]. Angewandte Chemie International Edition,2018,57(28):8525-8529. doi: 10.1002/anie.201803262 [54] WANG X, CHEN W, ZHANG L, et al. Uncoordinated amine groups of MOFs to anchor single Ru sites as chemoselective catalysts towards the hydrogenation of quinolone[J]. Journal of the American Chemical Society,2017,139(28):9419-9422. doi: 10.1021/jacs.7b01686 [55] CHEN Y, JI S, WANG Y, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition,2017,56(24):6937-6941. doi: 10.1002/anie.201702473 [56] WANG J, HAN G, WANG L, et al. ZIF-8 with ferrocene encapsulated: a promising precursor to single-atom Fe embedded nitrogen-doped carbon as highly efficient catalyst for oxygen electroreduction[J]. Small,2018,14(15):1704282-1704282. doi: 10.1002/smll.201704282 [57] ZHU Q L, XIA W, ZHENG L R, et al. Atomically dispersed Fe/N-doped hierarchical carbon architectures derived from a metal-organic framework composite for extremely efficient electrocatalysis[J]. ACS Energy Letters,2017,2(2):504-511. doi: 10.1021/acsenergylett.6b00686 [58] LAI Q, ZHENG L R, LIANG Y Y, et al. Metal-organic-framework-derived Fe-N/C electrocatalyst with five-coordinated Fe-Nx sites for advanced oxygen reduction in acid media[J]. ACS Catalysis,2017,7(3):1655-1663. doi: 10.1021/acscatal.6b02966 [59] QU Y, LI Z, CHEN W , et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms[J]. Nature Catalysis,2018,1:781-786. doi: 10.1038/s41929-018-0146-x [60] WEI S, LI A, LIU J C, et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms[J]. Nature Nanotechnology,2018,13:856-861. doi: 10.1038/s41565-018-0197-9 [61] YANG J, QIU Z, ZHAO C, et al. In-situ thermal atomization to transfer supported metal nanoparticles to surface enriched Ni single atom catalyst[J]. Angewandte Chemie International Edition,2018,57(43):14095-14100. doi: 10.1002/anie.201808049 [62] MA Y, WANG X, JIA Y, et al. Titanium dioxide-based nanoparticles for photocatalytic fuel generations[J]. Chemical Reviews,2014,114(19):9987-10043. doi: 10.1021/cr500008u [63] 曹军文, 张文强, 李一枫, 等. 中国制氢技术的发展现状[J]. 化学进展, 2021, 33(12):2215-2244.CAO Junwen, ZHANG Wenqiang, LI Yifeng, et al. Current status of hydrogen production in china[J]. Progress in Chemistry,2021,33(12):2215-2244(in Chinese). [64] JIANG R, LI L, SHENG T, et al. Edge-site engineering of atomically dispersed Fe-N4 by selective C-N bond cleavage for enhanced oxygen reduction reaction activities[J]. Journal of the American Chemical Society,2018,140(37):11594-11598. doi: 10.1021/jacs.8b07294 [65] XIAO M, CHEN Y, ZHU J, et al. Climbing the apex of the ORR volcano plot via binuclear site construction: electronic and geometric engineering[J]. Journal of the American Chemical Society,2019,141(44):17763-17770. doi: 10.1021/jacs.9b08362 [66] GONG Y N, JIAO L, QIAN Y, et al. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction[J]. Angewandte Chemie International Edition,2020,59(7):2705-2709. doi: 10.1002/anie.201914977 [67] YANG H B, HUNG S F, LIU S, et al. Atomically dispersed Ni(Ⅰ) as the active site for electrochemical CO2 reduction[J]. Nature Energy,2018,3:140-147. doi: 10.1038/s41560-017-0078-8 [68] 吴玉, 李轩, 杨恒攀, 等. 钴单原子的双重限域制备策略及高效CO2电还原性能[J]. 高等学校化学学报, 2022, 43(9):20220343.WU Yu, LI Xuan, YANG Hengpan, et al. Construction of cobalt single atoms via double-confinement strategy for high-performance electrocatalytic reduction of carbon dioxide[J]. Chemical Journal of Chinese Universities,2022,43(9):20220343(in Chinese). [69] GENG Z, LIU Y, KONG X, et al. Achieving a record-high yield rate of 120.9 µgNH3 mgcat. -1 h-1 for N2 electrochemical reduction over Ru single-atom catalysts[J]. Advanced Materials,2018,30(40):1803498. doi: 10.1002/adma.201803498 [70] LI J, ZHANG Y, LIU C, et al. 3.4% solar-to-ammonia efficiency from nitrate using Fe single atomic catalyst supported on MoS2 nanosheets[J]. Advanced Functional Materials,2022,32(18):2108316. doi: 10.1002/adfm.202108316 [71] XIA P, WANG C, HE Q, et al. MOF-derived single-atom catalysts: the next frontier in advanced oxidation for water treatment[J]. Chemical Engineering Journal,2023,452:139446-139471. doi: 10.1016/j.cej.2022.139446 [72] YU Z Y, DUAN Y, FENG X Y, et al. Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects[J]. Advanced Materials,2021,33(31):2007100. doi: 10.1002/adma.202007100 [73] CHEN H, LIANG X, LIU Y, et al. Active site engineering in porous electrocatalysts[J]. Advanced Materials,2020,32(44):2002435. doi: 10.1002/adma.202002435 [74] LI Y, TAN X, TAN H, et al. Phosphine vapor-assisted construction of heterostructured Ni2P/NiTe2 catalysts for efficient hydrogen evolution[J]. Energy & Environmental Science,2020,13(6):1799-1807. [75] CHEN W, PEI J, HE C-T, et al. Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution[J]. Advanced Materials,2018,30(30):1800396. doi: 10.1002/adma.201800396 [76] SHEN R, HAO L, HG Y H, et al. Heterogeneous N-coordinated single-atom photocatalysts and electrocatalysts[J]. Chinese Journal of Catalysis,2022,43(10):2453-2483. doi: 10.1016/S1872-2067(22)64104-4 [77] WAN J, ZHAO Z, SHANG H, et al. In situ phosphatizing of triphenylphosphine encapsulated within metal-organic frameworks to design atomic Co1-P1N3 interfacial structure for promoting catalytic performance[J]. Journal of the American Chemical Society,2020,142(18):8431-8439. doi: 10.1021/jacs.0c02229 [78] WANG Q, DOMEN K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies[J]. Chemical Reviews,2020,120(2):919-985. doi: 10.1021/acs.chemrev.9b00201 [79] ZHAO C, CHEN Z, SHI R, et al. Recent advances in conjugated polymers for visible-light-driven water splitting[J]. Advanced Materials,2020,32(28):1907296. doi: 10.1002/adma.201907296 [80] XUE Z H, LUAN D, ZHANG H, et al. Single-atom catalysts for photocatalytic energy conversion[J]. Joule,2022,6:92-133. doi: 10.1016/j.joule.2021.12.011 [81] YAN B, LIU D, FENG X, et al. Ru species supported on MOF-derived N-doped TiO2/C hybrids as efficient electrocatalytic/photocatalytic hydrogen evolution reaction catalysts[J]. Advanced Functional Materials,2020,30(31):2003007. doi: 10.1002/adfm.202003007 [82] YU H, SHI R, ZHAO Y, et al. Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution[J]. Advanced Materials,2017,29(16):1605148. doi: 10.1002/adma.201605148 [83] SUN Q, WANG N, XU Q, et al. Nanopore-supported metal nanocatalysts for efficient hydrogen generation from liquid-phase chemical hydrogen storage materials[J]. Advanced Materials,2020,32(44):2001818. doi: 10.1002/adma.202001818 [84] LI Z, XU Q. Metal-nanoparticle-catalyzed hydrogen generation from formic acid[J]. Accounts of Chemical Research,2017,50(6):1449-1458. doi: 10.1021/acs.accounts.7b00132 [85] BODDIEN A, MELLMANN D, GÄRTNER F, et al. Efficient dehydrogenation of formic acid using an iron catalyst[J]. Science,2011,333(6050):1733-1736. doi: 10.1126/science.1206613 [86] BODDIEN A, LOGES B, GÄRTNER F, et al. Iron-catalyzed hydrogen production from formic acid[J]. Journal of the American Chemical Society,2010,132(26):8924-8934. doi: 10.1021/ja100925n [87] WANG N, SUN Q, BAI R, et al. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation[J]. Jour-nal of the American Chemical Society,2016,138(24):7484-7487. doi: 10.1021/jacs.6b03518 [88] LIU Q, YANG X, HUANG Y, et al. A Schiff base modified gold catalyst for green and efficient H2 production from formic acid[J]. Energy & Environmental Science,2015,8(11):3204-3207. [89] LIU Q, YANG X, HUANG Y, et al. Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon[J]. ACS Catalysis,2018,8(12):12041-12045. doi: 10.1021/acscatal.8b03444 [90] TANG C, SURKUS A E, CHEN F, et al. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid[J]. Angewandte Chemie International Edition,2017,56(52):16616-16620. doi: 10.1002/anie.201710766 [91] LI X, SURKUS A E, RABEAH J, et al. Cobalt single-atom catalysts with high stability for selective dehydrogenation of formic acid[J]. Angewandte Chemie International Edition,2020,59(37):15849-15854. doi: 10.1002/anie.202004125 -





 下载:
下载: