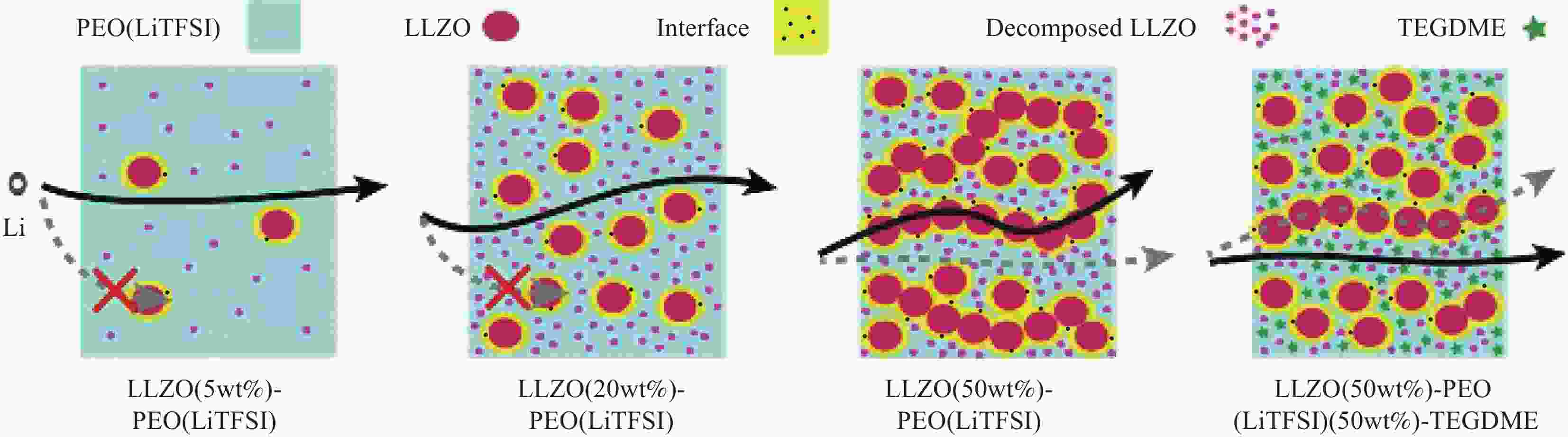
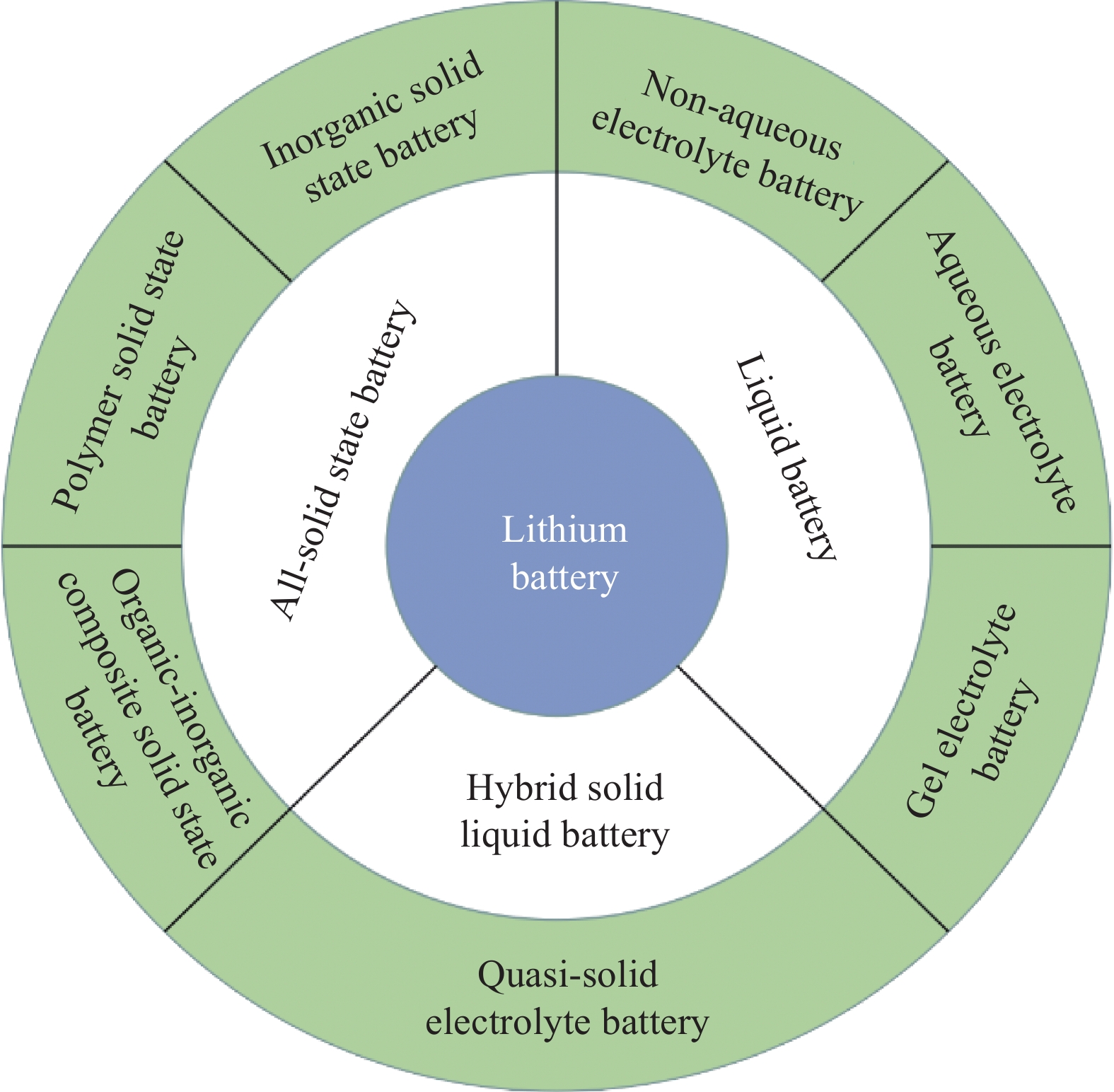
Research progress of organic-inorganic composite electrolytes for all-solid-state lithium batteries
-
摘要: 目前锂离子电池由于使用液态电解液面临着诸多问题,如工作温度范围窄、热稳定性差、容易泄露和生成锂枝晶等。发展全固态锂电池是提升电池能量密度和安全性的可行途径之一,而作为锂电池材料研究热点的有机-无机复合固态电解质,由于其兼具有机物和无机物的优点,有望运用于下一代全固态锂电池之中。本文首先概述了固态电解质的种类及传导机制,而后详细阐述了有机-无机复合固态电解质中聚合物基质和锂盐的选择以及不同维度无机填料对电解质性能尤其是力学性能的影响,最后提出了有机-无机复合固态电解质的研究总结与展望。Abstract: At present, lithium-ion batteries face many problems due to the use of liquid electrolytes, such as narrow operating temperature range, poor thermal stability, easy leakage, and formation of lithium dendrites. The development of all-solid-state lithium batteries is one of the feasible ways to improve the energy density and safety of batteries. In this paper, the types and conduction mechanisms of solid electrolytes are firstly summarized, and then the selection of polymer matrix and lithium salt in organic-inorganic composite solid electrolytes and the effects of inorganic fillers of different dimensions on electrolyte properties, especially mechanical properties, are described in detail. Finally, the research summary and prospect of organic-inorganic composite solid electrolytes are presented.
-
Key words:
- composite solid electrolyte /
- mechanical properties /
- inorganic fillers /
- polymer matrix /
- lithium salts
-
图 3 (a) Li+在聚合物固态电解质非晶区的传导[34];(b) Li+在聚合物固态电解质结晶区的传导(O代表络合位点,Mn+代表碱金属阳离子,Xn−代表阴离子)[34];(c) 聚环氧化乙烯(PEO)固态电解质中Li+传导机制[35]
Figure 3. (a) Li+ conduction in the amorphous region of the polymer solid electrolyte[34]; (b) Li+ conduction in the crystalline region of the polymer solid electrolyte (O represents complexation site, Mn+ represents alkali metal cation, Xn− represents anion)[34]; (c) Li+ conduction mechanism in poly(ethylene oxide) (PEO) solid electrolyte[35]
SPE—Solid polymer electrolyte
图 6 (a) 多孔乙烯基功能化硅(p-V-SiO2)/PEO交联复合聚合物电解质(CPE)合成流程图[82];(b) 不同p-V-SiO2(或p-SiO2)质量比的CPE应力-应变曲线[82];(c) 介孔SiO2纳米颗粒(MSNs)/聚碳酸丙烯脂(PPC)复合固态聚合物电解质制备合成图[83];(d) 不添加MSNs的聚合物固态电解质(SPE0)、含有4wt% MSNs复合固态聚合物电解质(CSPE4)在60℃时的应力-应变曲线[83];(e) 以SiO2空心纳米球颗粒为填料的复合固态电解质(SiSE)抑制Li枝晶生长的机制图[84];(f) Li/液态电解质/Li和Li/SiSE/Li对称电池在0.5 mA·cm−2电流密度下的恒电流充放电循环曲线[84]
Figure 6. (a) Schematic diagram of the synthetic route of porous vinyl-functionalized silicon (p-V-SiO2)/PEO cross-linked composite polymer electrolyte (CPE)[82]; (b) Stress-strain curves of CPEs with different mass ratios of p-V-SiO2 (or p-SiO2)[82]; (c) Synthesis diagram of mesoporous SiO2 nanoparticles (MSNs)/poly(propylene carbonate)(PPC) composite solid polymer electrolyte[83]; (d) Stress-strain curves of polymer solid electrolyte without adding MSNs (SPE0), and composite solid polymer electrolyte containing 4wt% MSNs (CSPE4) at 60℃[83]; (e) Mechanism of composite solid electrolyte with SiO2 hollow nanoparticles as filler (SiSE) as a solid electrolyte for inhibiting Li dendrite growth[84]; (f) Galvanostatic charge-discharge cycling curves of Li/liquid electrolyte/Li and Li/SiSE/Li symmetric cells at a current density of 0.5 mA·cm−2[84]
CTAB—Cetyltrimethylammonium bromide; PEGDA—Poly(ethylene glycol) diacrylate; AN—Acetonitrile; SEI—Solid electrolyte interphase
图 7 (a) 混合固态电解质(HSE)结构示意图[81];(b) 恒电流密度为0.5 mA·cm−2、温度为50℃,PEO和PEO/PEG-3Li10GeP2S12(3LGPS)作为固态电解质组装的对称电池的锂电镀/剥离测试曲线[81];((c)、(d)) 恒定电流密度为0.5 mA·cm−2的PEO/PEG-3LGPS的详细电压平台在50℃的不同循环阶段[81];(e) PEO/Li3/8Sr7/16Ta3/4Zr1/4O3(LSTZ)锂对称电池循环后的锂金属表面[86];(f) PEO/LiTFSI锂对称电池循环后的锂金属表面[86];(g) PEO/LSTZ锂对称电池的锂电镀/剥离测试曲线[86]
Figure 7. (a) Schematic diagram of hybrid solid electrolytes (HSE) structure[81]; (b) Lithium plating/peel test curves for a symmetric battery assembled as a solid electrolyte with a constant current density of 0.5 mA·cm−2 and a temperature of 50℃ with PEO and PEO/PEG-3LGPS[81]; ((c), (d)) Detailed voltage plateaus of PEO/PEG-3LGPS with a constant current density of 0.5 mA·cm−2 at different cycling stages at 50℃[81]; (e) Li metal surface after cycling in PEO/LSTZ lithium symmetric battery[86]; (f) Li metal surface after cycling in PEO/LiTFSI lithium symmetric battery[86]; (g) Lithium plating/stripping test curves for PEO/LSTZ lithium symmetric batteries[86]
CTMS—3-chloropropyl trimethoxysilane; PEG—Poly(ethylene glycol); M—Molecular mass
图 9 (a) 集成式全固态电池结构示意图[88];(b) PL(PEO、LiTFSI)、PLLM(PEO-LiTFSI-LLZO颗粒)和PLLN(PEO-LiTFSI-LLZO纳米线)固体电解质的应力-应变曲线[88];(c) 无机颗粒、随机纳米线、定向排列的纳米线Li+传导示意图[89];(d) 具有定向排列纳米线的CSEs制备流程图[89]
Figure 9. (a) Integrated all-solid-state battery structure diagram[88]; (b) Stress-strain curves of PL(PEO、LiTFSI), PLLM(PEO-LiTFSI-LLZO particles) and PLLN(PEO-LiTFSI-LLZO nanowires) solid electrolytes[88]; (c) Schematic diagram of Li+ conduction in inorganic particles, random nanowires, and aligned nanowires[89]; (d) Flowchart for the preparation of CSEs with aligned nanowires[89]
图 10 (a) 纳米压痕试验下蛭石片(VS)-CSEs和聚合物固态电解质的载荷-位移曲线[92];(b) VS-CSEs和聚合物固态电解质在拉伸试验下的应力-应变曲线[92];(c) 纳米压痕试验下PEO-SPE、层状蛭石骨架(Vr)/PEO-未溶胀的复合固态聚合物电解质(CSPE)和Vr/PEO-LCSE的载荷-位移曲线[93];(d) 拉伸试验下PEO-SPE、Vr/PEO-CSPE和Vr/PEO-LCSE的应力-应变曲线[93];(e) Vr/PEO-LCSE制备流程示意图[93]
Figure 10. (a) Load-displacement curves of vermiculite sheets (VS)-CSEs and polymer solid electrolytes under nanoindentation test[92]; (b) Stress-strain curves of VS-CSEs and polymer solid electrolytes under tensile tests[92]; (c) Load-displacement curves of PEO-SPE, laminar vermiculite framework (Vr)/PEO-composite solid polymer electrolyte without swelling (CSPE) and Vr/PEO-LCSE under nanoindentation test[93]; (d) Stress-strain curves of PEO-SPE, Vr/PEO-CSPE and Vr/PEO-LCSE under tensile test[93]; (e) Schematic diagram of the preparation process of Vr/PEO-LCSE[93]
图 11 (a) 聚合物固态电解质与单层双氢氧化物纳米片(SLN)-CSEs中Li+传导机制示意图(放大图是SLN与LiTFSI的相互作用机制)[5];(b) PVDF-HFP/SLN复合固态电解质和PVDF-HFP聚合物固态电解质的应力-应变曲线[5];(c) 2D 水辉石(HT)增强CSEs离子导电性的机制示意图[94];(d) PEO-10wt% 2D HT-Li和PEO-Li的拉伸应力-位移曲线[94]
Figure 11. (a) Schematic diagram of the Li+ conduction mechanism in the polymer solid electrolyte and single-layer layered-double-hydroxide nanosheets (SLN)-CSEs (Enlarged is the interaction mechanism between SLN and LiTFSI)[5]; (b) Stress-strain curves of PVDF-HFP/SLN composite solid electrolyte and PVDF-HFP polymer solid electrolyte[5]; (c) Schematic of the mechanism for 2D HT(hectorite) to enhance the ionic conductivity of CSEs[94]; (d) Tension stress-displacement curves of the PEO-10wt% 2D HT-Li and PEO-Li[94]
LDH—Layered double hydroxides
图 12 (a) PEO-琥珀腈(SN)-LiTFSI-玻璃纤维(GF) CSEs的结构及电化学性能[97];(b) PEO-SN25-LiTFSI10-GF CSEs的应力-应变图[97];(c) 3D石榴石型陶瓷骨架模型图和CSEs组装的固态电池在0.5 C下的典型充放电曲线[98];(d) 3D 石榴石型CSEs应力-应变图[98];(e) 3D LLZO-聚合物纳米纤维制备CSEs的结构示意图[99];(f) PVDF-PEO-LiTFSI聚合物固态电解质和3D LLZO-PVDF-PEO纳米纤维增强的CSEs应力-应变图[99]
Figure 12. (a) Structure and electrochemical properties of PEO-succinonitrile(SN)-LiTFSI-glass fiber(GF) CSEs[97]; (b) Stress-strain diagram of PEO-SN25-LiTFSI10-GF CSEs[97]; (c) 3D garnet-type ceramic framework model diagram and typical charge-discharge curves of solid-state batteries assembled with CSEs at 0.5 C[98]; (d) 3D garnet-type CSEs stress-strain diagram[98]; (e) Schematic diagram of the structure of CSEs prepared from 3D LLZO-polymer nanofibers[99]; (f) Stress-strain diagram of PVDF-PEO-LiTFSI polymer solid electrolyte and 3D LLZO-PVDF-PEO nanofibers reinforced CSEs[99]
σ—Conductivity; NCM811—Li(Ni0.8Co0.1Mn0.1)O2 powder ; LFP—LiFePO4 powder
图 13 活性陶瓷骨架的SEM形貌表征:((a)~(c)) 溶胶-凝胶法制备的LLZTO陶瓷块体(横截面图像);((d)~(f)) 静电纺丝制备的LLZTO纳米网络(俯视图);((g)~(i)) 三明治NCN陶瓷骨架(横截面图像)[100]
Figure 13. SEM morphological characterization of the active ceramic skeletons: ((a)-(c)) LLZTO ceramic bulk prepared by sol-gel method (Cross-section images); ((d)-(f)) LLZTO nano-network prepared by electrospinning (Top-view images); ((g)-(i)) sandwiched NCN ceramic skeleton (Cross-section images)[100]
图 14 ((a)~(c)) 在质量比 LLTO∶PEO=1∶1 的混合物中添加质量分数为 15wt%的 FEC 制备的复合固态电解质(CSSE-1115)的界面自愈过程[101];((d)~(f)) 质量比 LLTO∶PEO=1∶1 的复合固态电解质(CSSE-11)的锂枝晶生长进程[101]
Figure 14. ((a)-(c)) Interface self-healing process of the composite solid-state electrolytes was prepared by adding 15wt% FEC into the mixture of LLTO∶PEO=1∶1 (CSSE-1115)[101]; ((d)-(f)) Lithium dendrite growing process of composite solid-state electrolytes with mass ratio of LLTO∶PEO=1∶1 (CSSE-11)[101]
FEC—Fluoroethylene carbonate
表 1 常见聚合物材料玻璃化转变温度与熔点
Table 1. Glass transition temperature and melting point of common polymer materials
Polymer Glass transition
temperature Tg/℃Melting point Tm/℃ PEO −43 65 PAN 95 317 PMMA 105 Amorphous PPC 35 Amorphous PEC 5 Amorphous PEG −60 60 PVC 80 220 PVDF −40 171 PVDF-HFP −90 135 Notes: PAN—Poly(acrylonitrile); PMMA—Poly(methyl methacrylate); PPC—Poly(propylene carbonate); PEC—Poly(ethylene carbonate); PEG—Poly(ethylene glycol); PVC—Poly(vinyl carbonate); PVDF—Poly(vinylidene fluoride); HFP—Hexafluoropropylene. 表 2 有机-无机CSEs性能参数对比
Table 2. Comparison of performance parameters of polymer-inorganic CSEs
Polymer Lithium
saltFiller Dimension Ionic
conductivity/
(S·cm−1)Electro-
chemical
window/VThickness/
μmTensile
strength/
MPaElongation
at breaks/%Young's
modulus/
MPaRef. PEO LiTFSI p-V-SiO2 0D 5.08×10−4(60℃) 5.2 120 2.46 650 — [82] PPC LiTFSI MSN 0D 8.5×10−4(60℃) 4.8 200 4 900 — [83] PEO LiTFSI LGPS 0D 1.6×10−4(50℃) — 70 0.79 1230 — [81] PEO LiTFSI LLZO 1D 2.39×10−4(RT) 6.0 130 1 2029 — [88] PEO-PVDF LiNO3 LLTO 1D 6.02×10−4 (60℃) 4.87 — 20.67 5.41 109.87 [87] PEO LiTFSI VS 2D 1.2×10−3(60℃) 5.35 — 0.8 460 35.4 [92] PEO LiTFSI Vr 2D 1.22×10−5(25℃) 10 4.2 150 — [93] PEO LiClO4 HT 2D 9.47×10−4(60℃) — — 1.88 1270 — [94] PVDF-HFP LiTFSI SLN 2D 2.2×10−4(40℃ 4.9 — 13 450 — [5] PEO LiTFSI GN 3D 2.85×10−4(RT) 5.5 120 8.5 — 345 [97] PEO LiTFSI Ga-LLZO 3D 1.2×10−4(30℃) 5.6 262 21.8 22 — [98] PVDF-PEO LiTFSI LLZO 3D 1.05×10−4(50℃) 5.02 — 1.03 30.2 23.24 [99] Note: RT—Room temperature. -
[1] CHEN R, LI Q, YU X, et al. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces[J]. Chemical Reviews,2020,120(14):6820-6877. doi: 10.1021/acs.chemrev.9b00268 [2] MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications,2020,11(1):1-9. doi: 10.1038/s41467-019-13993-7 [3] ALBERTUS P, BABINEC S, LITZELMAN S, et al. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries[J]. Nature Energy,2018,3(1):16-21. doi: 10.1038/s41560-017-0047-2 [4] SHI Y, TAN D, LI M, et al. Nanohybrid electrolytes for high-energy lithium-ion batteries: Recent advances and future challenges[J]. Nanotechnology,2019,30(30):302002. doi: 10.1088/1361-6528/ab0fb2 [5] XIA S, YANG B, ZHANG H, et al. Ultrathin layered double hydroxide nanosheets enabling composite polymer electrolyte for all-solid-state lithium batteries at room temperature[J]. Advanced Functional Materials,2021,31(28):2101168. doi: 10.1002/adfm.202101168 [6] LIU F Q, WANG W P, YIN Y X, et al. Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries[J]. Science Advances,2018,4(10):eaat5383. doi: 10.1126/sciadv.aat5383 [7] TAN S J, YUE J, TIAN Y F, et al. In-situ encapsulating flame-retardant phosphate into robust polymer matrix for safe and stable quasi-solid-state lithium metal batteries[J]. Energy Storage Materials,2021,39:186-193. doi: 10.1016/j.ensm.2021.04.020 [8] 杨富杰, 王亮, 阮文红, 等. 石墨烯基聚合物复合电解质的设计, 性能及其应用研究进展[J]. 复合材料学报, 2021, 38(3):680-697.YANG Fujie, WANG Liang, RUAN Wenhong, et al. Research progress on the design, properties and applications of graphene-based polymer composite electrolytes[J]. Acta Materiae Compositae Sinica,2021,38(3):680-697(in Chinese). [9] 金英敏, 李栋, 贾政刚, 等. 用于全固态锂电池的有机-无机复合电解质[J]. 原子与分子物理学报, 2020, 37(6):958-973.JIN Yingmin, LI Dong, JIA Zhenggang, et al. Organic-inorganic composite electrolytes for all-solid-state lithium batteries[J]. Journal of Atomic and Molecular Physics,2020,37(6):958-973(in Chinese). [10] YU X, MANTHIRAM A. A review of composite polymer-ceramic electrolytes for lithium batteries[J]. Energy Storage Materials,2021,34:282-300. doi: 10.1016/j.ensm.2020.10.006 [11] KATO Y, HORI S, SAITO T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy,2016,1(4):1-7. doi: 10.1038/nenergy.2016.30 [12] LI J, ZHANG J, ZHAI H, et al. Rapid synthesis of garnet-type Li7La3Zr2O12 solid electrolyte with superior electrochemical performance[J]. Journal of the European Ceramic Society,2022,42(4):1568-1575. doi: 10.1016/j.jeurceramsoc.2021.11.028 [13] 温荣严, 高志浩, 门树林, 等. 聚偏氟乙烯基凝胶聚合物电解质的研究进展[J]. 储能科学与技术, 2021, 10(1):40-49. doi: 10.19799/j.cnki.2095-4239.2020.0234WEN Rongyan, GAO Zhihao, MEN Shulin, et al. Research progress on polyvinylidene fluoride-based gel polymer electrolytes[J]. Energy Storage Science and Technology,2021,10(1):40-49(in Chinese). doi: 10.19799/j.cnki.2095-4239.2020.0234 [14] CROCE F, CURINI R, MARTINELLI A, et al. Physical and chemical properties of nanocomposite polymer electrolytes[J]. The Journal of Physical Chemistry B,1999,103(48):10632-10638. doi: 10.1021/jp992307u [15] 许卓, 郑莉莉, 陈兵, 等. 固态电池复合电解质研究综述[J]. 储能科学与技术, 2021, 10(6):2117-2126. doi: 10.19799/j.cnki.2095-4239.2021.0178XU Zhuo, ZHENG Lili, CHEN Bing, et al. Review of research on composite electrolytes for solid-state batteries[J]. Energy Storage Science and Technology,2021,10(6):2117-2126(in Chinese). doi: 10.19799/j.cnki.2095-4239.2021.0178 [16] ZHANG B, LIN Z, DONG H, et al. Revealing cooperative Li-ion migration in Li1+xAlxTi2−x(PO4)3 solid state electrolytes with high Al doping[J]. Journal of Materials Chemistry A,2020,8(1):342-348. doi: 10.1039/C9TA09770H [17] LV R, KOU W, GUO S, et al. Preparing two-dimensional ordered Li0.33La0.557TiO3 crystal in interlayer channel of thin laminar inorganic solid-state electrolyte towards ultrafast Li+ transfer[J]. Angewandte Chemie International Edition,2022,61(7):e202114220. doi: 10.1002/anie.202114220 [18] WANG Z, XU H, XUAN M, et al. From anti-perovskite to double anti-perovskite: Tuning lattice chemistry to achieve super-fast Li+ transport in cubic solid lithium halogen-chalcogenides[J]. Journal of Materials Chemistry A,2018,6(1):73-83. doi: 10.1039/C7TA08698A [19] BUSCHMANN H, DÖLLE J, BERENDTS S, et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”[J]. Physical Chemistry Chemical Physics,2011,13(43):19378-19392. doi: 10.1039/c1cp22108f [20] ZHANG Z, SHAO Y, LOTSCH B, et al. New horizons for inorganic solid state ion conductors[J]. Energy & Environmental Science,2018,11(8):1945-1976. doi: 10.1039/c8ee01053f [21] 黄祯, 杨菁, 陈晓添, 等. 无机固体电解质材料的基础与应用研究[J]. 储能科学与技术, 2015, 4(1):1-18. doi: 10.3969/j.issn.2095-4239.2015.01.02HUANG Zhen, YANG Jing, CHEN Xiaotian, et al. Basic and applied research on inorganic solid electrolyte materials[J]. Energy Storage Science and Technology,2015,4(1):1-18(in Chinese). doi: 10.3969/j.issn.2095-4239.2015.01.02 [22] ZHANG Q, CAO D, MA Y, et al. Sulfide-based solid-state electrolytes: Synthesis, stability, and potential for all-solid-state batteries[J]. Advanced Materials,2019,31(44):1901131. doi: 10.1002/adma.201901131 [23] KAMAYA N, HOMMA K, YAMAKAWA Y, et al. A lithium superionic conductor[J]. Nature Materials,2011,10(9):682-686. doi: 10.1038/nmat3066 [24] MURAMATSU H, HAYASHI A, OHTOMO T, et al. Structural change of Li2S-P2S5 sulfide solid electrolytes in the atmosphere[J]. Solid State Ionics,2011,182(1):116-119. doi: 10.1016/j.ssi.2010.10.013 [25] ZHU Y, HE X, MO Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces,2015,7(42):23685-23693. [26] FAMPRIKIS T, CANEPA P, DAWSON J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials,2019,18(12):1278-1291. doi: 10.1038/s41563-019-0431-3 [27] CROCE F, APPETECCHI G B, PERSI L, et al. Nanocomposite polymer electrolytes for lithium batteries[J]. Nature,1998,394(6692):456-458. doi: 10.1038/28818 [28] LI Y, ZHANG B, YUAN Q. A comparative study of long and short GRBs. II. A multiwavelength method to distinguish type II (massive star) and type I (compact star) GRBs[J]. The Astrophysical Journal,2020,897(2):154. doi: 10.3847/1538-4357/ab96b8 [29] 闫雅婧. 锂离子电池用固态电解质的研究现状与展望[J]. 无机盐工业, 2020, 52(7):22-25. doi: 10.11962/1006-4990.2019-0676YAN Yajing. Research status and prospect of solid electrolytes for lithium-ion batteries[J]. Inorganic Salt Industry,2020,52(7):22-25(in Chinese). doi: 10.11962/1006-4990.2019-0676 [30] TAKEDA Y, YAMAMOTO O, IMANISHI N. Lithium dendrite formation on a lithium metal anode from liquid, polymer and solid electrolytes[J]. Electrochemistry,2016,84(4):210-218. doi: 10.5796/electrochemistry.84.210 [31] ZHOU D, SHANMUKARAJ D, TKACHEVA A, et al. Polymer electrolytes for lithium-based batteries: Advances and prospects[J]. Chemistry,2019,5(9):2326-2352. doi: 10.1016/j.chempr.2019.05.009 [32] GUPTA S, VARSHNEY P K. Effect of plasticizer on the conductivity of carboxymethyl cellulose-based solid polymer electrolyte[J]. Polymer Bulletin,2019,76(12):6169-6178. doi: 10.1007/s00289-019-02714-1 [33] FAN L Z, HE H, NAN C W. Tailoring inorganic-polymer composites for the mass production of solid-state batteries[J]. Nature Reviews Materials,2021,6(11):1003-1019. doi: 10.1038/s41578-021-00320-0 [34] ZHAO Q, STALIN S, ZHAO C Z, et al. Designing solid-state electrolytes for safe, energy-dense batteries[J]. Nature Reviews Materials,2020,5(3):229-252. doi: 10.1038/s41578-019-0165-5 [35] COSTA C M, LiZUNDIA E, LANCEROS-MÉNDEZ S. Polymers for advanced lithium-ion batteries: State of the art and future needs on polymers for the different battery components[J]. Progress in Energy and Combustion Science,2020,79:100846. doi: 10.1016/j.pecs.2020.100846 [36] HENDERSON W A, PASSERINI S. Ionic conductivity in crystalline-amorphous polymer electrolytes-P(EO)6:LiX phases[J]. Electrochemistry Communications,2003,5(7):575-578. doi: 10.1016/S1388-2481(03)00131-0 [37] XUE S, LIU Y, LI Y, et al. Diffusion of lithium ions in amorphous and crystalline poly(ethylene oxide)3: LiCF3SO3 polymer electrolytes[J]. Electrochimica Acta,2017,235:122-128. doi: 10.1016/j.electacta.2017.03.083 [38] 贾婉卿, 孙歌, 姚诗余, 等. 锂离子电池中有机–无机复合固态电解质的研究进展[J]. 硅酸盐学报, 2022, 50(1):121-133. doi: 10.14062/j.issn.0454-5648.20210622JIA Wanqing, SUN Ge, YAO Shiyu, et al. Research progress of organic-inorganic composite solid-state electrolytes in lithium-ion batteries[J]. Journal of the Chinese Ceramic Society,2022,50(1):121-133(in Chinese). doi: 10.14062/j.issn.0454-5648.20210622 [39] CARADANT L, VERDIER N, FORAN G, et al. Extrusion of polymer blend electrolytes for solid-state lithium batteries: A study of polar functional groups[J]. ACS Applied Polymer Materials,2021,3(12):6694-6704. doi: 10.1021/acsapm.1c01466 [40] LI Z, GUO D, LI F, et al. Nerve network-inspired solid polymer electrolytes (NN-SPE) for fast and single-ion lithium conduction[J]. Energy Storage Materials,2022,49:575-582. doi: 10.1016/j.ensm.2022.05.003 [41] ZHENG Y, LI X, LI C Y. A novel de-coupling solid polymer electrolyte via semi-interpenetrating network for lithium metal battery[J]. Energy Storage Materials,2020,29:42-51. doi: 10.1016/j.ensm.2020.04.002 [42] DEVAUX D, GLÉ D, PHAN T N T, et al. Optimization of block copolymer electrolytes for lithium metal batteries[J]. Chemistry of Materials,2015,27(13):4682-4692. doi: 10.1021/acs.chemmater.5b01273 [43] LIU F, LI T, YANG Y, et al. Investigation on the copolymer electrolyte of poly(1, 3-dioxolane-co-formaldehyde)[J]. Macromolecular Rapid Communications,2020,41(9):2000047. doi: 10.1002/marc.202000047 [44] DIRICAN M, YAN C, ZHU P, et al. Composite solid electrolytes for all-solid-state lithium batteries[J]. Materials Science and Engineering: R: Reports,2019,136:27-46. doi: 10.1016/j.mser.2018.10.004 [45] AO X, WANG X, TAN J, et al. Nanocomposite with fast Li+ conducting percolation network: Solid polymer electrolyte with Li+ non-conducting filler[J]. Nano Energy,2021,79:105475. doi: 10.1016/j.nanoen.2020.105475 [46] ZHENG J, HU Y Y. New insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes[J]. ACS Applied Materials & Interfaces,2018,10(4):4113-4120. doi: 10.1021/acsami.7b17301 [47] LI Z, HUANG H M, ZHU J K, et al. Ionic conduction in composite polymer electrolytes: Case of PEO:Ga-LLZO composites[J]. ACS Applied Materials & Interfaces,2018,11(1):784-791. doi: 10.1021/acsami.8b17279 [48] CHEN L, LI Y, LI S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”[J]. Nano Energy,2018,46:176-184. doi: 10.1016/j.nanoen.2017.12.037 [49] FENTON D E, PARKER J M, WRIGHT P V. Complexes of alkali metal ions with poly(ethylene oxide)[J]. Polymer,1973,14(11):589. doi: 10.1016/0032-3861(73)90146-8 [50] WANG Z, HUANG B, HUANG H, et al. Investigation of the position of Li+ ions in a polyacrylonitrile-based electrolyte by Raman and infrared spectroscopy[J]. Electrochimica Acta,1996,41(9):1443-1446. doi: 10.1016/0013-4686(95)00392-4 [51] CAZZANELLI E, MARIOTTO G, APPETECCHI G B, et al. Study of ion-molecule interaction in poly(methylmethacrylate) based gel electrolytes by raman spectroscopy[J]. Electrochimica Acta,1995,40(13-14):2379-2382. doi: 10.1016/0013-4686(95)00198-N [52] YUE H, LI J, WANG Q, et al. Sandwich-like poly(propylene carbonate)-based electrolyte for ambient-tempera-ture solid-state lithium ion batteries[J]. ACS Sustainable Chemistry & Engineering,2018,6(1):268-274. doi: 10.1021/acssuschemeng.7b02401 [53] KIMURA K, YAJIMA M, TOMINAGA Y. A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature[J]. Electrochemistry Communications,2016,66:46-48. doi: 10.1016/j.elecom.2016.02.022 [54] LIU H, MULDERRIG L, HALLINAN JR D, et al. Lignin-based solid polymer electrolytes: Lignin-graft-poly(ethylene glycol)[J]. Macromolecular Rapid Communications,2021,42(3):2000428. doi: 10.1002/marc.202000428 [55] SUKESHINI A M, NISHIMOTO A, WATANABE M. Transport and electrochemical characterization of plasticized poly(vinyl chloride) solid electrolytes[J]. Solid State Ionics,1996,86:385-393. [56] SABEL V F, MOHAMED R, ADNANE B, et al. Protic ionic liquids/poly(vinylidene fluoride) composite membranes for fuel cell application[J]. Journal of Energy Chemistry,2021,53(2):197-207. [57] SAIKIA D, WU H Y, PAN Y C, et al. Highly conductive and electrochemically stable plasticized blend polymer electrolytes based on PVDF-HFP and triblock copolymer PPG-PEG-PPG diamine for Li-ion batteries[J]. Journal of Power Sources,2011,196(5):2826-2834. doi: 10.1016/j.jpowsour.2010.10.096 [58] GONÇALVES R, MIRANDA D, ALMEIDA A M, et al. Solid polymer electrolytes based on lithium bis(trifluoromethanesulfonyl) imide/poly(vinylidene fluoride-co-hexafluoropropylene) for safer rechargeable lithium-ion batteries[J]. Sustainable Materials and Technologies,2019,21:e00104. doi: 10.1016/j.susmat.2019.e00104 [59] BESHAHWURED S L, WU Y S, TRUONG T B T, et al. A modified trilayer membrane for suppressing Li dendrite growth in all-solid-state lithium-metal batteries[J]. Chemical Engineering Journal,2021,426:131850. doi: 10.1016/j.cej.2021.131850 [60] LI Q, CHEN J, FAN L, et al. Progress in electrolytes for rechargeable Li-based batteries and beyond[J]. Green Energy & Environment,2016,1(1):18-42. [61] ZHU P, YAN C, DIRICAN M, et al. Li0.33La0.557TiO3 ceramic nanofiber-enhanced polyethylene oxide-based composite polymer electrolytes for all-solid-state lithium batteries[J]. Journal of Materials Chemistry A,2018,6(10):4279-4285. doi: 10.1039/C7TA10517G [62] HU P, CHAI J, DUAN Y, et al. Progress in nitrile-based polymer electrolytes for high performance lithium batteries[J]. Journal of Materials Chemistry A,2016,4(26):10070-10083. doi: 10.1039/C6TA02907H [63] 董甜甜, 张建军, 柴敬超, 等. 聚碳酸酯基固态聚合物电解质的研究进展[J]. 高分子学报, 2017(6):906-921. doi: 10.11777/j.issn1000-3304.2017.16333DONG Tiantian, ZHANG Jianjun, CHAI Jingchao, et al. Research progress of polycarbonate-based solid polymer electrolytes[J]. Acta Polymerica Sinica,2017(6):906-921(in Chinese). doi: 10.11777/j.issn1000-3304.2017.16333 [64] 赵莉, 杜蘅, 刘虎, 等. 纳米 SiO2 微球在 PMMA 凝胶聚合物电解质中的尺寸效应及其在全固态电致变色器件中的应用[J]. 复合材料学报, 2021, 38(5):1446-1454.ZHAO Li, DU Heng, LIU Hu, et al. Size effect of nano-SiO2 microspheres in PMMA gel polymer electrolyte and its application in all-solid-state electrochromic devices[J]. Acta Materiae Compositae Sinica,2021,38(5):1446-1454(in Chinese). [65] KOBAYASHI K, PAGOT G, KETI V, et al. Effect of plasticizer on the ion-conductive and dielectric behavior of poly(ethylene carbonate)-based Li electrolytes[J]. Polymer Journal,2020,53(1):149-155. [66] CHAI J, LIU Z, MA J, et al. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries[J]. Advanced Science,2017,4(2):1600377. doi: 10.1002/advs.201600377 [67] ZHENG F, LI H T, ZHENG Y Z, et al. Trimethyl phosphate-enhanced polyvinyl carbonate polymer electrolyte with improved interfacial stability for solid-state lithium battery[J]. Rare Metals,2022,41(6):1889-1898. doi: 10.1007/s12598-021-01928-5 [68] GAO Z, WEN R, DENG H, et al. Composite membrane of poly(vinylidene fluoride) and 2D Ni(OH)2 nanosheets for high-performance lithium-ion battery[J]. ACS Applied Polymer Materials,2022,4(2):960-970. doi: 10.1021/acsapm.1c01413 [69] 苏月, 刘旭华, 曾芳磊, 等. 聚偏氟乙烯/聚偏氟乙烯磺酸锂/锂盐复合固态电解质的制备及其性能[J]. 储能科学与技术, 2021, 10(6):2069. doi: 10.19799/j.cnki.2095-4239.2021.0160SU Yue, LIU Xuhua, ZENG Fanglei, et al. Preparation and properties of polyvinylidene fluoride/lithium polyvinylidene fluoride sulfonate/lithium salt composite solid electrolyte[J]. Energy Storage Science and Technology,2021,10(6):2069(in Chinese). doi: 10.19799/j.cnki.2095-4239.2021.0160 [70] LI S, LI N, SUN C. A flexible three-dimensional composite nanofiber enhanced quasi-solid electrolyte for high-performance lithium metal batteries[J]. Inorganic Chemistry Frontiers,2021,8(2):361-367. doi: 10.1039/D0QI01159B [71] LIU W, YI C, LI L, et al. Designing polymer-in-salt electrolyte and fully infiltrated 3D electrode for integrated solid-state lithium batteries[J]. Angewandte Chemie,2021,133(23):13041-13050. doi: 10.1002/ange.202101537 [72] 王星星, 宋子钰, 吴浩, 等. 固态聚合物电解质导电锂盐的研究进展[J]. 储能科学与技术, 2022, 11(4):1226-1235. doi: 10.19799/j.cnki.2095-4239.2022.0038WANG Xingxing, SONG Ziyu, WU Hao, et al. Research progress of conductive lithium salts in solid polymer electrolytes[J]. Energy Storage Science and Technology,2022,11(4):1226-1235(in Chinese). doi: 10.19799/j.cnki.2095-4239.2022.0038 [73] ZHANG S S, XU K, JOW T R. EIS study on the formation of solid electrolyte interface in Li-ion battery[J]. Electrochimica Acta,2006,51(8-9):1636-1640. doi: 10.1016/j.electacta.2005.02.137 [74] LESTARININGSIH T, SABRINA Q, RATRI C R, et al. Structure, thermal and electrical properties of PVDF-HFP/LiBOB solid polymer electrolyte[J]. Journal of Physics Conference Series,2019,1191(1):012026. doi: 10.1088/1742-6596/1191/1/012026 [75] XU K, ZHANG S, JOW T R, et al. LiBOB as salt for lithium-ion batteries: A possible solution for high temperature operation[J]. Electrochemical and Solid-State Letters,2001,5(1):A26. [76] ULUTAŞ K, YAHSI U, DELIGÖZ H, et al. Dielectric properties and conductivity of PVDF-co-HFP/LiClO4 polymer electrolytes[J]. Canadian Journal of Physics,2018,96(7):786-791. doi: 10.1139/cjp-2017-0678 [77] 赵丹妮, 田芳禺, 于雅琳, 等. 锂离子电解液/环氧乙烯基酯树脂固态电解质的制备与性能[J]. 复合材料学报, 2018, 35(2):253-259.ZHAO Danni, TIAN Fangyu, YU Yalin, et al. Preparation and properties of lithium ion electrolyte/epoxy vinyl ester resin solid electrolyte[J]. Acta Materiae Compositae Sinica,2018,35(2):253-259(in Chinese). [78] ESHETU G G, JUDEZ X, LI C, et al. Ultrahigh performance all solid-state lithium sulfur batteries: Salt anion’s chemistry-induced anomalous synergistic effect[J]. Journal of the American Chemical Society,2018,140(31):9921-9933. doi: 10.1021/jacs.8b04612 [79] RAVI M, KIM S, RAN F, et al. Hybrid gel polymer electrolyte based on 1-methyl-1-propylpyrrolidinium bis(trifluoromethanesulfonyl) imide for flexible and shape-variant lithium secondary batteries[J]. Journal of Membrane Science,2021,621:119018. doi: 10.1016/j.memsci.2020.119018 [80] TAO S D, LI J, HU R, et al. 3Li2S-2MoS2 filled composite polymer PVDF-HFP/LiODFB electrolyte with excellent interface performance for lithium metal batteries[J]. Applied Surface Science,2021,536:147794. doi: 10.1016/j.apsusc.2020.147794 [81] PAN K, ZHANG L, QIAN W, et al. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries[J]. Advanced Materials,2020,32(17):2000399. doi: 10.1002/adma.202000399 [82] ZHAN H, WU M, WANG R, et al. Excellent performances of composite polymer electrolytes with porous vinyl-functionalized SiO2 nanoparticles for lithium metal batteries[J]. Polymers,2021,13(15):2468. doi: 10.3390/polym13152468 [83] DIDWAL P N, SINGHBABU Y N, VERMA R, et al. An advanced solid polymer electrolyte composed of poly(propylene carbonate) and mesoporous silica nanoparticles for use in all-solid-state lithium-ion batteries[J]. Energy Storage Materials,2021,37:476-490. doi: 10.1016/j.ensm.2021.02.034 [84] ZHOU D, LIU R, HE Y B, et al. SiO2 hollow nanosphere-based composite solid electrolyte for lithium metal batteries to suppress lithium dendrite growth and enhance cycle life[J]. Advanced Energy Materials,2016,6(7):1502214. doi: 10.1002/aenm.201502214 [85] CHOUDHURY S, MANGAL R, AGRAWAL A, et al. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles[J]. Nature Communications,2015,6:10101. doi: 10.1038/ncomms10101 [86] XU H, CHIEN P H, SHI J, et al. High-performance all-solid-state batteries enabled by salt bonding to perovskite in poly(ethylene oxide)[J]. Proceedings of the National Academy of Sciences,2019,116(38):18815-18821. doi: 10.1073/pnas.1907507116 [87] NOURISABET T, AVAL H J, SHIDPOUR R, et al. Fabrication of a PEO-PVDF blend based polymer composite electrolyte with extremely high ionic conductivity via the addition of LLTO nanowires[J]. Solid State Ionics,2022,377:115885. doi: 10.1016/j.ssi.2022.115885 [88] WAN Z, LEI D, YANG W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder[J]. Advanced Functional Materials,2019,29(1):1805301. doi: 10.1002/adfm.201805301 [89] LIU W, LEE S W, LIN D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires[J]. Nature Energy,2017,2(5):1-7. [90] LI X, CHEN W, QIAN Q, et al. Electrospinning-based strategies for battery materials[J]. Advanced Energy Materials,2021,11(2):2000845. doi: 10.1002/aenm.202000845 [91] PAN P, ZHANG M, CHENG Z, et al. Garnet ceramic fabric-reinforced flexible composite solid electrolyte derived from silk template for safe and long-term stable all-solid-state lithium metal batteries[J]. Energy Storage Materials,2022,47:279-287. doi: 10.1016/j.ensm.2022.02.018 [92] TANG W, TANG S, ZHANG C, et al. Simultaneously enhancing the thermal stability, mechanical modulus, and electrochemical performance of solid polymer electrolytes by incorporating 2D sheets[J]. Advanced Energy Materials,2018,8(24):1800866. doi: 10.1002/aenm.201800866 [93] ZHAI P, PENG N, SUN Z, et al. Thin laminar composite solid electrolyte with high ionic conductivity and mechanical strength towards advanced all-solid-state lithium-sulfur battery[J]. Journal of Materials Chemistry A,2020,8(44):23344-23353. doi: 10.1039/D0TA07630A [94] PEI X, MU J, HONH J, et al. Solution-processed 2D hectorite nanolayers for high-efficient composite solid-state electrolyte[J]. Applied Clay Science,2022,216:106363. doi: 10.1016/j.clay.2021.106363 [95] CHENG M, JIANG Y. 3D-printed solid-state electrolytes for electrochemical energy storage devices[J]. Journal of Materials Research,2021,36:4547-4564. [96] CAO L, WU H, YANG P, et al. Graphene oxide-based solid electrolytes with 3D prepercolating pathways for efficient proton transport[J]. Advanced Functional Materials,2018,28(50):1804944. doi: 10.1002/adfm.201804944 [97] WANG J, YANG J, SHEN L, et al. Synergistic effects of plasticizer and 3 framework toward high-performance solid polymer electrolyte for room-temperature solid-state lithium batteries[J]. ACS Applied Energy Materials,2021,4(4):4129-4137. doi: 10.1021/acsaem.1c00468 [98] LI Z, SHA W X, GUO X. Three-dimensional garnet framework-reinforced solid composite electrolytes with high lithium-ion conductivity and excellent stability[J]. ACS Applied Materials & Interfaces,2019,11(30):26920-26927. [99] ZHANG M, PAN P, CHENG Z, et al. Flexible, mechanically robust, solid-state electrolyte membrane with conducting oxide-enhanced 3D nanofiber networks for lithium batteries[J]. Nano Letters,2021,21(16):7070-7078. doi: 10.1021/acs.nanolett.1c01704 [100] FAN R, LIAO W, FAN S, et al. Regulating interfacial Li-ion transport via an integrated corrugated 3D skeleton in solid composite electrolyte for all-solid-state lithium metal batteries[J]. Advanced Science,2022,9(8):2104506. doi: 10.1002/advs.202104506 [101] LI H, LIU W, YANG X, et al. Fluoroethylene carbonate-Li-ion enabling composite solid-state electrolyte and lithium metal interface self-healing for dendrite-free lithium deposition[J]. Chemical Engineering Journal,2021,408:127254. doi: 10.1016/j.cej.2020.127254 -






 下载:
下载: