Research progress of photocatalytic CO2 reduction based on CsPbBr3 perovskite
-
摘要: 探索绿色发展、解决能源危机已成为近年来商业发展的趋势。金属卤化物钙钛矿因其独特的光催化性能而备受关注。其中,CsPbBr3钙钛矿具有较高的光催化活性和优异的稳定性,在光催化CO2还原方面发展迅速。在能源发展趋势下,减少碳排放和催化还原CO2作为燃料是研究热点和主要途径。然而,纯CsPbBr3较差的CO2吸附还原能力、严重的电荷复合和较低的电荷效率严重阻碍了钙钛矿光催化的商业化。为了解决纯CsPbBr3材料光催化中的一系列问题,对CsPbBr3钙钛矿进行表面改性或构建多组分复合材料是目前最经济、最有前景的解决方案。本文讨论了CsPbBr3钙钛矿的光催化反应原理及所面临稳定性和还原能力的阻碍,对CsPbBr3钙钛矿及其复合物的光催化CO2还原研究进行了系统的回顾。最后对构建更加稳定、高效及可持续性的CO2还原光催化剂新的探索方向进行了展望。Abstract: Exploring green development and solving the energy crisis has become a trend of commercial development in recent years. Metal halide perovskites have attracted great attentions due to their unique photocatalytic properties. Among them, CsPbBr3 perovskite has high photocatalytic activity and excellent stability, and has developed rapidly in photocatalytic CO2 reduction. Under the trend of energy development, reducing carbon emissions and catalytic reduction of CO2 as fuels are research hotspots and main approaches. However, the poor CO2 adsorption capacity, severe charge recombination, and low charge efficiency of pure CsPbBr3 seriously hinder the commercialization of perovskite photocatalysis. In order to solve a series of problems in photocatalysis of pure CsPbBr3 materials, surface modification the of CsPbBr3 perovskite or construct on of multicomponent composites is currently the most economical and promising solution. In this review, we systematically review the latest research on photocatalytic CO2 reduction of CsPbBr3 perovskites and their composites, discuss the photocatalytic reaction mechanism of CsPbBr3 perovskites, and then propose obstacles to development. Finally, we expect this review to provide new exploration directions for building more stable, efficient and sustainable photocatalysts for CO2 emission reduction.
-
Key words:
- photocatalytic CO2 reduction /
- perovskites /
- catalysts /
- surface modification /
- composite material /
- CsPbBr3
-
图 3 不同样品的CO产量(a)和 CH4产量(b)图作为反应时间的函数;(c) CsPbBr3/沸石咪唑酯骨架结构材料 (ZIFs) 的制备过程和CO2光还原过程的示意图[45];(d) 钴掺杂CsPbBr3@石墨二炔 (GDY)的合成及其光氧化还原反应示意图[47]
Figure 3. CO yield (a) and CH4 yield (b) plots of different samples as the function of reaction time; (c) Schematic illustration of the fabrication process and CO2 photoreduction process of CsPbBr3/zeolite imidazolate framework (ZIFs)[45]; (d) Illustration of the synthesis of cobalt doped CsPbBr3@graphdiyne (GDY) and its photoredox reactions[47]
2-Hmim—2-Methylimidazole; Eg—Electronic transition
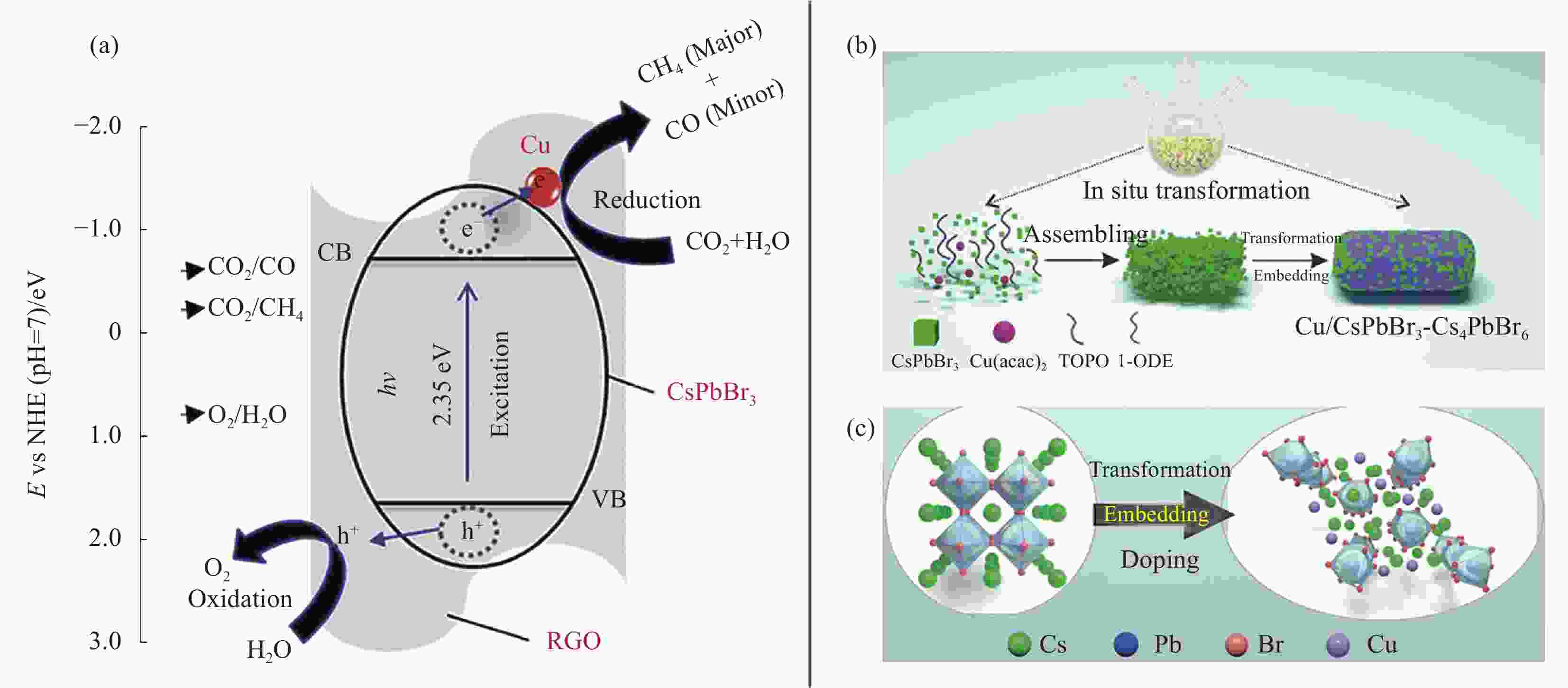
图 4 (a) 模拟阳光下CsPbBr3-Cu-还原氧化石墨烯(RGO)纳米复合材料CO2还原电荷分离与转移机制示意图[55];(b) Cu/CsPbBr3-Cs4PbBr6合成过程示意图;(c)原位转化、嵌入和掺杂过程的原子结构转换示意图[56]
Figure 4. (a) Schematic diagram for the charge separation and transfer mechanism of CO2 reduction on CsPbBr3-Cu-reduced graphene oxide (RGO) nanocomposites under simulated sunlight[55]; (b) Schematic diagram of Cu/CsPbBr3-Cs4PbBr6 synthesis process; (c) Schematic atomic structures conversion for in-situ transformation, embedding and doping processes[56]
NHE—Normal hydrogen electrode; Cu(acac)2—Cupric(II) acetylacetonate; TOPO—Trioctylphosphine oxide; 1-ODE—1-octadecene; E—Potential
图 5 (a) CsPbBr3-Au纳米复合材料的制备示意图[60];(b) 块体CsPbBr3、三维有序大孔(3DOM) CsPbBr3和3DOM Au-CsPbBr3的XRD图谱及CsPbBr3和Au的标准PDF卡;(c) 3DOM Au-CsPbBr3光催化剂的合成过程示意图;(d) 光催化反应过程中可能的电荷转移途径[61];(e) Fe掺杂CsPbBr3和未掺杂CsPbBr3纳米晶光催化剂的示意图[63]
Figure 5. (a) Sketch of the fabrication of CsPbBr3-Au nanocomposites[60]; (b) XRD patterns of bulk CsPbBr3, 3DOM CsPbBr3 and 3DOM Au-CsPbBr3 and the standard PDF cards of CsPbBr3 and Au; (c) Illustration of the synthesis process of three dimensional ordered macropore (3DOM) Au-CsPbBr3 photocatalyst; (d) Possible charge transfer route during the photocatalytic reaction[61]; (e) Schematic presentation of Fe-doped CsPbBr3 and undoped CsPbBr3 nanocrystal photocatalysts[63]
NPs—Nanoparticles; NCs—Nanocrystallines; MPA—3-mercaptopropionic acid; λ—Wavelength
图 6 (a) TiO2/CsPbBr3异质结示意图:内电场(IEF)诱导的电荷转移、分离和在紫外可见光照射下形成S型异质结用于CO2光还原[77];(b) 静电自组装制备的2D/2D CsPbBr3/Bi2WO6异质结示意图[78];(c) α-Fe2O3/Amine-RGO/CsPbBr3制备过程示意图-固态Z型光催化剂[79];(d) 三元WO3/CsPbBr3/ZIF-67异质结构制备过程示意图[80]
Figure 6. (a) Schematic illustration of TiO2/CsPbBr3 heterojunction: Internal electric field (IEF)-induced charge transfer, separation, and the formation of S-scheme heterojunction under UV-visible-light irradiation for CO2 photoreduction[77]; (b) Schematic diagram of 2D/2D CsPbBr3/Bi2WO6 heterojunction prepared via electrostatic self-assembly process[78]; (c) Schematic illustration of the fabrication procedure of α-Fe2O3/Amine-RGO/CsPbBr3 all-solid-state Z-scheme photocatalyst[79]; (d) Schematic illustration of the preparation process of the ternary WO3/CsPbBr3/ZIF-67 heterostructure[80]
EF—Electric potential; NS—Nanosheets; IPA—Isopropanol; [BMIM]BF4—1-butyl-3-methylimidazolium tetrafluoroborate; E—Potential
图 8 CsPbBr3 QDs (BZA & BA)和CsPbBr3 QDs (BZA & BA)/BP NSs ((a), (b))、CsPbBr3 QDs (OA & OAm)和CsPbBr3 QDs (OA & OAm)/BP NSs ((c), (d))、CsPbBr3 QDs (APS) (e)和CsPbBr3 QDs (APS)/BP NSs (f)的PL图谱和时间分辨PL图谱[86]
Figure 8. PL spectra and time-resolved PL spectra of CsPbBr3 QDs (BZA & BA) and CsPbBr3 QDs (BZA & BA)/BP NSs ((a), (b)), CsPbBr3 QDs (OA & OAm) and CsPbBr3 QDs (OA & OAm)/BP NSs ((c), (d)), CsPbBr3 QDs (APS) (e) and CsPbBr3 QDs (APS)/BP NSs (f)[86]
OA—Oleic acid; OAm—Oleylamine; APS—Modified ligand 3-aminopropyltriethoxysilane with glutaric anhydride; T1-T3—Time; A1, A2—Charge transfer contribution
图 9 (a) CsPbBr3 钙钛矿量子点 (PQDs) 和CsPbBr3/聚苯胺 (PANI)复合电极在Na2SO4水溶液(0.1 mol/L,pH=6.8)中的瞬态光电流响应;(b) CsPbBr3 PQDs和CsPbBr3/PANI复合材料的电化学阻抗谱(EIS)[90];(c) 电流测量电流密度-时间曲线;(d) EIS奈奎斯特图[93]
Figure 9. (a) Transient photocurrent responses of CsPbBr3 perovskite quantum dots (PQDs) and CsPbBr3/polyaniline (PANI) composite electrodes in Na2SO4 aqueous solution (0.1 mol/L, pH=6.8); (b) Electrochemical impedance spectra (EIS) of CsPbBr3 PQDs and the CsPbBr3/PANI composite[90]; (c) Amperometric current density-time curves; (d) EIS Nyquist plots[93]
P3 HT—Poly(3-hexylthiophene-2,5-diyl); Z''—Imaginary reactance of impedance Z; Z'—Real part resistance of impedance Z
-
[1] DONG F, HUA Y, YU B. Peak carbon emissions in China: Status, key factors and countermeasures—A literature review[J]. Sustainability,2018,10(8):2895. doi: 10.3390/su10082895 [2] ZHU J F, XU X, ZHU Z, et al. A wind-light-fire co-generation strategy considering dual carbon targets and emission costs[J]. Journal of Physics: Conference Series,2021,2030:012078. doi: 10.1088/1742-6596/2030/1/012078 [3] HUANG R, ZHANG S, WANG P. Key areas and pathways for carbon emissions reduction in Beijing for the “Dual Carbon” targets[J]. Energy Policy,2022,164:112873. doi: 10.1016/j.enpol.2022.112873 [4] CHEN L, MSIGWA G, YANG M, et al. Strategies to achieve a carbon neutral society: A review[J]. Environmental Chemistry Letters,2022,20:2277-2310. doi: 10.1007/s10311-022-01435-8 [5] CHEN J M. Carbon neutrality: Toward a sustainable future[J]. The Innovation,2021,2(3):100127. doi: 10.1016/j.xinn.2021.100127 [6] ABZIEHER T. Best research-cell efficiency chart[EB/OL]. (2021-12-23) [2022-07-29]. https://www.nrel.gov/pv/cell-efficiency.html. [7] SCHANZE K S, KAMAT P V, YANG P, et al. Progress in perovskite photocatalysis[J]. ACS Energy Letters,2020,5(8):2602-2604. doi: 10.1021/acsenergylett.0c01480 [8] PARK S, CHANG W J, LEE C W, et al. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution[J]. Nature Energy,2017,2:16185. doi: 10.1038/nenergy.2016.185 [9] LIU D, SHAO Z, LI C, et al. Structural properties and stability of inorganic CsPbI3 perovskites[J]. Small Structures,2021,2(3):2000089. doi: 10.1002/sstr.202000089 [10] 穆延飞. 卤化钙钛矿基高效催化剂的可控制备及其光催化CO2还原性能研究[D]. 天津: 天津理工大学, 2021.MU Yanfei. Controlled preparation of halide perovskite based catalysts and their photocatalytic performances for CO2 reduction[D]. Tianjin: Tianjin University of Technology, 2021(in Chinese). [11] HUANG H, PRADHAN B, HOFKENS J, et al. Solar-driven metal halide perovskite photocatalysis: Design, stability, and performance[J]. ACS Energy Letters,2020,5(4):1107-1123. doi: 10.1021/acsenergylett.0c00058 [12] XU Y F, YANG M Z, CHEN B X, et al. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction[J]. Journal of the American Chemical Society,2017,139(16):5660-5663. doi: 10.1021/jacs.7b00489 [13] LIANG J, ZHAO P Y, WANG C X, et al. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability[J]. Journal of the American Chemical Society,2017,139(40):14009-14012. doi: 10.1021/jacs.7b07949 [14] LI C, LU X G, DING W Z, et al. Formability of ABX3 (X= F, Cl, Br, I) halide perovskites[J]. Acta Crystallographica Section B: Structural Science,2008,64(6):702-707. doi: 10.1107/S0108768108032734 [15] KIESLICH G, SUN S, CHEETHAM A K. Solid-state principles applied to organic-inorganic perovskites: New tricks for an old dog[J]. Chemical Science,2014,5(12):4712-4715. doi: 10.1039/C4SC02211D [16] BARTEL C J, SUTTON C, GOLDSMITH B R, et al. New tolerance factor to predict the stability of perovskite oxides and halides[J]. Science Advances,2019,5(2):eaav0693. doi: 10.1126/sciadv.aav0693 [17] STOUMPOS C C, MALLIAKAS C D, PETERS J A, et al. Crystal growth of the perovskite semiconductor CsPbBr3: A new material for high-energy radiation detection[J]. Crystal Growth Design,2013,13(7):2722-2727. doi: 10.1021/cg400645t [18] BERTOLOTTI F, PROTESESCU L, KOVALENKO M V, et al. Coherent nanotwins and dynamic disorder in cesium lead halide perovskite nanocrystals[J]. ACS Nano,2017,11(4):3819-3831. doi: 10.1021/acsnano.7b00017 [19] JU M G, DAI J, MA L, et al. Lead-free mixed tin and germanium perovskites for photovoltaic application[J]. Journal of the American Chemical Society,2017,139(23):8038-8043. doi: 10.1021/jacs.7b04219 [20] WEI Y, CHENG Z Y, LIN J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs[J]. Chemical Society Reviews,2019,48(1):310-350. doi: 10.1039/C8CS00740C [21] TUREDI B, LEE K J, DURSUN I, et al. Water-induced dimensionality reduction in metal-halide perovskites[J]. The Journal of Physical Chemistry C,2018,122(25):14128-14134. doi: 10.1021/acs.jpcc.8b01343 [22] BULYK L I, GAMERNYK R, CHORNODOLSKYY J, et al. Influence of the degradation processes on luminescent and photoelectrical properties of CsPbBr3 single crystals[J]. Journal of Alloys Compounds,2021,884:161023. doi: 10.1016/j.jallcom.2021.161023 [23] SHRESTHA S, TSAI H, YOHO M, et al. Role of the metal-semiconductor interface in halide perovskite devices for radiation photon counting[J]. ACS Applied Materials Interfaces,2020,12(40):45533-45540. doi: 10.1021/acsami.0c11805 [24] ZHANG Z J, ZHU Y M, WANG W L, et al. Aqueous solution growth of millimeter-sized nongreen-luminescent wide bandgap Cs4PbBr6 bulk crystal[J]. Crystal Growth Design,2018,18(11):6393-6398. doi: 10.1021/acs.cgd.8b00817 [25] SHEN W, RUAN L F, SHEN Z T, et al. Reversible light-mediated compositional and structural transitions between CsPbBr3 and CsPb2Br5 nanosheets[J]. Chemical Communications,2018,54(22):2804-2807. doi: 10.1039/C8CC00139A [26] YIN J, YANG H Z, SONG K P, et al. Point defects and green emission in zero-dimensional perovskites[J]. The Journal of Physical Chemistry Letters,2018,9(18):5490-5495. doi: 10.1021/acs.jpclett.8b02477 [27] XIANG Q J, CHENG B, YU J G. Graphene-based photocatalysts for solar-fuel generation[J]. Angewandte Chemie International Edition,2015,54(39):11350-11366. doi: 10.1002/anie.201411096 [28] YAASHIKAA P, KUMAR P S, VARJANI S J, et al. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products[J]. Journal of CO2 Utilization,2019,33:131-147. doi: 10.1016/j.jcou.2019.05.017 [29] KUMAR N, RANI J, KURCHANIA R. Advancement in CsPbBr3 inorganic perovskite solar cells: Fabrication, efficiency and stability[J]. Solar Energy,2021,221:197-205. doi: 10.1016/j.solener.2021.04.042 [30] QIAN J Y, XU B, TIAN W J. A comprehensive theoretical study of halide perovskites ABX3[J]. Organic Electronics,2016,37:61-73. doi: 10.1016/j.orgel.2016.05.046 [31] PROTESESCU L, YAKUNIN S, BODNARCHUK M I, et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut[J]. Nano Letters,2015,15(6):3692-3696. doi: 10.1021/nl5048779 [32] KAMAT P V, KUNO M. Halide ion migration in perovskite nanocrystals and nanostructures[J]. Accounts of Chemical Research,2021,54(3):520-531. doi: 10.1021/acs.accounts.0c00749 [33] CHEN C, FU Q Y, GUO P J, et al. Ionic transport characteristics of large-size CsPbBr3 single crystals[J]. Materials Research Express,2019,6(11):115808. doi: 10.1088/2053-1591/ab4d79 [34] ZHANG B B, WANG F B, ZHANG H J, et al. Defect proliferation in CsPbBr3 crystal induced by ion migration[J]. Applied Physics Letters,2020,116(6):063505. doi: 10.1063/1.5134108 [35] KOH T M, FU K W, FANG Y, et al. Formamidinium-containing metal-halide: An alternative material for near-IR absorption perovskite solar cells[J]. The Journal of Physical Chemistry C,2014,118(30):16458-16462. doi: 10.1021/jp411112k [36] ULLAH S, WANG J, YANG P, et al. All-inorganic CsPbBr3 perovskite: A promising choice for photovoltaics[J]. Materials Advances,2021,2(2):646-683. doi: 10.1039/D0MA00866D [37] ZHANG X Y, PANG G T, XING G C, et al. Temperature dependent optical characteristics of all-inorganic CsPbBr3 nanocrystals film[J]. Materials Today Physics,2020,15:100259. doi: 10.1016/j.mtphys.2020.100259 [38] CHEN J S, LIU D Z, AL-MARRI M J, et al. Photo-stability of CsPbBr3 perovskite quantum dots for optoelectronic application[J]. Science China Materials,2016,59(9):719-727. doi: 10.1007/s40843-016-5123-1 [39] SUN Y F, ZHANG H D, ZHU K, et al. Research on the influence of polar solvents on CsPbBr3 perovskite QDs[J]. RSC Advances,2021,11(44):27333-27337. doi: 10.1039/D1RA04485K [40] LI J H, XU L M, WANG T, et al. 50-fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control[J]. Advanced Materials,2017,29(5):1603885. doi: 10.1002/adma.201603885 [41] RAVI V K, SANTRA P K, JOSHI N, et al. Origin of the substitution mechanism for the binding of organic ligands on the surface of CsPbBr3 perovskite nanocubes[J]. The Journal of Physical Chemistry Letters,2017,8(20):4988-4994. doi: 10.1021/acs.jpclett.7b02192 [42] YAN D D, SHI T C, ZANG Z G, et al. Ultrastable CsPbBr3 perovskite quantum dot and their enhanced amplified spontaneous emission by surface ligand modification[J]. Small,2019,15(23):1901173. [43] ZHONG G H, LIU D X, ZHANG J Y. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts[J]. Journal of Materials Chemistry A,2018,6(5):1887-1899. doi: 10.1039/C7TA08268A [44] ZHANG H F, ZHAO M, LIN Y S. Stability of ZIF-8 in water under ambient conditions[J]. Microporous Mesoporous Materials,2019,279:201-210. doi: 10.1016/j.micromeso.2018.12.035 [45] KONG Z C, LIAO J F, DONG Y J, et al. Core@shell CsPbBr3@zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction[J]. ACS Energy Letters,2018,3(11):2656-2662. doi: 10.1021/acsenergylett.8b01658 [46] KONG Z C, ZHANG H H, LIAO J F, et al. Immobilizing Re(CO)3Br(dcbpy) complex on CsPbBr3 nanocrystal for boosted charge separation and photocatalytic CO2 reduction[J]. Solar RRL,2020,4(1):1900365. doi: 10.1002/solr.201900365 [47] SU K, DONG G X, ZHANG W, et al. In situ coating CsPbBr3 nanocrystals with graphdiyne to boost the activity and stability of photocatalytic CO2 reduction[J]. ACS Applied Materials Interfaces,2020,12(45):50464-50471. doi: 10.1021/acsami.0c14826 [48] WANG J C, LI N Y, IDRIS A M, et al. Surface defect engineering of CsPbBr3 nanocrystals for high efficient photocatalytic CO2 reduction[J]. Solar RRL,2021,5(7):2100154. doi: 10.1002/solr.202100154 [49] CHENG J L, MU Y F, WU L Y, et al. Acetate-assistant efficient cation-exchange of halide perovskite nanocrystals to boost the photocatalytic CO2 reduction[J]. Nano Research,2022,15:1845-1852. doi: 10.1007/s12274-021-3775-3 [50] JEON N J, NOH J H, KIM Y C, et al. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells[J]. Nature Materials,2014,13(9):897-903. doi: 10.1038/nmat4014 [51] CHEN Y X, XU Y F, WANG X D, et al. Solvent selection and Pt decoration towards enhanced photocatalytic CO2 reduction over CsPbBr3 perovskite single crystals[J]. Sustainable Energy Fuels,2020,4(5):2249-2255. doi: 10.1039/C9SE01218D [52] YOU S Q, GUO S H, ZHAO X, et al. All-inorganic perovskite/graphitic carbon nitride composites for CO2 photoreduction into C1 compounds under low concentrations of CO2[J]. Dalton Transactions,2019,48(37):14115-14121. doi: 10.1039/C9DT02468A [53] CHEN Q, LAN X F, MA Y C, et al. Boosting CsPbBr3-driven superior and long-term photocatalytic CO2 reduction under pure water medium: Synergy effects of multifunctional melamine foam and graphitic carbon nitride (g-C3N4)[J]. Solar RRL,2021,5(7):2100186. doi: 10.1002/solr.202100186 [54] ZHANG Z J, SHU M Y, JIANG Y, et al. Fullerene modified CsPbBr3 perovskite nanocrystals for efficient charge separation and photocatalytic CO2 reduction[J]. Chemical Engineering Journal,2021,414:128889. doi: 10.1016/j.cej.2021.128889 [55] KUMAR S, REGUE M, ISAACS M A, et al. All-inorganic CsPbBr3 nanocrystals: Gram-scale mechanochemical synthesis and selective photocatalytic CO2 reduction to methane[J]. ACS Applied Energy Materials,2020,3(5):4509-4522. doi: 10.1021/acsaem.0c00195 [56] LI L J, ZHANG Z H. In-situ fabrication of Cu doped dual-phase CsPbBr3-Cs4PbBr6 inorganic perovskite nanocomposites for efficient and selective photocatalytic CO2 reduction[J]. Chemical Engineering Journal,2022,434:134811. doi: 10.1016/j.cej.2022.134811 [57] THOMAS N, MATHEW S, NAIR K M, et al. 2D MoS2: Structure, mechanisms, and photocatalytic applications[J]. Materials Today Sustainability,2021,13:100073. doi: 10.1016/j.mtsust.2021.100073 [58] WANG Z C, LIU J H, CHEN W. Plasmonic Ag/AgBr nanohybrid: Synergistic effect of SPR with photographic sensitivity for enhanced photocatalytic activity and stability[J]. Dalton Transactions,2012,41(16):4866-4870. doi: 10.1039/c2dt12089e [59] ZHANG Z H, ZHANG L B, HEDHILI M N, et al. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting[J]. Nano Letters,2013,13(1):14-20. doi: 10.1021/nl3029202 [60] LIAO J F, CAI Y T, LI J Y, et al. Plasmonic CsPbBr3-Au nanocomposite for excitation wavelength dependent photocatalytic CO2 reduction[J]. Journal of Energy Chemistry,2021,53:309-315. doi: 10.1016/j.jechem.2020.04.017 [61] TANG R, SUN H, ZHANG Z, et al. Incorporating plasmonic Au-nanoparticles into three-dimensionally ordered macroporous perovskite frameworks for efficient photocatalytic CO2 reduction[J]. Chemical Engineering Journal,2022,429:132137. doi: 10.1016/j.cej.2021.132137 [62] TANG C, CHEN C Y, XU W W, et al. Design of doped cesium lead halide perovskite as a photo-catalytic CO2 reduction catalyst[J]. Journal of Materials Chemistry A,2019,7(12):6911-6919. doi: 10.1039/C9TA00550A [63] SHYAMAL S, DUTTA S K, PRADHAN N. Doping iron in CsPbBr3 perovskite nanocrystals for efficient and product selective CO2 reduction[J]. The Journal of Physical Chemistry Letters,2019,10(24):7965-7969. doi: 10.1021/acs.jpclett.9b03176 [64] CHENG R, DEBROYE E, HOFKENS J, et al. Efficient photocatalytic CO2 reduction with MIL-100 (Fe)-CsPbBr3 composites[J]. Catalysts,2020,10(11):1352. doi: 10.3390/catal10111352 [65] DONG G X, ZHANG W, MU Y F, et al. A halide perovskite as a catalyst to simultaneously achieve efficient photocatalytic CO2 reduction and methanol oxidation[J]. Chemical Communications,2020,56(34):4664-4667. doi: 10.1039/D0CC01176B [66] XI Y M, ZHANG X W, SHEN Y, et al. Aspect ratio dependent photocatalytic enhancement of CsPbBr3 in CO2 reduction with two-dimensional metal organic framework as a cocatalyst[J]. Applied Catalysis B: Environmental,2021,297:120411. doi: 10.1016/j.apcatb.2021.120411 [67] YANG L, ZENG X F, WANG W C, et al. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells[J]. Advanced Functional Materials,2018,28(7):1704537. doi: 10.1002/adfm.201704537 [68] DING M, FLAIG R W, JIANG H L, et al. Carbon capture and conversion using metal-organic frameworks and MOF-based materials[J]. Chemical Society Reviews,2019,48(10):2783-2828. doi: 10.1039/C8CS00829A [69] LI N, ZHAI X P, YAN W K, et al. Boosting cascade electron transfer for highly efficient CO2 photoreduction[J]. Solar RRL,2021,5(11):2100558. doi: 10.1002/solr.202100558 [70] WANG Q L, TAO L M, JIANG X X, et al. Graphene oxide wrapped CH3NH3PbBr3 perovskite quantum dots hybrid for photoelectrochemical CO2 reduction in organic solvents[J]. Applied Surface Science,2019,465:607-613. doi: 10.1016/j.apsusc.2018.09.215 [71] CHEN Y H, YE J K, CHANG Y J, et al. Mechanisms behind photocatalytic CO2 reduction by CsPbBr3 perovskite-graphene-based nanoheterostructures[J]. Applied Catalysis B: Environmental,2021,284:119751. doi: 10.1016/j.apcatb.2020.119751 [72] PARZINGER E, MILLER B, BLASCHKE B, et al. Photocatalytic stability of single-and few-layer MoS2[J]. ACS Nano,2015,9(11):11302-11309. doi: 10.1021/acsnano.5b04979 [73] XIA D D, GONG F, PEI X, et al. Molybdenum and tungsten disulfides-based nanocomposite films for energy storage and conversion: A review[J]. Chemical Engineering Journal,2018,348:908-928. doi: 10.1016/j.cej.2018.04.207 [74] SINGH R, GIRI A, PAL M, et al. Perovskite solar cells with an MoS2 electron transport layer[J]. Journal of Materials Chemistry A,2019,7(12):7151-7158. doi: 10.1039/C8TA12254G [75] WANG X D, HE J, MAO L, et al. CsPbBr3 perovskite nanocrystals anchoring on monolayer MoS2 nanosheets for efficient photocatalytic CO2 reduction[J]. Chemical Engineering Journal,2021,416:128077. doi: 10.1016/j.cej.2020.128077 [76] MISHRA R, BERA S, CHATTERJEE R, et al. A review on Z/S-scheme heterojunction for photocatalytic applications based on metal halide perovskite materials[J]. Applied Surface Science Advances,2022,9:100241. doi: 10.1016/j.apsadv.2022.100241 [77] XU F Y, MENG K, CHENG B, et al. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction[J]. Nature Communications,2020,11(1):1-9. doi: 10.1038/s41467-019-13993-7 [78] JIANG Y, CHEN H Y, LI J Y, et al. Z-scheme 2D/2D heterojunction of CsPbBr3/Bi2WO6 for improved photocatalytic CO2 reduction[J]. Advanced Functional Materials,2020,30(50):2004293. doi: 10.1002/adfm.202004293 [79] JIANG Y, LIAO J F, CHEN H Y, et al. All-solid-state Z-scheme α-Fe2O3/amine-RGO/CsPbBr3 hybrids for visible-light-driven photocatalytic CO2 reduction[J]. Chemistry,2020,6(3):766-780. doi: 10.1016/j.chempr.2020.01.005 [80] DONG Y J, JIANG Y, LIAO J F, et al. Construction of a ternary WO3/CsPbBr3/ZIF-67 heterostructure for enhanced photocatalytic carbon dioxide reduction[J]. Science China Materials,2022,65:1550-1559. doi: 10.1007/s40843-021-1962-9 [81] LEI J C, ZHANG X, ZHOU Z. Recent advances in MXene: Preparation, properties, and applications[J]. Frontiers of Physics,2015,10(3):276-286. doi: 10.1007/s11467-015-0493-x [82] PAN A Z, MA X Q, HUANG S Y, et al. CsPbBr3 perovskite nanocrystal grown on MXene nanosheets for enhanced photoelectric detection and photocatalytic CO2 reduction[J]. The Journal of Physical Chemistry Letters,2019,10(21):6590-6597. doi: 10.1021/acs.jpclett.9b02605 [83] LIU H, NEAL A T, ZHU Z, et al. Phosphorene: An unexplored 2D semiconductor with a high hole mobility[J]. ACS Nano,2014,8(4):4033-4041. doi: 10.1021/nn501226z [84] WANG X D, HE J, LI J Y, et al. Immobilizing perovskite CsPbBr3 nanocrystals on black phosphorus nanosheets for boosting charge separation and photocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental,2020,277:119230. doi: 10.1016/j.apcatb.2020.119230 [85] ZHAO Y T, WANG H Y, HUANG H, et al. Surface coordination of black phosphorus for robust air and water stability[J]. Angewandte Chemie,2016,128(16):5087-5091. doi: 10.1002/ange.201512038 [86] GONG Y Q, SHEN J H, ZHU Y H, et al. Tailoring charge transfer in perovskite quantum dots/black phosphorus nanosheets photocatalyst via aromatic molecules[J]. Applied Surface Science,2021,545:149012. doi: 10.1016/j.apsusc.2021.149012 [87] ZHANG Y Q, DONG N N, TAO H C, et al. Exfoliation of stable 2D black phosphorus for device fabrication[J]. Chemistry of Materials,2017,29(15):6445-6456. doi: 10.1021/acs.chemmater.7b01991 [88] CHOUDHARY R B, ANSARI S, PURTY B. Robust electrochemical performance of polypyrrole (PPy) and polyindole (PIn) based hybrid electrode materials for supercapacitor application: A review[J]. Journal of Energy Storage,2020,29:101302. doi: 10.1016/j.est.2020.101302 [89] DANG M T, HIRSCH L, WANTZ G. P3 HT: PCBM, best seller in polymer photovoltaic research[J]. Advanced Materials, 2011, 23(31): 3597-3602. [90] ZHANG Z J, LI L, LIU L H, et al. Water-stable and photoelectrochemically active CsPbBr3/polyaniline composite by a photocatalytic polymerization process[J]. The Journal of Physical Chemistry C,2020,124(40):22228-22234. doi: 10.1021/acs.jpcc.0c05774 [91] ZHANG Z J, LIU L H, HUANG H R, et al. Encapsulation of CsPbBr3 perovskite quantum dots into PPy conducting polymer: Exceptional water stability and enhanced charge transport property[J]. Applied Surface Science,2020,526:146735. doi: 10.1016/j.apsusc.2020.146735 [92] BAI X J, SUN C P, WU S L, et al. Enhancement of photocatalytic performance via a P3 HT-g-C3N4 heterojunction[J]. Journal of Materials Chemistry A,2015,3(6):2741-2747. doi: 10.1039/C4TA04779F [93] LI L, ZHANG Z J, DING C, et al. Boosting charge separation and photocatalytic CO2 reduction of CsPbBr3 perovskite quantum dots by hybridizing with P3 HT[J]. Chemical Engineering Journal,2021,419:129543. doi: 10.1016/j.cej.2021.129543 -





 下载:
下载: