Preparation of functionalized nanocomposites Fe3O4@SiO2-3-aminopropyltrimethoxysilane and its adsorption to Pb(Ⅱ)
-
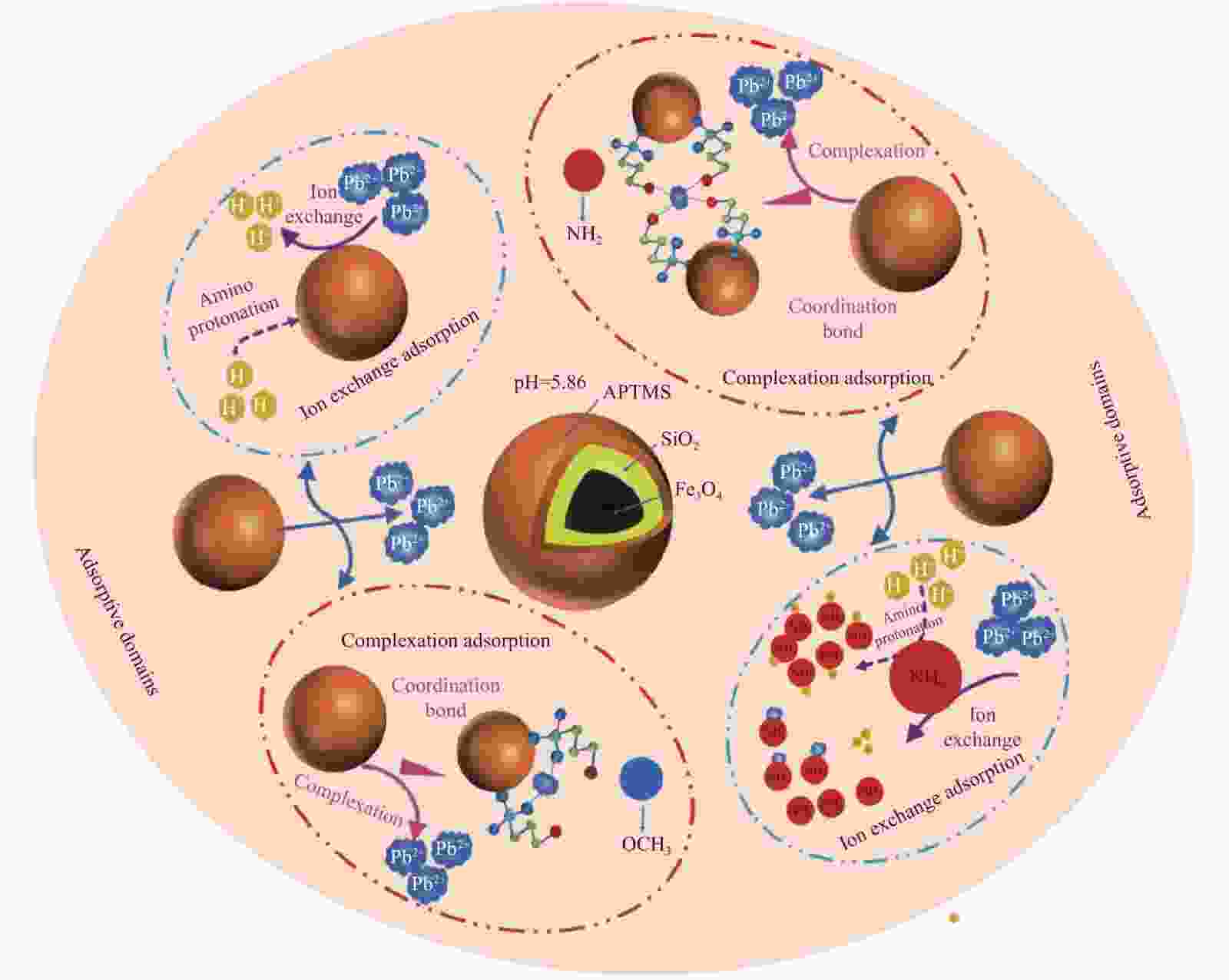
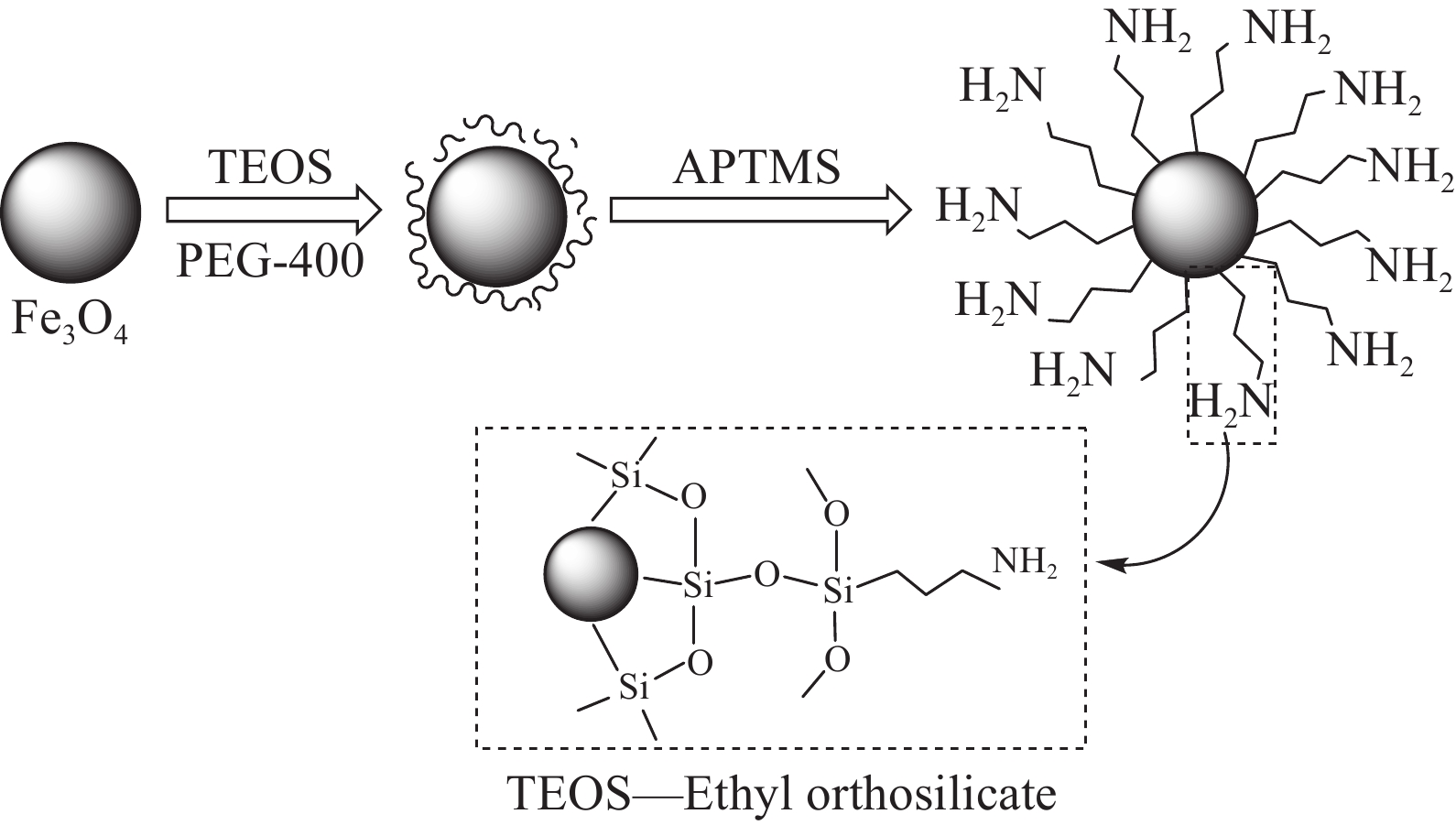
摘要: 为解决磁性纳米Fe3O4颗粒易腐蚀、团聚等问题,对其进行功能化修饰改进。在超声波辐照下以FeCl3和FeSO4为原料,氨水为沉淀剂,然后加入正硅酸乙酯(TEOS)和3-氨丙基三甲氧基硅烷(APTMS)进行功能化修饰,制备得到SiO2包覆的氨基功能化纳米复合材料Fe3O4@SiO2-APTMS,并采用TEM、FTIR、VSM、TGA、低温氮吸附、XRD等对其进行表征测试,证实了超声波辐照下制备的复合材料具有磁响应强度强、耐酸碱性强、分散性高、比表面积大、粒径小等特点,同时探究了纳米复合材料对Pb(Ⅱ)的吸附性能。结果表明:溶液初始pH值为5.86,吸附剂投加量为1.0~1.5 g·L−1时Pb(Ⅱ)吸附效果较好;Langmuir模型适合模拟该等温吸附过程,吉布斯自由能变∆G0<0,吸附过程是一个自发过程;准二级动力学可以较好地描述Pb(Ⅱ)在复合材料上的吸附行为,准二级动力学常数k2=0.0401 g·mg−1·min−1,达到吸附平衡时的吸附量qe=80.041 mg·g−1;推测得到吸附机制主要为离子交换和络合吸附。Abstract: In order to solve the indefects that magnetic nano-Fe3O4 particles were corroded and agglomerated easily, functional modification was carried out. FeCl3 and FeSO4 were used as raw materials and ammonia as preci-pitant in the presence of ultrasonic irradiation, then functionalized by ethyl orthosilicate (TEOS) and 3-aminopropyltrimethoxysilane (APTMS) to prepare SiO2-coated amino-functional nanocomposites Fe3O4@SiO2-APTMS. The magnetic nanocomposites were characterized by TEM, FTIR, VSM, TGA, low temperature nitrogen adsorption and XRD, etc. The characterized results show that the magnetic nanocomposites prepared by ultrasonic strengthening method have the characteristics of strong magnetic response, strong acid and alkali resistance, high dispersion, large specific surface area and small particle size.Meanwhile, the adsorption effects of magnetic nanocomposites for Pb(Ⅱ) were investigated. The results show that the initial pH value of the solution and the dosage of adsorbent have greatest effects on the adsorption effect of Pb(Ⅱ) with the initial pH value of the solution 5.86 and the dosage of adsorbent 1.0-1.5 g·L−1. The Langmuir model is suitable for simulating the isothermal adsorption process, and the adsorption process is a spontaneous process when Gibbs free energy change ∆G0<0. The adsorption behavior of Pb(Ⅱ) can be well described by quasi-second-order kinetics on the composites, Quasi-second-order kinetic constant k2=0.0401 g·mg−1·min−1, equilibrium adsorption capacity qe=80.041 mg·g−1; it is speculated that the adsorption mechanism is mainly complex adsorption and ion exchange.
-
Key words:
- ultrasonic /
- magnetic nanocomposites /
- complex adsorption /
- adsorption kinetics /
- lead ions
-
表 1 不同吸附剂对Pb(Ⅱ)的吸附效果比较
Table 1. Comparison of adsorption effects of different adsorbents for Pb (Ⅱ)
表 2 Fe3O4@SiO2-APTMS吸附Pb(Ⅱ)热力学常数
Table 2. Thermodynamic constants of Pb(Ⅱ) adsorbed by Fe3O4@SiO2-APTMS
C0/(mg·L−1) ΔG0/(kJ·mol−1) ΔH0/(kJ·mol−1) ΔS0/(J·mol−1·K−1) 283 K 293 K 303 K 313 K 50 −3.555 −4.373 −4.827 −5.292 12.513 57.125 100 −3.746 −4.067 −4.609 −5.115 9.391 46.227 150 −3.767 −4.313 −4.871 −5.423 11.871 55.271 Notes: C0—Initial concentration of Pb (Ⅱ) solution; ∆G0—Gibbs free energy change; ∆H0—Enthalpy change; ∆S0—Entropy change. 表 3 Fe3O4@SiO2-APTMS对Pb(Ⅱ)吸附动力学方程拟合结果
Table 3. Fitting results of Pb (Ⅱ) adsorption kinetic equation by Fe3O4@SiO2-APTMS
Quasi-first order kinetics Quasi-second-order kinetics Internal diffusion equation k1 qe,cal qe,exp R2 k2 qe,cal qe,exp R2 kp C R2 0.0165 2.714 80.041 0.789 0.0401 79.99 80.041 0.999 kp1=2.0639 69.675 0.952 kp2=0.0616 79.223 0.969 kp3=0.0092 79.823 0.980 Notes: qe,cal—Theoretical saturated adsorption capacity; qe,exp—Experimental saturated adsorption capacity; k1—Quasi-first-order kinetic constant; k2—Quasi-second-order kinetic constant; C—Constant related to thickness and boundary layer. 表 4 Fe3O4@SiO2-APTMS对Pb(Ⅱ)的吸附等温线拟合结果
Table 4. Adsorption isotherm fitting results of Pb(Ⅱ) by Fe3O4@SiO2-APTMS
T/K Langmuir Freundlich Temkin KL qm RL R2 KF R2 Kt Bl R2 308 0.0387 401.606 0.0252-0.615 0.991 47.83 0.905 0.534 74.27 0.989 298 0.0323 390.625 0.0312-0.570 0.993 39.57 0.916 0.373 79.25 0.988 288 0.0311 366.300 0.0300-0.602 0.995 33.41 0.946 0.299 81.02 0.977 Notes: KL—Langmuir adsorption coefficient (L·mg−1); KF—Freundlich adsorption coefficient (mg1−(1/n)·L1/n·g−1); Kt, Bl—Temkin adsorption isotherm constant; qm—Saturated adsorption capacity (mg·g−1); RL—Separation constant; R2—linear correlation coefficient. 表 5 复合材料耐酸碱腐蚀性研究
Table 5. Study on acid and alkali corrosion resistance of compound materials
Condition Solution Fe3O4@SiO2 Fe3O4@SiO2-APTMS Fe/(mg·L−1) Fe/(mg·L−1) TOC/(mg·L−1) 298 K, soak, 24 h Water 0.135 0.015 0.025 0.1 mol·L−1 HCl 3.598 0.206 3.420 1 mol·L−1 HCl 25.542 2.729 8.866 0.1 mol·L−1 NaOH 0.137 0.149 6.712 1 mol·L−1 NaOH 0.276 0.186 8.106 313 K, stirring, 72 h Water 0.138 0.016 0.027 1 mol·L−1 HCl 24.842 2.629 8.886 1 mol·L−1 NaOH 0.281 0.189 8.126 313 K, stirring, 96 h Water 0.140 0.017 0.029 1 mol·L−1 HCl 25.552 2.749 8.916 1 mol·L−1 NaOH 0.296 0.191 8.173 Note: TOC—Total organic carbon. -
[1] 徐峥. 重金属污染水体的环境保护处理技术分析[J]. 信息记录材料, 2021, 22(9):235-237. doi: 10.16009/j.cnki.cn13-1295/tq.2021.09.111XU Zheng. Analysis of environmental protection treatment technology of water body polluted by heavy metals[J]. Information Recording Material,2021,22(9):235-237(in Chinese). doi: 10.16009/j.cnki.cn13-1295/tq.2021.09.111 [2] TADJARODI A, ABBASZADEH A, TAGHIZADEHB M, et al. Solid phase extraction of Cd(II) and Pb(II) ions based on a novel functionalized Fe3O4@SiO2 core-shell nanoparticles with the aid of multivariate optimization methodology[J]. Materials Science and Engineering C,2015,49:416-421. doi: 10.1016/j.msec.2015.01.013 [3] 郭健, 姚云, 赵小旭, 等. 粮食中重金属铅离子、镉离子的污染现状及对人体的危害[J]. 粮食科技与经济, 2018, 43(3):33-35, 85.GUO Jian, YAO Yun, ZHAO Xiaoxu, et al. Pollution status and harm to human body of heavy metal lead ion and cadmium ion in grain[J]. Grain Science, Technology and Economy,2018,43(3):33-35, 85(in Chinese). [4] CENDROWSKIA K, SIKORAB P, ZIELINSKAA B, et al. Chemical and thermal stability of core-shelled magnetite nanoparticles and solid silica[J]. Applied Surface Science,2017,407:391-397. doi: 10.1016/j.apsusc.2017.02.118 [5] 许端平, 陈丽媛, 孔岳. 纳米级四氧化三铁回收水中铅离子实验[J]. 应用化工, 2021, 50(1):75-77, 82. doi: 10.3969/j.issn.1671-3206.2021.01.018XU Duanping, CHEN Liyuan, KONG Yue. Experiment on recovery of lead ion from water by nanometer iron tetroxide[J]. Applied Chemical Industry,2021,50(1):75-77, 82(in Chinese). doi: 10.3969/j.issn.1671-3206.2021.01.018 [6] 罗超. 二氧化锰和四氧化三铁对碳纳米管改性及其对Cd(Ⅱ)和Cr(Ⅵ)的吸附研究[D]. 兰州: 兰州大学, 2014.LUO Chao. Modification of carbon nanotubes by manganese dioxide and ferric oxide and their adsorption for Cd(Ⅱ) and Cr(Ⅵ)[D]. Lanzhou: Lanzhou University, 2014(in Chinese). [7] LIU J, ZHANG J, XING L, et al. Magnetic Fe3O4/attapulgite hybrids for Cd(II) adsorption: Performance, mechanism and recovery[J]. Journal of Hazardous Materials,2021,412(14):125-237. [8] DUGOSZ O, SZOSTAK K, KRUPINSKI M, et al. Synthesis of Fe3O4/ZnO nanoparticles and their application for the photodegradation of anionic and cationic dyes[J]. International Journal of Environmental Science and Technology,2021,18(3):561-574. doi: 10.1007/s13762-020-02852-4 [9] JIN S Y, PARK B C, HAM W S, et al. Effect of the magnetic core size of amino-functionalized Fe3O4-mesoporous SiO2 core-shell nanoparticles on the removal of heavy metal ions[J]. Colloids and Surfaces A,2017,531:133-140. [10] 王朝辉, 杨芳. 磺酸基功能化磁性纳米粒子的制备、表征及除去水中Cu(II)的研究[J]. 化学工程师, 2012, 202(7):15-19. doi: 10.3969/j.issn.1002-1124.2012.07.005WANG Zhaohui, YANG Fang. Preparation, characterization and removal of Cu(II) from water by sulfonic acid functionalized magnetic nanoparticles[J]. Chemical Engineer,2012,202(7):15-19(in Chinese). doi: 10.3969/j.issn.1002-1124.2012.07.005 [11] 赵永纲, 沈昊宇, 李勍, 等. 氨基功能化纳米Fe3O4磁性高分子吸附剂对废水中Cr(VI)的吸附研究[J]. 化学学报, 2009, 67(13):1509-1514. doi: 10.3321/j.issn:0567-7351.2009.13.018ZHAO Yonggang, SHEN Haoyu, LI Gou, et al. Study on the adsorption of Cr(VI) in wastewater by amino-functionalized nano-Fe3O4 magnetic polymer adsorbent[J]. Journal of Chemistry,2009,67(13):1509-1514(in Chinese). doi: 10.3321/j.issn:0567-7351.2009.13.018 [12] LIN S, LIU L, YANG Y, et al. Comparison of the adsorption preference using superparamagnetic Fe3O4-SH nanoparticles to remove aqueous heavy metal contaminants[J]. Chemical Engineering Research and Design,2017,125:319-327. doi: 10.1016/j.cherd.2017.07.027 [13] ZHANG J, ZHAI S, SHI L, et al. Pb(II) removal of Fe3O4@SiO2-NH2 core-shell nanomaterials prepared via a controllable sol-gel process[J]. Chemical Engineering Journal,2013,215:461-471. [14] 雷婷. 官能化磁性Fe3O4纳米颗粒的制备及其对污水中重金属离子的吸附性能研究[D]. 昆明: 云南大学, 2020.LEI Ting. Preparation of functional magnetic Fe3O4 nanoparticles and their adsorption properties for heavy metal ions in wastewater[D]. Kunming: Yunnan University, 2020(in Chinese). [15] ABAZARI R, MAHJOUB A R, MOLAIE S, et al. The effect of different parameters under ultrasound irradiation for synthesis of new nanostructured Fe3O4@bio-MOF as an efficient anti-leishmanial in vitro and in vivo conditions[J]. Ultrasonics Sonochemistry,2018,43:248-261. doi: 10.1016/j.ultsonch.2018.01.022 [16] ZHAO D M, LI M, ZHANG D X, et al. Reductive dechlorination of 2, 4-dichlorophenol by Pd/Fe nanoparticles prepared in the presence of ultrasonic irradiation[J]. Ultrasonics Sonochemistry,2013,20:864-871. [17] 窦国金, 郑莹, 麦欣欣, 等. 二甲酚橙分光光度法测定化学镀镍液中的铅浓度[J]. 材料保护, 2012, 45(5):72-74, 88. doi: 10.16577/j.cnki.42-1215/tb.2012.05.001DOU Guojin, ZHENG Ying, MAI Xinxin, et al. Determination of lead in electroless nickel plating bath by xylenol orange spectrophotometry[J]. Material Protection,2012,45(5):72-74, 88(in Chinese). doi: 10.16577/j.cnki.42-1215/tb.2012.05.001 [18] TA T K H, TRINH M T, LONG N V. Synthesis and surface functionalization of Fe3O4-SiO2 core-shell nanoparticles with 3-glycidoxypropyltrimethoxysilane and 1, 1’-carbonyldiimidazole for bio-applications[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2016,504:376-383. [19] 聂阳, 王永花, 胡良锋, 等. Fe3O4@SiO2-NH2磁性复合材料对水中全氟化合物的检测研究[J]. 分析测试学报, 2016(1):1-7. doi: 10.3969/j.issn.1004-4957.2016.01.001NIE Yang, WANG Yonghua, HU Liangfeng, et al. Detection of perfluorinated compounds in water by Fe3O4@SiO2-NH2 magnetic composites[J]. Journal of Analysis and Testing,2016(1):1-7(in Chinese). doi: 10.3969/j.issn.1004-4957.2016.01.001 [20] 关桦楠, 宋岩, 龚德状. 功能化Fe3O4纳米粒子去除水中Cu2+的研究[J]. 化学工程师, 2018, 32(10):11-14.GUAN Hua'nan, SONG Yan, GONG Dezhuang. Study on removal of Cu2+ from Water by Functionalized Fe3O4 nanoparticles[J]. Chemical Engineer,2018,32(10):11-14(in Chinese). [21] 胡超凡, 贾丽. 硅烷化试剂修饰Fe3O4磁性微粒的研究进展[J]. 激光生物学报, 2009, 18(4):561-569. doi: 10.3969/j.issn.1007-7146.2009.04.026HU Chaofan, JIA Li. Research progress of Fe3O4 magnetic particles modified by silanization reagents[J]. Journal of Laser Biology,2009,18(4):561-569(in Chinese). doi: 10.3969/j.issn.1007-7146.2009.04.026 [22] 陈华军, 王锐, 丁梧秀, 等. 聚乙二醇聚合度对介孔η-Al2O3纤维形貌及吸附能力的影响[J]. 复合材料学报, 2014, 31(3):845-850.CHEN Huajun, WANG Rui, DING Wuxiu, et al. Effect of degree of polymerization of polyethylene glycol on the morphology and adsorption capacity of mesoporous η-Al2O3 fiber[J]. Acta Materiae Compositae Sinica,2014,31(3):845-850(in Chinese). [23] 何飞, 赫晓东, 李垚. 掺杂二氧化硅干凝胶孔结构的分形特性[J]. 复合材料学报, 2007(1):81-85. doi: 10.3321/j.issn:1000-3851.2007.01.014HE Fei, HE Xiaodong, LI Yao. Fractal characteristics of pore structure of doped silica xerogel[J]. Journal of Composite Materials,2007(1):81-85(in Chinese). doi: 10.3321/j.issn:1000-3851.2007.01.014 [24] 李美兰, 豆小喻, 何娇, 等. 羧基化磁性Fe3O4复合材料的制备及其对水体中Pb2+的吸附研究[J]. 中国塑料, 2021, 35(10):37-44.LI Meilan, DOU Xiaoyu, HE Jiao, et al. Preparation of carboxylated magnetic Fe3O4 composites and their adsorption of Pb2+ in water[J]. China Plastics,2021,35(10):37-44(in Chinese). [25] 刘崇敏, 黄益宗, 于方明, 等. 改性沸石及添加CaCl2和MgCl2对重金属离子Pb2+吸附特性的影响[J]. 环境化学, 2013, 32(5):803-809.LIU Chongmin, HUANG Yizong, YU Fangming, et al. Effect of modified zeolite and addition of CaCl2 and MgCl2 on the adsorption characteristics of heavy metal ion Pb2+[J]. Environmental Chemistry,2013,32(5):803-809(in Chinese). [26] 孙玉坤. 功能化Fe3O4@PAMAM纳米复合材料的制备及其对重金属离子的去除[D]. 杭州: 浙江大学, 2019.SUN Yukun. Preparation of functionalized Fe3O4@PAMAM nanocomposites and their removal of heavy metal ions[D]. Hangzhou: Zhejiang University, 2019(in Chinese). [27] 曹玮, 周航, 邓贵友, 等. 改性谷壳生物炭负载磁性Fe去除废水中Pb2+的效果及机制[J]. 环境工程学报, 2017(3):1437-1444. doi: 10.12030/j.cjee.201511081CAO Wei, ZHOU Hang, DENG Guiyou, et al. Effect and mechanism of Pb2+ removal from wastewater by magnetic Fe loaded with modified chaff biochar[J]. Journal of Envi-ronmental Engineering,2017(3):1437-1444(in Chinese). doi: 10.12030/j.cjee.201511081 [28] FENG Z G, ZHU S, GODOI D, et al. Adsorption of Cd2+ on carboxyl-terminated suerparamagnetic iron oxide nanoparticles[J]. Analytical Chemistry,2012,84(8):3764-3770. doi: 10.1021/ac300392k [29] 张立志, 易平, 方丹丹, 等. 超顺磁性纳米Fe3O4@SiO2功能化材料对镉的吸附机制[J]. 环境科学, 2021(6):2917-2927.ZHANG Lizhi, YI Ping, FANG Dandan, et al. Adsorption mechanism of cadmium on superparamagnetic nano-Fe3O4@SiO2 functionalized materials[J]. Environmental Science,2021(6):2917-2927(in Chinese). [30] NASROLLAHZADEH M, ISSAABADI Z, SAJADI S M. Green synthesis of Pd/Fe3O4 nanocomposite using Hibiscus tiliaceus L. extract and its application for reductive catalysis of Cr(VI) and nitrocompounds[J]. Separation and Purification Technology,2018,197:253-260. doi: 10.1016/j.seppur.2018.01.010 [31] MA J, JIA K F, CHENG G L, et al. Solid-phase extraction of Pb(II) ions based on L-cysteine functionalized Fe3O4/SiO2 core-shell nanoparticles[J]. Journal of Environmental Engineering,2016,142(11):04016062. doi: 10.1061/(ASCE)EE.1943-7870.0001062 [32] AKPOMIE K G, DAWODU F A. Efficient abstraction of nickel (II) and manganese (II) ions from solution onto an alkaline-modified montmorillonite[J]. Journal of Taibah University for Science,2014,8(4):343-356. doi: 10.1016/j.jtusci.2014.05.001 [33] 刘嘉丽. 氨基改性Fe3O4@mC复合材料的制备及其对Pb2+的吸附性能研究[D]. 湘潭: 湘潭大学, 2020.LIU Jiali. Preparation of amino modified Fe3O4@mC composites and their adsorption properties for Pb2+[D]. Xiangtan: Xiangtan University, 2020(in Chinese). [34] XIANG W, LIU X J, XIAO C W, et al. Triethylenetetramine-modified hollow Fe3O4/SiO2/chitosan magnetic nanocomposites for removal of Cr(VI) ions with high adsorption capacity and rapid rate[J]. Microporous and Mesoporous Materials,2020,297(C):110041. [35] ZHANG W B, DENG M, SUN C X, et al. Ultrasound-enhanced adsorption of chromium(VI) on Fe3O4 magnetic particles[J]. Industrial and Engineering Chemistry Research,2014,53(1):333-339. doi: 10.1021/ie401497k [36] CRUZLOPES L P, MACENA M, ESTEVES B, et al. Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+ in aqueous solution with different adsorbent materials[J]. Open Agriculture,2021,6(1):115-123. doi: 10.1515/opag-2021-0225 [37] 王彦惠, 冷阳春, 成建峰, 等. Fe3O4@SiO2-NH2粒子对铀(Ⅵ)在阿拉善水相中的吸附性能研究[J]. 核科学与工程, 2020, 40(4):688-695. doi: 10.3969/j.issn.0258-0918.2020.04.025WANG Yanhui, LENG Yangchun, CHENG Jianfeng, et al. Study on the adsorption of uranium(VI) by Fe3O4@SiO2-NH2 particles in alxa aqueous phase[J]. Nuclear Science and Engineering,2020,40(4):688-695(in Chinese). doi: 10.3969/j.issn.0258-0918.2020.04.025 [38] 包炳钦, 张军, 宋卫锋, 等. 磁性复合凝胶球对Pb(Ⅱ)的吸附特性与机制[J]. 复合材料学报, 2021, 38(6):1929-1938. doi: 10.13801/j.cnki.fhclxb.20200924.001BAO Bingqin, ZHANG Jun, SONG Weifeng, et al. Adsorption characteristics and mechanism of magnetic compo-site gel spheres for Pb(Ⅱ)[J]. Acta Materiae Compositae Sinica,2021,38(6):1929-1938(in Chinese). doi: 10.13801/j.cnki.fhclxb.20200924.001 [39] 王申宛, 钟爽, 郑丽丽, 等. 共热解法制备方解石/生物炭复合材料及其吸附Pb(II)性能和机制[J]. 复合材料学报, 2021, 38(12):4282-4293. doi: 10.13801/j.cnki.fhclxb.20210309.002WANG Shenwan, ZHONG Shuang, ZHENG Lili, et al. Calcite/biochar composites prepared by co-pyrolysis and their adsorption properties and mechanism of Pb(Ⅱ)[J]. Acta Materiae Compositae Sinica,2021,38(12):4282-4293(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210309.002 [40] 李安生. 聚天冬氨酸的合成及其与金属离子络合沉淀的研究[D]. 西安: 西北大学, 2005.LI Ansheng. Synthesis of polyaspartic acid and its complexation with metal ions[D]. Xi'an: Northwestern University, 2005(in Chinese). [41] 刘立华, 周智华, 吴俊, 等. 两性高分子螯合絮凝剂与Cu(Ⅱ)、Pb(Ⅱ)、Cd(Ⅱ)、Ni(Ⅱ)的螯合稳定性[J]. 环境科学学报, 2013(1):79-87.LIU Lihua, ZHOU Zhihua, WU Jun, et al. The chelating stability of amphoteric polymer chelating flocculant with Cu (Ⅱ), Pb(Ⅱ), Cd (Ⅱ) and Ni(Ⅱ)[J]. Journal of Environmental Science,2013(1):79-87(in Chinese). -






 下载:
下载: