Study of the degradation of tetracycline by visible photo-Fenton catalyzed by ultrasound-assisted LaFeO3/PS
-
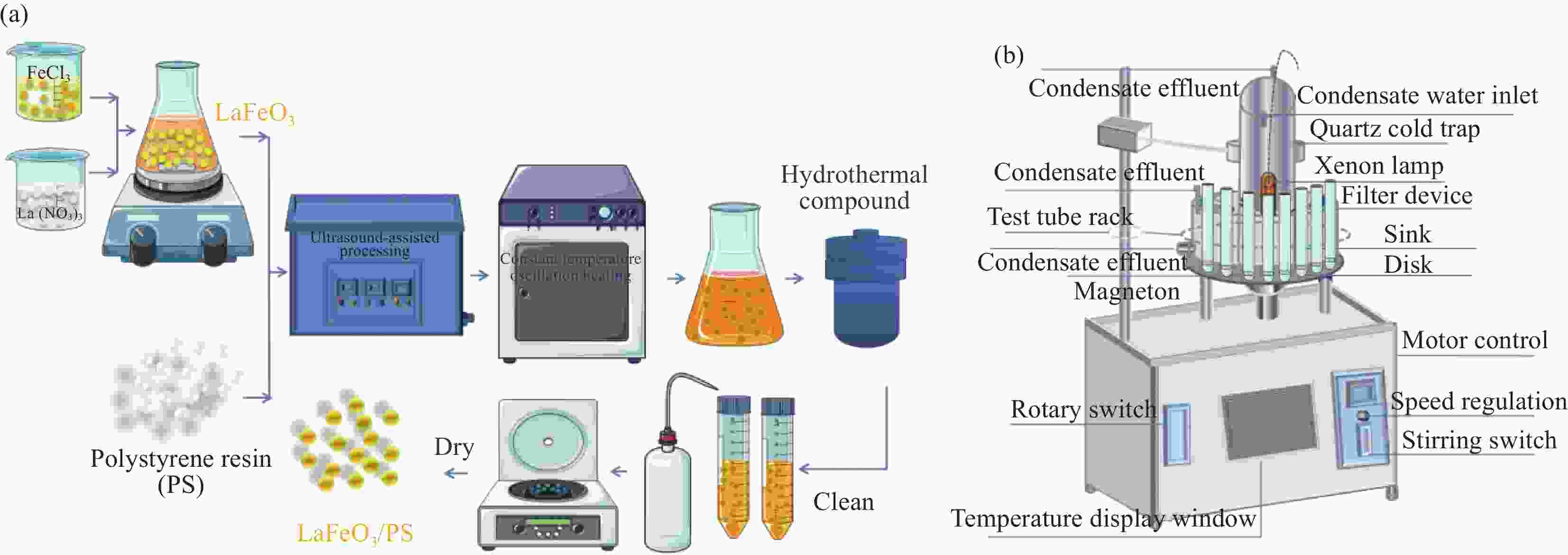
摘要: 粉末状LaFeO3材料具有易团聚、分离困难等缺点,规模化应用中受到限制。聚苯乙烯树脂(PS)上沉积粉末状催化剂,弥补了以上粉末材料的不足。为此,本文采用超声辅助溶胶-凝胶和水热法,在PS上沉积了自组装形成的LaFeO3凝胶微球,制得了LaFeO3/PS催化剂,并对其结构和性能进行了系统的研究。LaFeO3凝胶微球在PS上的分散分布使LaFeO3的禁带宽度变宽,从而增加了氧化还原能力,并解决了催化剂团聚等问题,提高了LaFeO3的光芬顿催化活性。在La∶Fe∶柠檬酸(CA)摩尔比=1∶1∶2、超声40 min、水热时间18 h、水热温度90℃、LaFeO3起始剂/PS质量比=32∶1的制备条件制得的复合材料,在可见光下催化芬顿降解盐酸四环素(TC),TC的去除率可达96.51%(降解速率k=0.0160 min−1)。自由基捕获实验表明,•O2−是主要活性物种。结合捕获实验提出了TC的降解机制。通过LC/MS分析,得到了TC的降解路径。该技术提高了催化剂的稳定性,高效利用了太阳能,是一种很有前途的有机污染物降解技术。Abstract: The powdered LaFeO3 material had shortcomings such as easy agglomeration and difficult separation, so it was limited in large-scale applications. The deposition of powdered catalysts in polystyrene resin (PS) made up for the above shortcomings of powdered materials. Therefore, in this study, the self-assembled LaFeO3 gel was deposited on the PS through ultrasound-assisted sol-gel and hydrothermal methods. The dispersion and distribution of LaFeO3 on PS broadens the forbidden band width of LaFeO3, improves its redox ability, solves the problem of agglomeration, and consequently improves its photo-Fenton catalytic activity. The LaFeO3/PS composite prepared under the following experimental conditions shows the highest photocatalytic activity: La∶Fe∶Citric acid(CA) molar ratio=1∶1∶2, ultrasonic time 40 min, hydrothermal temperature 90℃, hydrothermal time 18 h, LaFeO3 initiator/polystyrene mass ratio=32∶1. The removal rate of tetracycline hydrochloride (TC) is up to 96.51%(rate of degradation k=0.0160 min−1) under visible light irradiation in the Fenton process catalyzed by LaFeO3/PS. Free radical capture experiments show that •O2− is the main active species. According to the capture experiment, the degradation mechanism of TC was proposed. Through LC/MS analysis, the degradation path of TC was obtained. The photo-Fenton process catalyzed by LaFeO3/PS is a promising technology for the degradation of organic pollutants due to the high stability of the catalyst and the efficient use of solar energy.
-
Key words:
- tetracycline hydrochloride /
- photo Fenton /
- PS /
- lanthanum ferrite /
- catalytic degradation
-
图 2 La∶ Fe∶ CA的摩尔比对LaFeO3/PS催化性能的影响(超声波功率100 W,频率40 kHz,超声辅助时间40 min,水热温度90℃,水热时间18 h,LaFeO3起始剂/PS质量比=32∶1)
Figure 2. Influence of the molar ratio of La∶ Fe∶ CA on the catalytic performance of LaFeO3/PS (P=100 W, f=40 kHz, tu=40 min, T=90°C, th=18 h, LaFeO3 initiator/PS mass ratio=32∶1. P—Power; f—Frequency; tu—Time of ultrasonic; T—Temperature of the hydrothermal; th—Time of the hydrothermal)
CA—Citric acid; C0—Initial concentration of tetracycline hydrochloride (TC); Ct—Concentration of TC at time t; k—Degradation rate; R—Correlation index
图 3 (a) 超声时间对LaFeO3/PS催化性能的影响(La∶Fe∶CA的摩尔比=1∶1∶2,超声功率100 W,频率40 kHz,水热温度90℃,水热时间18 h,LaFeO3起始剂/聚苯乙烯质量比=32∶1);(b) 水热温度对LaFeO3/PS催化性能的影响(La∶Fe∶CA的摩尔比=1∶1∶2,超声功率为100 W,频率40 kHz,超声辅助时间为40 min,水热时间18 h,LaFeO3起始剂/PS质量比=32∶1)
Figure 3. (a) Effect of ultrasound time on the catalytic performance of LaFeO3/PS (Molar ratio of La∶Fe∶CA=1∶1∶2, P=100 W, f=40 kHz, T=90°C, th=18 h, LaFeO3 initiator/polystyrene mass ratio=32∶1); (b) Influence of hydrothermal temperature on the catalytic performance of LaFeO3/PS (Molar ratio of La∶Fe∶CA = 1∶1∶2, P= 100 W, f=40 kHz, tu=40 min, T=90°C ,th=18 h, LaFeO3 initiator/PS mass ratio=32∶1)
图 8 LaFeO3/PS和PS样品的SEM图像、负载前后实际图像及SEM元素映射图像:((a)、(b)) PS的SEM图像;((c)、(d)) LaFeO3/PS的SEM图像;(e) PS and LaFeO3/PS的照片;(f) LaFeO3/PS的映射点;((g)、(h)) LaFeO3/PS的元素映射
Figure 8. SEM images of the LaFeO3/PS and PS sample, the actual pictures before and after loading, and SEM element mapping images: ((a), (b)) SEM images of PS; ((c), (d)) SEM images of LaFeO3/PS; (e) Pictures of PS and LaFeO3/PS; (f) Mapping spot of LaFeO3/PS; ((g), (h)) Elemental mapping of LaFeO3/PS
图 12 (a) 不同捕获剂对光芬顿降解TC的影响;(b) LaFeO3/PS催化光芬顿降解TC的机制图
Figure 12. (a) Effects of different trapping agents on degradation of TC by photo-Fenton; (b) Mechanism diagram of the degradation of TC by LaFeO3/PS catalyzed by photo-Fenton
TBA—Tertiary butyl alcohol; PBQ—p-Benzoquinone; DMSO—Dimethyl sulfoxide; CB—Conduction band; h—Planck's constant; ν—Frequency
表 1 LaFeO3、LaFeO3/PS和PS材料的物理性质和零电荷点
Table 1. Physical properties and zero charge point of LaFeO3, LaFeO3/PS and PS materials
Sample Specific surface area/(m2·g−1) Micropore
area/(m2·g−1)Pore volume/
(cm3·g−1)Average pore
diameter/nmPoint of zero charge (pHpzc) PS 837.41 4.71 1.15000 5.74 6.74 LaFeO3/PS 756.89 53.67 1.02000 5.62 3.08 LaFeO3 0.03 0.13 0.00007 0.70 3.00 表 2 LaFeO3/PS催化光芬顿降解TC去除率同近年来报道的方法的比较
Table 2. Removal efficiency of TC by LaFeO3/PS catalyzed by photo-Fenton compared with approaches reported in recent years
Material Method Removal efficiency Parameter Reference Biochar/Geopolymer Fenton 92.55% pH=5.0, 11 h, 50 mg/L [41] LaFeO3/Persulfate Photo-Fenton 92.60% pH=4.4, 1 h, 40 mg/L [42] s-MnFe2O4 Fenton 87.60% (TOCRT=47.50%) pH=3.0, 3 h, 30 mg/L [43] Fe3O4@C UV-Fenton 79.25% (TOCRT=43.50%) pH=4.0-5.0, 2 h, 43.7 mg/L [44] CuFeO2/Biochar Photo-Fenton 97.40% (TOCRT=39.00%) pH=4.0-5.0, 2 h, 20 mg/L [45] LaFeO3/DiaionTM HP21 Photo-Fenton 90.80% (TOCRT=73.00%),
73.00% (3rd cycle)pH=4.0-5.0, 3 h, 10 mg/L [18] LaFeO3/PS Photo-Fenton 98.01% (TOCRT=73.25%),
90.93% (7rd cycle)pH=4.75, 5 h, 16.73 mg/L This study Note: TOCRT—TOC removal rate. -
[1] MACHULEK A J, MORAES J E F, VAUTIER-GIONGO C, et al. Abatement of the inhibitory effect of chloride anions on the photo-Fenton process[J]. Environmental Science & Eechnology,2007,41(24):8459-8463. doi: 10.1021/es071884q [2] HU J Y, TIAN K, JIANG H. Improvement of phenol photodegradation efficiency by a combined g-C3N4/Fe(III)/persulfate system[J]. Chemosphere,2016,148:34-40. doi: 10.1016/j.chemosphere.2016.01.002 [3] HABIBI-YANGJEH A, MOUSAVI M, NAKATA K. Boosting visible-light photocatalytic performance of g-C3N4/Fe3O4 anchored with CoMoO4 nanoparticles: Novel magnetically recoverable photocatalysts[J]. Journal of Photochemistry and Photobiology A: Chemistry,2019,368:120-136. doi: 10.1016/j.jphotochem.2018.09.026 [4] 刘庆生, 游拯, 国辉, 等. LaFeO3复合氧化物的制备与红外辐射性能[J]. 中国有色金属学报, 2017, 27(4):781-788.LIU Qingsheng, YOU Zheng, GUO Hui, et al. Preparation and infrared radiation performance of LaFeO3 composite oxide[J]. Transactions of Nonferrous Metals Society of China,2017,27(4):781-788(in Chinese). [5] SHEN H F, LI Q M, WANG Y M, et al. Preparation of lanthanum ferrite via ultrasound-assisted sol-gel method and its photocatalytic activity[J]. Bulletin of the Chinese Ceramic Society,2018,46(3):402-412. doi: 10.14062/j.issn.0454-5648.2018.03.14 [6] JAUHAR S, DHIMAN M, BANSAL S, et al. Mn3+ ion in perovskite lattice: A potential Fenton's reagent exhibiting remarkably enhanced degradation of cationic and anionic dyes[J]. Journal of Sol-Gel Science and Technology,2015,75:124-133. doi: 10.1007/s10971-015-3682-8 [7] DAS S, MAHALINGAM H. Dye degradation studies using immobilized pristine and waste polystyrene-TiO2/rGO/g-C3N4 nanocomposite photocatalytic film in a novel airlift reactor under solar light[J]. Journal of Environmental Chemical Engineering,2019,7(5):103289. doi: 10.1016/j.jece.2019.103289 [8] MUHAMMAD H, NING S, FAZAL R, et al. Synthesis of ZnO/Bi-doped porous LaFeO3 nanocomposites as highly efficient nano-photocatalysts dependent on the enhanced utilization of visible-light-excited electrons[J]. Applied Catalysis B: Environmental,2018,231:23-33. doi: 10.1016/j.apcatb.2018.02.060 [9] WANG K, NIU H, CHEN J, et al. Immobilizing LaFeO3 nanoparticles on carbon spheres for enhanced heterogeneous photo-Fenton like performance[J]. Applied Surface Science,2017,404:138-145. doi: 10.1016/j.apsusc.2017.01.223 [10] REN X, YANG H, GEN S, et al. Controlled growth of LaFeO3 nanoparticles on reduced graphene oxide for highly efficient photocatalysis[J]. Nanoscale,2016,8(2):752-756. doi: 10.1039/C5NR06338H [11] MIHAI O, CHEN D, HOLMEN A, et al. Preparation of stable cubic LaFeO3 nanoparticles using carbon nanotubes as templates[J]. Journal of Materials Chemistry A,2013,1(24):7006-7011. doi: 10.1039/c3ta10828g [12] PHAN T T N, NIKOLOSKI A N, BAHRI P A, et al. Adsorption and photo-Fenton catalytic degradation of organic dyes over crystalline LaFeO3-doped porous silica[J]. RSC Advances,2018,8(63):36181-36190. doi: 10.1039/c8ra07073c [13] PHAN T T N, NIKOLOSKI A N, BAHRI P A, et al. Enhanced removal of organic using LaFeO3-integrated modified natural zeolites via heterogeneous visible light photo-Fenton degradation[J]. Journal of Environmental Management,2019,233:471-480. doi: 10.1016/j.jenvman.2018.12.051 [14] PENG K, FU L, YANG H, et al. Perovskite LaFeO3/montmorillonite nanocomposites: Synthesis, interface characteristics and enhanced photocatalytic activity[J]. Scientific Reports,2016,6:19723. [15] SADAKANE M, HORIUCHI T, KATO N, et al. Preparation of three-dimensionally ordered macroporous perovskite-type lanthanum-iron-oxide LaFeO3 with tunable pore diameters: High porosity and photonic property[J]. Journal of Solid State Chemistry,2010,183(6):1365-1371. doi: 10.1016/j.jssc.2010.04.012 [16] VAIANO V, MATARANGOLO M, SACCO O. UV-LEDs floating-bed photoreactor for the removal of caffeine and paracetamol using ZnO supported on polystyrene pellets[J]. Chemical Engineering Journal,2018,350:703-713. doi: 10.1016/j.cej.2018.06.011 [17] ZHANG J, LI L, XIAO Z, et al. Hollow sphere TiO2-ZrO2 prepared by self-assembly with polystyrene colloidal template for both photocatalytic degradation and H2 evolution from water splitting[J]. ACS Sustainable Chemistry & Engineering,2016,4(4):2037-2046. doi: 10.1021/acssuschemeng.5b01359 [18] ALPAY A, TUNA Ö, SIMSEK E B. Deposition of perovskite-type LaFeO3 particles on spherical commercial polystyrene resin: A new platform for enhanced photo-Fenton-catalyzed degradation and simultaneous wastewater purification[J]. Environmental Technology & Innovation,2020,20:101175. doi: 10.1016/j.eti.2020.101175 [19] XU H, ZEIGER B W, SUSLICK K S. Sonochemical synthesis of nanomaterials[J]. Chemical Society Reviews,2013,42(7):2555-2567. doi: 10.1039/C2CS35282F [20] 沈宏芳, 李清明, 王燕民, 等. 超声波辅助溶胶-凝胶法合成纳米铁酸镧及其光催化性能[J]. 硅酸盐学报, 2018, 46(3): 402-412.SHEN Hongfang, LI Qingming, WANG Yanmin, et al. Ultrasonic-assisted sol-gel synthesis of nano-lanthanum ferrite and its photocatalytic properties[J]. Journal of the Chinese Ceramic Society, 2018, 46(3): 402-412(in Chinese). [21] PHAN T T N, NIKOLOSKI A N, BAHRI P A, et al. Heterogeneous photo-Fenton degradation of organics using highly efficient Cu-doped LaFeO3 under visible light[J]. Journal of Industrial and Engineering Chemistry,2018,61:53-64. doi: 10.1016/j.jiec.2017.11.046 [22] THIRUMALAIRAJAN S, GIRIJA K, GANESH I, et al. Controlled synthesis of perovskite LaFeO3 microsphere composed of nanoparticles via self-assembly process and their associated photocatalytic activity[J]. Chemical Engineering Journal,2012,209:420-428. doi: 10.1016/j.cej.2012.08.012 [23] DANG F, KATO K, IMAI H, et al. Growth of BaTiO3 nanoparticles in ethanol-water mixture solvent under an ultrasound-assisted synthesis[J]. Chemical Engineering Journal,2011,170(1):333-337. doi: 10.1016/j.cej.2011.03.076 [24] FU Z, POPOV V. Parametric study of acoustically-driven microbubble cavitations in a sonochemical reactor[J]. Ultrasonics Sonochemistry,2014,21(1):415-427. doi: 10.1016/j.ultsonch.2013.07.001 [25] KATO H, KUDO A. Visible-light-response and photocatalytic activities of TiO2 and SrTiO3 photocatalysts codoped with antimony and chromium[J]. Journal of Physical Chemistry B,2002,106(19):5029-5034. doi: 10.1021/jp0255482 [26] WU Y, LI X, ZHAO H, et al. 2D/2D FeNi-layered double hydroxide/bimetal-MOFs nanosheets for enhanced photo-Fenton degradation of antibiotics: Performance and synergetic degradation mechanism[J]. Chemosphere,2022,287:132061. doi: 10.1016/j.chemosphere.2021.132061 [27] TIWARI S, SHARMA N, SAXENA R. On-line speciation of chromium using a modified chelating resin and determination in industrial water samples by flame atomic absorption spectrometry[J]. New Journal of Chemistry,2015,40(2):1412-1419. doi: 10.1039/C5NJ02283E [28] 刘艳, 高洋, 赵昕, 等. 凝胶型树脂载纳米水合氧化铁复合材料的制备与除As(V)特性[J]. 高分子学报, 2018(7):939-948.LIU Yan, GAO Yang, ZHAO Xin, et al. Preparation of gel-type resin-loaded nano-hydrated iron oxide composite material and its As(V) removal characteristics[J]. Acta Polymerica Sinica,2018(7):939-948(in Chinese). [29] ESRA B S, ÖZLEM T, ZEYNEP B. Construction of stable perovskite-type LaFeO3 particles on polymeric resin with boosted photocatalytic Fenton-like decaffeination under solar irradiation[J]. Separation and Purification Technology,2020,237:116384. doi: 10.1016/j.seppur.2019.116384 [30] SUN B, ZHOU W, LI H, et al. Synthesis of particulate hierarchical tandem heterojunctions toward optimized photocatalytic hydrogen production[J]. Advanced Materials,2018,30(43):1804282. doi: 10.1002/adma.201804282 [31] XU Y, LI H, SUN B, et al. Surface oxygen vacancy defect-promoted electron-hole separation for porous defective ZnO hexagonal plates and enhanced solar-driven photocatalytic performance[J]. Chemical Engineering Journal,2020,379:122295. [32] 吴丹, 詹海鹃, 刘宇凤, 等. LaNixFe(1-x)O3钙钛矿光催化降解碱性品红[J]. 硅酸盐通报, 2019, 38(6):1832-1838.WU Dan, ZHAN Haijuan, LIU Yufeng, et al. LaNixFe(1-x)O3 perovskite photocatalytic degradation of basic fuchsin[J]. Silicate Bulletin,2019,38(6):1832-1838(in Chinese). [33] 郝强, 郝思濛, 牛秀秀, 等. 通过rGO与g-C3N4的π–π堆积作用提高氮化碳光化学氧化能力[J]. 催化学报, 2017, 38(2):278-286.HAO Qiang, HAO Simeng, NIU Xiuxiu, et al. Improving the photochemical oxidation ability of carbon nitride through the π-π stacking interaction of rGO and g-C3N4[J]. Chinese Journal of Catalysis,2017,38(2):278-286(in Chinese). [34] AUGUGLIARO V, LITTER M, PALMISANO L, et al. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews,2006,7(4):127-144. doi: 10.1016/j.jphotochemrev.2006.12.001 [35] POURETEDAL H R, TOFANGSAZI Z, KESHAVARZ M H. Photocatalytic activity of mixture of ZrO2/SnO2, ZrO2/CeO2 and SnO2/CeO2 nanoparticles Generic[J]. Journal of Alloys and Compounds,2011,513:359-564. [36] 段丽媛, 李国强, 张舒婷, 等. 二次等温热缩聚改性对g-C3N4光催化剂性能的影响[J]. 化工进展, 2021, 40(6):3389-3400.DUAN Liyuan, LI Guoqiang, ZHANG Shuting, et al. Effect of secondary isothermal thermal polycondensation modification on the performance of g-C3N4 photocatalyst[J]. Chemical Progress,2021,40(6):3389-3400(in Chinese). [37] 綦毓文, 魏砾宏, 石冬妮, 等. UiO-66/BiVO4复合光催化剂的制备及其对四环素的光解[J]. 中国环境科学, 2021, 41(3):1162-1171. doi: 10.3969/j.issn.1000-6923.2021.03.019QI Yuwen, WEI Lihong, SHI Dongni, et al. Preparation of UiO-66/BiVO4 composite photocatalyst and its photolysis of tetracycline[J]. China Environmental Science,2021,41(3):1162-1171(in Chinese). doi: 10.3969/j.issn.1000-6923.2021.03.019 [38] 王丹, 赵利霞, 张辉, 等. 二氧化钛光催化产生超氧自由基的形态分布研究[J]. 分析化学, 2017, 45(12):1882-1887. doi: 10.11895/j.issn.0253-3820.171378WANG Dan, ZHAO Lixia, ZHANG Hui, et al. Study on the speciation distribution of superoxide radicals generated by titanium dioxide photocatalysis[J]. Analytical Chemistry,2017,45(12):1882-1887(in Chinese). doi: 10.11895/j.issn.0253-3820.171378 [39] LIU D, WANG J, WANG Y, et al. An anion exchange strategy for construction of a novel Bi2SiO5/Bi2MoO6 heterostructure with enhanced photocatalytic performance[J]. Catalysis Science & Technology,2018,8(13):3278-3285. [40] WANG S, ZHAO L, HUANG W, et al. Solvothermal synthesis of CoO/BiVO4 p-n heterojunction with micro-nano spherical structure for enhanced visible light photocatalytic activity towards degradation of tetracycline[J]. Materials Research Bulletin,2021,135:111161. doi: 10.1016/j.materresbull.2020.111161 [41] 黄嘉绮, 葛圆圆, 李志礼, 等. 生物炭/地聚物复合膜的制备及其对四环素的去除[J]. 化工进展, 2022, 41(1): 427-434.HUANG Jiaqi, GE Yuanyuan, LI Zhili, et al. Preparation of biochar/geopolymer composite membrane and its removal of tetracycline[J]. Chemical Progress, 2022, 41(1): 427-434(in Chinese). [42] FENG Q, ZHOU J, LUO W, et al. Photo-Fenton removal of tetracycline hydrochloride using LaFeO3 as a persulfate activator under visible light[J]. Ecotoxicology and Environmental Safety,2020,198:110661. doi: 10.1016/j.ecoenv.2020.110661 [43] 秦航道, 肖榕, 吴思展, 等. MnFe2O4磁性纳米棒非均相Fenton催化降解水中四环素的研究[J]. 环境科学学报, 2020, 40(11):3913-3921.QIN Hangdao, XIAO Rong, WU Sizhan, et al. Study on heterogeneous Fenton catalytic degradation of tetracycline in water by MnFe2O4 magnetic nanorods[J]. Journal of Environmental Science,2020,40(11):3913-3921(in Chinese). [44] KAKAVANDI B, TAKDASTAN A, JAAFARZADEH N, et al. Application of Fe3O4@C catalyzing heterogeneous UV-Fenton system for tetracycline removal with a focus on optimization by a response surface method[J]. Journal of Photochemistry and Photobiology A: Chemistry,2016,314:178-188. doi: 10.1016/j.jphotochem.2015.08.008 [45] XIN S, MA B, LIU G, et al. Enhanced heterogeneous photo-Fenton-like degradation of tetracycline over CuFeO2/biochar catalyst through accelerating electron transfer under visible light[J]. Journal of Environmental Management,2021,285:112093. doi: 10.1016/j.jenvman.2021.112093 [46] JIANG J, WANG X, LIU Y, et al. Photo-Fenton degradation of emerging pollutants over Fe-POM nanoparticle/porous and ultrathin g-C3N4 nanosheet with rich nitrogen defect: Degradation mechanism, pathways, and products toxicity assessment[J]. Applied Catalysis B: Environmental,2020,278:119349. doi: 10.1016/j.apcatb.2020.119349 [47] WANG Y, RAO L, WANG P, et al. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment[J]. Applied Catalysis B: Environmental,2020,262:118308. doi: 10.1016/j.apcatb.2019.118308 -





 下载:
下载:















