Preparation of magnetic γ-Fe2O3/corn stalk starch and its adsorption performance for U(VI) in wastewater
-
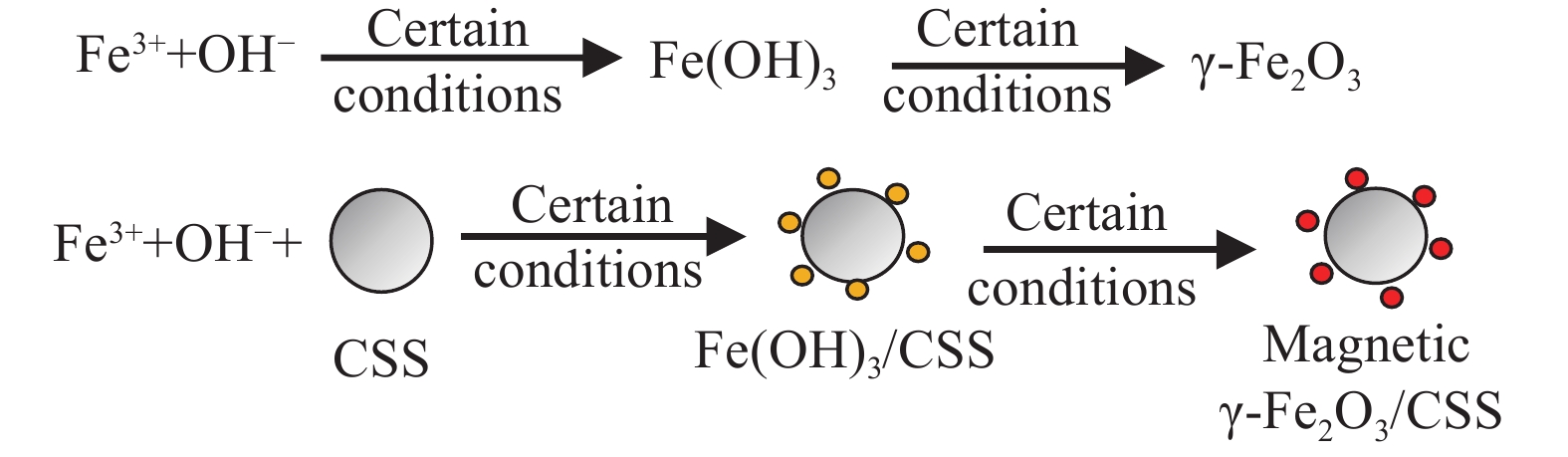
摘要: 随着核能发展,放射性核素铀通过各种途径流入环境,对人类的身体健康构成潜在威胁。以废弃玉米秸秆为原料自制玉米秸秆淀粉(CSS),采用共沉淀法将磁性γ-Fe2O3包裹在CSS表面合成磁性γ-Fe2O3/CSS,并用于溶液中U(VI)的吸附。考察初始pH值、投加量、时间、初始浓度、温度和共存离子等因素对γ-Fe2O3/CSS吸附U(VI)性能的影响,并加以分析。采用SEM、FTIR、XPS对吸附前后的磁性γ-Fe2O3/CSS进行表征分析,深入研究其对吸附U(VI)的技术机制。结果表明:在适宜条件下,γ-Fe2O3/CSS对U(VI)的最大吸附量达到214.1 mg/g。准二级动力学模型更准确地描述其吸附过程即以化学吸附为主。磁性γ-Fe2O3/CSS对U(VI)吸附符合Langmuir模型和Freundlich模型。吸附机制主要为U(VI)与γ-Fe2O3/CSS的羟基、羧基发生络合反应与离子交换作用。通过4次吸附解吸实验表明,U(VI)吸附率仍在78.60%以上,说明磁性γ-Fe2O3/CSS具有一定的再生能力。Abstract: With the development of nuclear energy, the radionuclide uranium flows into the environment through various channels, posing a potential threat to human health. Using waste corn stalks as raw materials, self-made corn stalk starch (CSS) was used, and magnetic γ-Fe2O3 was wrapped on the surface of CSS by co-precipitation method to synthesize magnetic γ-Fe2O3/CSS, which was used to adsorb U(VI) in the solution. The effects of factors such as initial pH value, dosage, time, initial concentration, temperature and coexisting ions on the adsorption performance of γ-Fe2O3/CSS for U(VI) were investigated and analyzed. The SEM, FTIR, XPS were used to characterize and analyze the magnetic γ-Fe2O3/CSS before and after adsorption, and the technical mechanism of adsorption of U(VI) was deeply studied. The results show that the maximum adsorption capacity of γ-Fe2O3/CSS for U(VI) reaches 214.1 mg/g in certain conditions. The quasi-second-order kinetic model describes the adsorption process more accurately, that is, chemical adsorption is the main. The adsorption of magnetic γ-Fe2O3/CSS on U(VI) satisfies Langmuir model and Freundlich model. The adsorption mechanism is mainly complex reaction and ion exchange between U(VI) and the hydroxyl and carboxyl groups of γ-Fe2O3/CSS. Four adsorption and desorption experiments show that the U(VI) adsorption rate is still above 78.60%, indicating that the magnetic γ-Fe2O3/CSS has a certain regeneration ability.
-
Key words:
- γ-Fe2O3 /
- corn stalk starch /
- uranium /
- adsorption performance /
- ion exchange
-
图 7 时间对γ-Fe2O3/CSS吸附U(VI)影响 (a) 和准一级 (b)、准二级(c)动力学拟合曲线
Figure 7. Influence of time on the adsorption of U(VI) by γ-Fe2O3/CSS (a) and the kinetic fitting curves of quasi-first-order (b) and quasi-second-order (c)
q—Adsorption capacity; qt—Adsorption capacity of adsorbent to U(VI) at time t; qe—Equilibrium adsorption capacity to U(VI)
表 1 γ-Fe2O3/CSS对U(VI)吸附动力学参数
Table 1. Adsorption kinetics parameters of U(VI) by γ-Fe2O3/CSS
Adsorbent qe(Experiment)/
(mg·g−1)Quasi-first-order dynamics model Quasi-second-order dynamics model K1/min−1 qe(Calculation)/
(mg·g−1)R2 K2/(g·mg−1·min−1) qe(Calculation)/
(mg·g−1)R2 γ-Fe2O3/CSS 74.76 0.0419 6.15 0.9518 0.0241 75.02 0.9999 Notes: qe—Equilibrium adsorption capacity to U(VI); K1—Adsorption rate constant of quasi-first-order model; K2—Adsorption rate constant of quasi-second-order model; R2—Correlation coefficient. 表 2 γ-Fe2O3/CSS对U(VI)的吸附等温线拟合参数
Table 2. Adsorption isotherm parameters of U(VI) by γ-Fe2O3/CSS
Freundlich Langmuir KF 1/n R2 qm/(mg·g−1) KL R2 116.7 0.238 0.9866 214.1 1.99 0.9818 Notes: KF—Adsorption equilibrium constant of Freundlich model; KL—Adsorption equilibrium constant of Langmuir model; 1/n—Empirical parameter related to adsorption strength; qm—Maximum adsorption capacity of γ-Fe2O3/CSS to U(VI). -
[1] 杨军, 张恩昊, 郭志恒, 等. 全球核能科技前沿综述[J]. 科技导报, 2020, 38(20):35-49. doi: 10.3981/j.issn.1000-7857.2020.20.010YANG Jun, ZHANG Enhao, GUO Zhiheng, et al. Recent progress of frontier nuclear energy science and technology[J]. Science & Technology Review,2020,38(20):35-49(in Chinese). doi: 10.3981/j.issn.1000-7857.2020.20.010 [2] WU Y H, PANG H W, YAO W, et al. Synthesis of rod-like metal-organic framework (MOF-5) nanomaterial for efficient removal of U(VI): Batch experiments and spectroscopy study[J]. Science Bulletin,2018,63(13):831-839. doi: 10.1016/j.scib.2018.05.021 [3] YU S J, WANG J, SONG S, et al. One-pot synthesis of graphene oxide and Ni-Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater[J]. Science China (Chemistry),2017,60(3):415-422. doi: 10.1007/s11426-016-0420-8 [4] 庞宏伟, 唐昊, 王佳琦, 等. 三元水滑石负载的硫化纳米零价铁对铀的高效去除与机理研究[J]. 无机材料学报, 2020, 35(3):381-389.PANG Hongwei, TANG Hao, WANG Jiaqi, et al. Ternary layered double hydroxide supported sulfide NZVI: Efficient U(VI) elimination and mechanism[J]. Journal of Inorganic Materials,2020,35(3):381-389(in Chinese). [5] LI J, WANG X X, ZHAO G X, et al. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions[J]. Chemical Society Reviews,2018,47(7):2322-2356. doi: 10.1039/C7CS00543A [6] 刘玥, 吴忆涵, 庞宏伟, 等. 石墨相氮化碳材料在水环境污染物去除中的研究[J]. 化学进展, 2019, 31(6):831-846.LIU Yue, WU Yihan, PANG Hongwei, et al. Study on the removal of water pollutants by graphite phase carbon nitride materials[J]. Progress in Chemistry,2019,31(6):831-846(in Chinese). [7] BASELGA-CERVERA B, ROMERO-LOPEZ J, GARCIA-BALBOA C, et al. Improvement of the uranium sequestration ability of a chlamydomonas sp. (ChlSP strain) isolated from extreme uranium mine tailings through selection for potential bioremediation application[J]. Frontiers in Microbiology,2018,9:523. doi: 10.3389/fmicb.2018.00523 [8] 蒋丽, 高慧慧, 曹茹雅, 等. 三维大孔g-C3N4吸附和光催化还原U(VI)性能研究[J]. 无机材料学报, 2020, 35(3):359-366.JIANG Li, GAO Huihui, CAO Ruya, et al. Construction of novel three dimensionally macroporous g-C3N4 for efficient adsorption/photocatalytic reduction of U(VI)[J]. Journal of Inorganic Materials,2020,35(3):359-366(in Chinese). [9] 彭国文, 肖方竹, 丁德馨, 等. 新型功能化吸附剂G-PA-SBA-15对U(VI)的吸附机理[J]. 中国有色金属学报, 2015, 25(11):3237-3244.PENG Guowen, XIAO Fangzhu, DING Dexin, et al. Adsorption mechanisms of uranium(VI) from aqueous solution by G-PA-SBA-15[J]. The Chinese Journal of Nonferrous Metals,2015,25(11):3237-3244(in Chinese). [10] 谢新玲, 熊海武, 童张法, 等. 5种改性淀粉吸附Cu2+性能及动力学研究[J]. 功能材料, 2017, 48(2):2009-2012, 2019. doi: 10.3969/j.issn.1001-9731.2017.02.003XIE Xinling, XIONG Haiwu, TONG Zhangfa, et al. Adsorption properties and kinetic study of Cu2+ removal using five kinds of modified starches[J]. Journal of Functional Materials,2017,48(2):2009-2012, 2019(in Chinese). doi: 10.3969/j.issn.1001-9731.2017.02.003 [11] 汪秀丽, 张玉荣, 王玉忠. 淀粉基高分子材料的研究进展[J]. 高分子学报, 2011(1):24-37.WANG Xiuli, ZHANG Yurong, WANG Yuzhong. Recent progress in starch-based polymeric materials[J]. Acta Polymerica Sinica,2011(1):24-37(in Chinese). [12] SU X X, HU J L, ZHANG J, et al. Investigating the adsorption behavior and mechanisms of insoluble Humic acid/starch composite microspheres for metal ions from water[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2020,610:125672. doi: 10.1016/j.colsurfa.2020.125672 [13] 邹皓, 谢水波, 刘迎九, 等. 回生淀粉吸附U(VI)的特性及机理分析[J]. 科学技术与工程, 2017, 17(13):97-101. doi: 10.3969/j.issn.1671-1815.2017.13.018ZOU Hao, XIE Shuibo, LIU Yingjiu, et al. Adsorption behavior and mechanism of uranium(VI) absorbed by retrograde starch[J]. Science Technology and Engineering,2017,17(13):97-101(in Chinese). doi: 10.3969/j.issn.1671-1815.2017.13.018 [14] 王星, 朱春山, 张弘, 等. 磁性淀粉微球对水中Cr(VI)的吸附性能研究[J]. 河南工业大学学报(自然科学版), 2013, 34(6):43-50.WANG Xing, ZHU Chunshan, ZHANG Hong, et al. Adsorption behavior of magnetic starch microspheres on hexavalent chromium in wastewater[J]. Journal of Henan University of Technology (Natural Science Edition),2013,34(6):43-50(in Chinese). [15] 阳鹏飞, 黎杰鑫, 朱春霞. 磁性纳米材料处理含铀废水的研究进展[J]. 化工进展, 2020, 39(1):206-215.YANG Pengfei, LI Jiexin, ZHU Chunxia. Research progress in treating uranium containing wastewater with magnetic materials[J]. Chemical Industry and Engineering Progress,2020,39(1):206-215(in Chinese). [16] 於照惠, 戴仲然, 尤青, 等. 纳米赤铁矿吸附剂吸附低浓度U(VI)的行为与机理[J]. 南华大学学报(自然科学版), 2019, 33(3):1-7.YU Zhaohui, DAI Zhongran, YOU Qing, et al. Adsorption behavior and mechanism of U(VI) by nano hematite from low concentration wastewater[J]. Journal of University of South China (Science and Technology),2019,33(3):1-7(in Chinese). [17] 静远. 玉米秸秆制淀粉[J]. 资源开发与保护, 1988(4):81.JING Yuan. Preparation of starch from corn stover[J]. Resource Development & Market,1988(4):81(in Chinese). [18] 袁土贵. 纳米零价铁处理含铀废水的研究[D]. 广州: 广州大学, 2013.YUAN Tugui. Removal of uranium from aqueous solution using zero-valent iron nanoparticles[D]. Guangzhou: Guangzhou University, 2013(in Chinese). [19] 谭洪卓, 谭斌, 刘明, 等. 甘薯粉丝生产中多糖胶与甘薯淀粉相互作用机理[J]. 食品科学, 2008(5):49-55. doi: 10.3321/j.issn:1002-6630.2008.05.002TAN Hongzhuo, TAN Bin, LIU Ming, et al. Mechanism of interaction between polysaccharide gums and sweet potato starch in production of its noodles[J]. Food Science,2008(5):49-55(in Chinese). doi: 10.3321/j.issn:1002-6630.2008.05.002 [20] 雷增江, 杨金辉, 戴漾泓, 等. 铝污泥对废水中铀(VI)的吸附机理研究[J]. 应用化工, 2020, 49(3):572-577. doi: 10.3969/j.issn.1671-3206.2020.03.009LEI Zengjiang, YANG Jinhui, DAI Yanghong, et al. Study on the adsorption mechanism of uranium(VI) in wastewater by aluminum sludge[J]. Applied Chemical Industry,2020,49(3):572-577(in Chinese). doi: 10.3969/j.issn.1671-3206.2020.03.009 [21] 李妍, 崔维建, 赵城彬, 等. 玉米淀粉-玉木耳多糖复配体系理化及结构性质[J]. 食品科学, 2021, 42(4):58-64.LI Yan, CUI Weijian, ZHAO Chengbin, et al. Physicochemi-cal and structural properties of corn starch-auricularia cornea ehrenb polysaccharide blends[J]. Food Science,2021,42(4):58-64(in Chinese). [22] 黄文富, 梁宁, 蔡锦源. 玉米淀粉-丙烯酸接枝共聚物的制备及其吸水性能研究[J]. 化工技术与开发, 2019, 48(12):12-16.HUANG Wenfu, LIANG Ning, CAI Jinyuan. Preparation and water absorption of corn starch acrylic acid graft copolymer[J]. Technology & Development of Chemical Industry,2019,48(12):12-16(in Chinese). [23] 王立东, 肖志刚. 气流粉碎对玉米淀粉结构及理化性质的影响[J]. 农业工程学报, 2016, 32(24):276-281. doi: 10.11975/j.issn.1002-6819.2016.24.037WANG Lidong, XIAO Zhigang. Effect of jet-milling on structure and physicochemical properties of maize starch[J]. Transactions of the Chinese Society of Agricultural Engi-neering,2016,32(24):276-281(in Chinese). doi: 10.11975/j.issn.1002-6819.2016.24.037 [24] 王泽敏, 刘勇, 戢明, 等. γ-Fe2O3纳米粉末的光谱吸收特性研究[J]. 功能材料, 2006(2):284-286, 291. doi: 10.3321/j.issn:1001-9731.2006.02.035WANG Zemin, LIU Yong, JI Ming, et al. Spectrum absorption studies of γ-Fe2O3 nanoparticles[J]. Journal of Functional Materials,2006(2):284-286, 291(in Chinese). doi: 10.3321/j.issn:1001-9731.2006.02.035 [25] 莫官海, 农海杜, 胡青, 等. 硝酸改性污泥基生物炭除U(VI)效果及机理分析[J]. 精细化工, 2021, 38(2):395-403.MO Guanhai, NONG Haidu, HU Qing, et al. U(VI) removal efficiency and mechanism by acidified sewage sludge-derived biochar[J]. Fine Chemicals,2021,38(2):395-403(in Chinese). [26] YAMASHITA T, HAYES P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials[J]. Applied Surface Science,2008,254(8):2441-2449. doi: 10.1016/j.apsusc.2007.09.063 [27] 王佳琦, 庞宏伟, 唐昊, 等. 碳热还原法制备的碳载零价铁对水中U(VI)的去除研究[J]. 无机材料学报, 2020, 35(3):373-380.WANG Jiaqi, PANG Hongwei, TANG Hao, et al. Carbothermic synthesis of carbon-supported zero-valent iron material for removal of U(VI) from aqueous solution[J]. Journal of Inorganic Materials,2020,35(3):373-380(in Chinese). [28] 高翔, 谢水波, 刘迎久, 等. 壳聚糖-生物炭复合材料对U(VI)的吸附性能试验研究[J]. 原子能科学技术, 2019, 53(8):1350-1358.GAO Xiang, XIE Shuibo, LIU Yingjiu, et al. Experimental investigation on adsorption of U(VI) by chitosan-biochar composite[J]. Atomic Energy Science and Technology,2019,53(8):1350-1358(in Chinese). [29] BELGACEM A, REBIAI R, HADOUN H, et al. The removal of uranium(VI) from aqueous solutions onto activated carbon developed from grinded used tire[J]. Environmental Science and Pollution Research,2014,21(1):684-694. doi: 10.1007/s11356-013-1940-2 [30] 刘学, 李小燕, 陈玉洁, 等. 石墨负载纳米零价铁去除溶液中U(VI)[J]. 中国有色金属学报, 2020, 30(8):1967-1973.LIU Xue, LI Xiaoyan, CHEN Yujie, et al. Removal of U(VI) in aqueous solution by graphite loading nano-zero-valent iron[J]. The Chinese Journal of Nonferrous Metals,2020,30(8):1967-1973(in Chinese). [31] YU S J, LIU Y, AI Y J, et al. Rational design of carbonaceous nanofiber/Ni-Al layered double hydroxide nanocomposites for high-efficiency removal of heavy metals from aqueous solutions[J]. Environmental Pollution,2018,242(Part A):1-11. doi: 10.1016/j.envpol.2018.06.031 [32] 王慧. 氧化石墨烯及其功能化改性材料富集水中重金属离子机理研究[D]. 长沙: 湖南大学, 2016.WANG Hui. Application of graphene oxide and its functional composites for enrichment of heavy metal ions from aqueous solution[D]. Changsha: Hunan University, 2016(in Chinese). [33] 彭国文, 丁德馨, 胡南, 等. 化学修饰啤酒酵母菌对铀的吸附特性[J]. 化工学报, 2011, 62(11):3201-3206. doi: 10.3969/j.issn.0438-1157.2011.11.032PENG Guowen, DING Dexin, HU Nan, et al. Adsorption characteristics of uranium by Saccharomyces cerevisiae by chemical modification[J]. CIESC Journal,2011,62(11):3201-3206(in Chinese). doi: 10.3969/j.issn.0438-1157.2011.11.032 [34] 彭国文. 新型功能化吸附剂的制备及其吸附铀的试验研究[D]. 长沙: 中南大学, 2014.PENG Guowen. Construction of novel functional adsorbents and application of wastewater treatment in uranium mining and hydrometallurgy[D]. Changsha: Central South University, 2014(in Chinese). -






 下载:
下载: