δ-MnO2 supported on low-grade Palygorskite clay from Linze as a catalyst for formaldehyde catalytic oxidation at room temperature
-
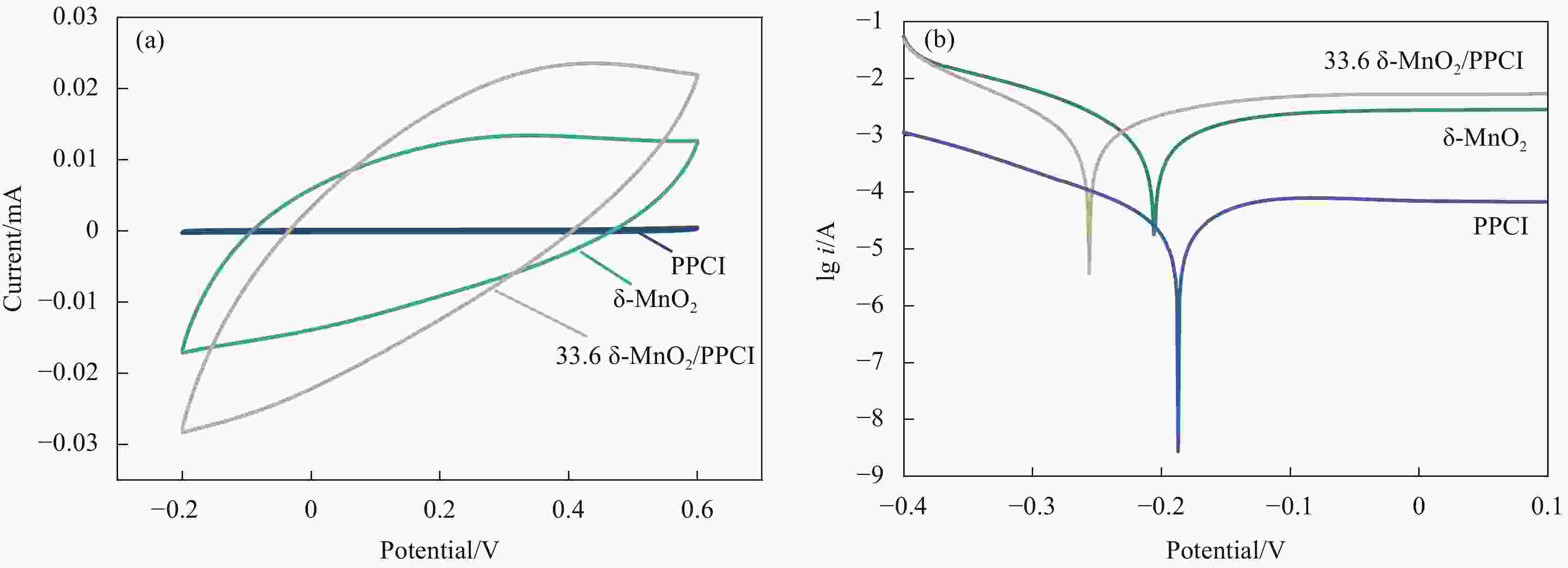
摘要: 针对临泽地区低品位凹凸棒石黏土利用率低的问题,用预富集处理的临泽红色低品位凹凸棒石黏土(PPCI)为载体,利用高锰酸钾和草酸铵为反应前驱体,通过氧化还原法制备MnOx/PPCI复合催化剂,并用于常温下降解室内空气中甲醛效果评价。结果表明,Mn负载量为33.6wt%的复合催化剂具有优异性能,动态实验中,当进气甲醛浓度为1.22 mg/m3时,去除率在720 min内保持99%以上,而未负载的δ-MnO2在相同条件下去除甲醛的效率仅为87%;静态实验中对初始浓度为146.6 mg/m3的甲醛气体去除率高达95%以上,PPCI负载δ-MnO2可以显著提高锰基催化剂室温降解甲醛效果。δ-MnO2/PPCI复合催化剂降解甲醛反应遵循二级动力学。由于δ-MnO2在凹凸棒石黏土矿物表面高度分散,有更大的比表面积(73.2 m2/g),可以暴露出更多Mn3+/Mn4+活性电对,从而提高了复合催化剂的氧化还原能力和电化学活性,并最终促进甲醛的降解。通过原位红外光谱(In situ-DRFTS)研究了甲醛在复合催化剂表面中间产物的生成和转化过程,结果表明甲醛首先被表面羟基转化为亚甲基二氧(DOM),进而被表面活性氧氧化为甲酸盐物种(HCOO-),最终被氧化为CO2和H2O,并且催化反应消耗表面羟基可通过凹凸棒石表面吸附水与表面活性氧反应再生。本研究通过开发低品位凹凸棒石黏土,提高资源利用率,并为开发高效复合室内空气净化材料提供新思路。Abstract: Aiming at the low utilization rate of low-grade palygorskite in Linze area, MnOx/purifing palygorskite clay rich iron (PPCI) hybrid catalysts were prepared by the redox reaction method between KMnO4 and (NH4)2C2O4 via using low-grade PPCI from Linze as the support. The δ-MnO2/PPCI catalyst with Mn loading of 33.6wt% has excellent performance for formaldehyde oxidation at room temperature, which maintains more than effective 99% formaldehyde removal rate within 720 minutes. In contrast, the effective formaldehyde removal rate of the unloaded δ-MnO2 sample is only 87% after 720 min. Also, the removal rate of formaldehyde with initial concentration of 146.6 mg/m3 is more than 95% in 1h. The above results reveal that the introduction of palygorskite clay as the support greatly improves the efficiency of the MnOx/PPCI hybrid catalysts at room temperature. The formaldehyde oxidation kinetics results follow the second-order kinetics. Manganese oxide was highly distributed on the surface of palygorskite, resulting in a larger specific surface area (73.2 m2/g) and expose more Mn3+/Mn4+ couples, which improves the redox capacity and electrochemical activity of the composite catalyst and contributes to the process of formaldehyde degradation eventually. Based on the analysis results of in-situ DRFTS, the formation and conversion of formaldehyde on the surface of catalysts were revealed. Formaldehyde is first converted to dioxymethylene (DOM) by surface hydroxyl groups (–OH), and then oxidized to formate species (HCOO−) by surface active oxygen, HCOO− is finally oxidized to CO2 and H2O. The consumed -OH groups can be compensated from the activation of O2 via water and surface-active oxygen species reaction. This work paves a new road to utilizing low-grade palygorskite clay as compo-site catalysts for air purification at room temperature.
-
Key words:
- low-grade palygorskite clay /
- MnOx /
- formaldehyde /
- oxidative degradation /
- room temperature
-
图 5 催化剂去除甲醛的动态效能 (a)、33.6 δ-MnO2/PPCI长时间动态稳定性测试 (b)、静态效能 (c) 和33.6 δ-MnO2/PPCI 静态去除甲醛过程中CO2浓度变化 (d)
Figure 5. Dynamic performance (a), long-term stability of 33.6 δ-MnO2/PPCI under dynamic test (b), static performance (c) and change of the CO2 concertration during formaldehyde removal by 33.6 δ-MnO2/PPCI under static test (d)
C—Concentration
表 1 MnOx/预富集处理的临泽红色低品位凹凸棒石黏土(PPCI)的命名
Table 1. Naming of MnOx
/purifing palygorskite clay rich iron (PPCI) Sample Mn/wt% KMnO4/g PPCI/g 6.2 δ-MnO2/PPCI 6.2 0.55 3 11.6 δ-MnO2/PPCI 11.6 1.18 3 15.7 δ-MnO2/PPCI 15.7 1.64 3 23.0 δ-MnO2/PPCI 23.0 2.57 3 26.7 δ-MnO2/PPCI 26.7 2.87 3 33.6 δ-MnO2/PPCI 33.6 4.44 3 35.6 δ-MnO2/PPCI 35.6 4.85 3 表 2 MnO2/PPCI复合材料的比表面积
Table 2. Specific surface area of MnO2/PPCI
Sample Surface area/(m2·g−1) PPCI 64.3 δ-MnO2 41.4 6.2 δ-MnO2/PPCI 46.6 11.6 δ-MnO2/PPCI 52.7 15.7 δ-MnO2/PPCI 55.4 23.0 δ-MnO2/PPCI 58.2 26.7 δ-MnO2/PPCI 67.8 33.6 δ-MnO2/PPCI 73.2 35.6 δ-MnO2/PPCI 59.8 表 3 一级动力学和二级动力学模型的拟合数据
Table 3. Fitting data of the first-order and second-order kinetic models
Order Parameter PPCI δ-MnO2 15.7 δ-MnO2/PPCI 33.6 δ-MnO2/PPCI First k1 −0.0012 −0.039 −0.038 −0.041 R2 0.7744 0.8031 0.8453 0.8108 Second k2 1.45×10−4 2.18×10−3 1.59×10−3 1.86×10−3 R2 0.8800 0.9891 0.9946 0.9872 Notes: k1—Reaction rate constants of first-kinetic model; k2—Reaction rate constants of second-kinetic model; R2—Correlation. -
[1] LU N, PEI J J, ZHAO Y X, et al. Performance of a biological degradation method for indoor formaldehyde removal[J]. Building and Environment,2012,57:253-258. doi: 10.1016/j.buildenv.2012.05.007 [2] MA C J, LI X, ZHU T L. Removal of low-concentration formaldehyde in air by adsorption on activated carbon modified by hexamethylene diamine[J]. Carbon,2011,49(8):2873-2875. doi: 10.1016/j.carbon.2011.02.058 [3] NOHMAN A K H, ISAMIL H M, HUSSEIN G A M. Thermal and chemical events in the decomposition course of manganese compounds[J]. Journal of Analytical and Applied Pyrolysis,1995,34(2):265-278. doi: 10.1016/0165-2370(95)00896-M [4] SHIE J L, LEE C H, CHIOU C, CHANG C S, et al. Photodegradation kinetics of formaldehyde using light sources of UVA, UVC and UVLED in the presence of composed silver titanium oxide photocatalyst[J]. Journal of Hazardous Materials,2008,155(1-2):164-172. doi: 10.1016/j.jhazmat.2007.11.043 [5] LIU P, HE H P, WEI G, et al. Effect of Mn substitution on the promoted formaldehyde oxidation over spinel ferrite: Catalyst characterization, performance and reaction mechanism[J]. Applied Catalysis B: Environmental,2016,182:476-484. doi: 10.1016/j.apcatb.2015.09.055 [6] 刘亚茹, 黄宇. MnO2基材料常温催化降解甲醛研究进展[J]. 地球环境学报, 2020, 11(1):14-30.LIU Yaru, HUANG Yu. Research progress on room-temperature catalytic degradation of formaldehyde over MnO2-based catalysts[J]. Journal of Earth Environment,2020,11(1):14-30(in Chinese). [7] LIU P, HE H P, WEI G H, et al. An efficient catalyst of manganese supported on diatomite for toluene oxidation: Manganese species, catalytic performance, and structure-activity relationship[J]. Microporous and Mesoporous Materials,2017,239:101-110. doi: 10.1016/j.micromeso.2016.09.053 [8] LIANG X L, LIU P, HE H P, et al. The variation of cationic microstructure in Mn-doped spinel ferrite during calcination and its effect on formaldehyde catalytic oxidation[J]. Journal of Hazardous Materials. 2016, 306: 305-312. [9] 陈天虎, 徐晓春, 岳书仓. 苏皖凹凸棒石粘土纳米矿物学及地球化学[M]. 北京: 科学出版社, 2004.CHEN Tianhu, XU Xiaochun, YUE Shucang. Nanometer scale mineralogy and geochemistry of palygorskite clays in the border of Jiangsu and Anhui Provinces[M]. Beijing: Science Press, 2004(in Chinese). [10] 王文波, 牟斌, 张俊平, 等. 凹凸棒石: 从矿物材料到功能材料[J]. 中国科学: 化学, 2018, 48(12):1432-1451. doi: 10.1360/N032018-00193WANG Wenbo, MU Bin, ZHANG Junpin, et al. Attapulgite: from clay minerals to functional materials[J]. Scientia Sinica Chimica,2018,48(12):1432-1451(in Chinese). doi: 10.1360/N032018-00193 [11] YIN H B, REN C, LI W. Introducing hydrate aluminum into porous thermally-treated calcium-rich attapulgite to enhance its phosphorus sorption capacity for sediment internal loading management[J]. Chemical Engineering Journal, 2018, 348: 704-712. [12] WANG H, WANG X J, LI J, et al. Comparison of palygorskite and struvite supported palygorskite derived from phosphate recovery in wastewater for in-situ immobilization of Cu, Pb and Cd in contaminated soil[J]. Journal of Hazardous Materials,2018,346:273-284. doi: 10.1016/j.jhazmat.2017.12.042 [13] XU C B, QI J, YANG W. et al. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay[J]. Science of The Total Environment, 2019, 686: 476-483. [14] WEI Y X, SONG M, YU L, et al. Hydroxyl-promoter on hydrated Ni-(Mg, Si) attapulgite with high metal sintering resistance for biomass derived gas reforming[J]. International Journal of Hydrogen Energy, 2019, 44 (36): 20056-20067. [15] CHEN Y, CHEN T H, LIU H B, et al. High catalytic perfor-mance of the Al-promoted Ni/Palygorskite catalysts for dry reforming of methane[J]. Applied Clay Science, 2020, 188: 105498-105508. [16] 白国梁, 陶海兵, 蔡思敏, 等. 凹凸棒石(PG)负载V2O5催化剂脱除气态Hg0的研究[J]. 环境科学学报, 2019, 39(7):2369-2376.BAI Guoliang, TAO Haibing, CAI Simin, et al. Removal of vapor-phase Hg0 over a V2O5/PG catalyst[J]. Acta Scientiae Circumstantiae,2019,39(7):2369-2376(in Chinese). [17] ZOU X H, CHEN T H, ZHANG P, et al. High catalytic performance of Fe-Ni/palygorskite in the steam reforming of toluene for hydrogen production[J]. Applied Energy,2018,226:827-837. doi: 10.1016/j.apenergy.2018.06.005 [18] ZHU L, WANG J L, RONG S P, et al. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature[J]. Applied Catalysis B: Environmental,2017,211:212-221. doi: 10.1016/j.apcatb.2017.04.025 [19] LIU P, WEI G L, LIANG X L, et al. et al. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance[J]. Applied Clay Science,2018,161:265-273. doi: 10.1016/j.clay.2018.04.032 [20] WANG C, CHEN T H, LIU H B, et al. Promotional catalytic oxidation of airborne formaldehyde over mineral-supported MnO2 at ambient temperature[J]. Applied Clay Science,2019,182:105289-105301. doi: 10.1016/j.clay.2019.105289 [21] 任珺, 刘丽莉, 陶玲, 等. 甘肃地区凹凸棒石的矿物组成分析[J]. 硅酸盐通报, 2013, 32(11):2362-2365.REN Jun, LIU Lili, TAO Lin, et al. Mineral composition analysis of attapulgite from Gansu area[J]. Bulletin of The Chinese Ceramic Society,2013,32(11):2362-2365(in Chinese). [22] 张帅, 刘莉辉, 乔志川, 等. 临泽县杨台洼滩新近系白杨河组凹凸棒石的成因[J]. 矿物学报, 2019, 39(6):690-696.ZHANG Shuai, LIU Lihui, QIAO Zhichuan, et al. Genesis of attapulgite from the Neogene Baiyanghe Formation in the Yangtaiwatan area, Linze County, Gansu Province, China[J]. Acta Mineralogica Sinica,2019,39(6):690-696(in Chinese). [23] ZHANG Z F, WANG W B, KANG Y R, et al. Structure evolution of brick-red palygorskite induced by hydroxylammonium chloride[J]. Powder Technology,2018,327:246-254. doi: 10.1016/j.powtec.2017.12.067 [24] LU Y S, DONG W K, WANG W K, et al. A comparative study of different natural palygorskite clays for fabricating cost-efficient and eco-friendly iron red composite pigments[J]. Applied Clay Science,2019,167:50-59. doi: 10.1016/j.clay.2018.10.008 [25] LIU H B, CHEN T H, CHANG D Y, et al. Characterization and catalytic performance of Fe3Ni8/palygorskite for catalytic cracking of benzene[J]. Applied Clay Science,2013,74:135-140. doi: 10.1016/j.clay.2012.04.005 [26] DING J J, HUANG D J, WANG W B, et al. Effect of removing coloring metal ions from the natural brick-red palygorskite on properties of alginate/palygorskite nanocompo-site film[J]. International Journal of Biological Macromolecules, 2019, 122: 684-694. [27] BROOKS H R, STEPHEN E K, JOHN M R. Dolomite dissolution: An alternative diagenetic pathway for the formation of palygorskite clay[J]. Sedimentology, 2019, 66 (5): 1803-1824. [28] WANG J L, LI J G, JIANG C J, et al. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air[J]. Applied Catalysis B: Environmental,2017,204:147-155. doi: 10.1016/j.apcatb.2016.11.036 [29] LIU Z P, MA R Z, EBINA Y S, et al. Synthesis and delamination of layered manganese oxide nanobelts[J]. Chemistry of Materials,2007,19(26):6504-6512. doi: 10.1021/cm7019203 [30] WANG C, ZOU X H, LIU H B, et al. A highly efficient catalyst of palygorskite-supported manganese oxide for formaldehyde oxidation at ambient and low temperature: Performance, mechanism and reaction kinetics[J]. Applied Surface Science,2019,486:420-430. doi: 10.1016/j.apsusc.2019.04.257 [31] CHU Q X, WANG X F, ZHANG X H, et al. Buckled layers in K0.66Mn2O4·0.28H2O and K0.99Mn3O6·1.25H2O synthesized at high pressure: Implication for the mechanism of layer-to-tunnel transformation in manganese oxides[J]. Inorganic Chemistry,2011,50:2049-2051. doi: 10.1021/ic102282v [32] HSU Y U, CHEN Y C, LIN Y G, et al. Reversible phase transformation of MnO2 nanosheets in an electrochemical capacitor investigated by in situ Raman spectroscopy[J]. Che-mical Communications, 2011, 47: 1252-1254. [33] ZOU X H, MA Z Y, LIU H B, et al. Green synthesis of Ni supported hematite catalysts for syngas production from catalytic cracking of toluene as a model compound of biomass tar[J]. Fuel,2018,217:343-351. doi: 10.1016/j.fuel.2017.12.063 [34] DU X, LI C, ZHAO L, ZHANG J, et al. Promotional removal of HCHO from simulated flue gas over Mn-Fe oxides modified activated coke[J]. Applied Catalysis B: Environmental,2018,232:37-48. doi: 10.1016/j.apcatb.2018.03.034 [35] 张浩, 高青, 韩祥祥, 等. 基于XRF和XRD的热闷渣改性活性炭降解甲醛机理分析[J]. 光谱学与光谱分析, 2020, 40(5):1447-1451.ZHANG Hao, GAO Qin, HAN Xiangxiang, et al. Mechanism analysis of formaldehyde degradation by hot braised slag modified activated carbon based on XRF and XRD[J]. Spectroscopy and Spectral Analysis,2020,40(5):1447-1451(in Chinese). [36] ZHU L, WANG J L, RONG S P, et al. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature[J]. Applied Catalysis B: Environmental,2017,b, 211:212-221. [37] LI J P, ZHANG P, WANG J L, et al. Birnessite-type manganese oxide on granular activated carbon for formaldehyde removal at room temperature[J]. The Journal of Physical Chemistry C,2016,120:24121-24129. doi: 10.1021/acs.jpcc.6b07217 [38] JIA J B, ZHANG P Y, CHEN L. The effect of morphology of α-MnO2 on catalytic decomposition of gaseous ozone[J]. Catalysis Science & Technology,2016,6:5841-5847. [39] WANG J L, YUNUS R, Li J G, et al. In situ synthesis of manganese oxides on polyester fiber for formaldehyde decomposition at room temperature[J]. Applied Surface Science,2015,357:787-794. doi: 10.1016/j.apsusc.2015.09.109 [40] LIU F, RONG S P, ZHANG P Y, et al. One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature[J]. Applied Catalysis B: Environmental,2018,235:158-167. doi: 10.1016/j.apcatb.2018.04.078 [41] MIAO L, WANG J L, ZHANGP Y. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde[J]. Applied Surface Science,2018,446:441-453. [42] GUO J H, LIN C X, JIANG C J, et al. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature[J]. Applied Surface Science,2019,475:237-255. doi: 10.1016/j.apsusc.2018.12.238 [43] GUAN S N, LI W Z, MA J R, et al. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation[J]. Journal of Industrial and Engineering Chemistry,2018,66:126-140. doi: 10.1016/j.jiec.2018.05.023 [44] WANG M, ZHANG L X, HUANG W M, et al. The catalytic oxidation removal of low-concentration formaldehyde at high space velocity by partially crystallized mesoporous MnOx[J]. Chemical Engineering Journal,2017,320:667-676. doi: 10.1016/j.cej.2017.03.098 [45] YOSHIKA S. Oxidative decomposition of formaldehyde by metal oxides at room temperature[J]. Atmospheric Environment,2002,36(35):5543-5547. doi: 10.1016/S1352-2310(02)00670-2 -






 下载:
下载: