Preparation and visible light catalytic performance of WO3/g-C3N4 composite photocatalyst
-
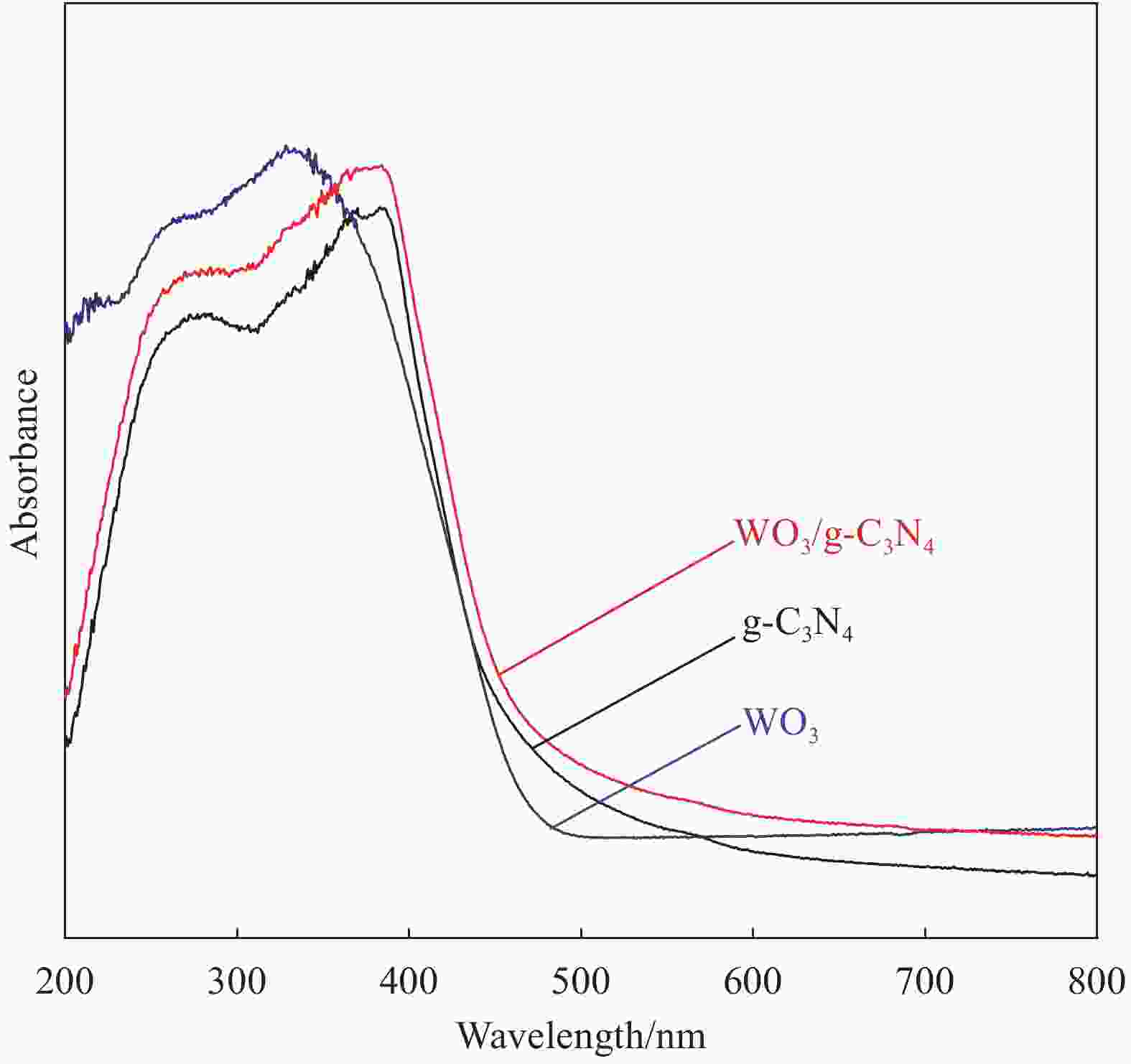
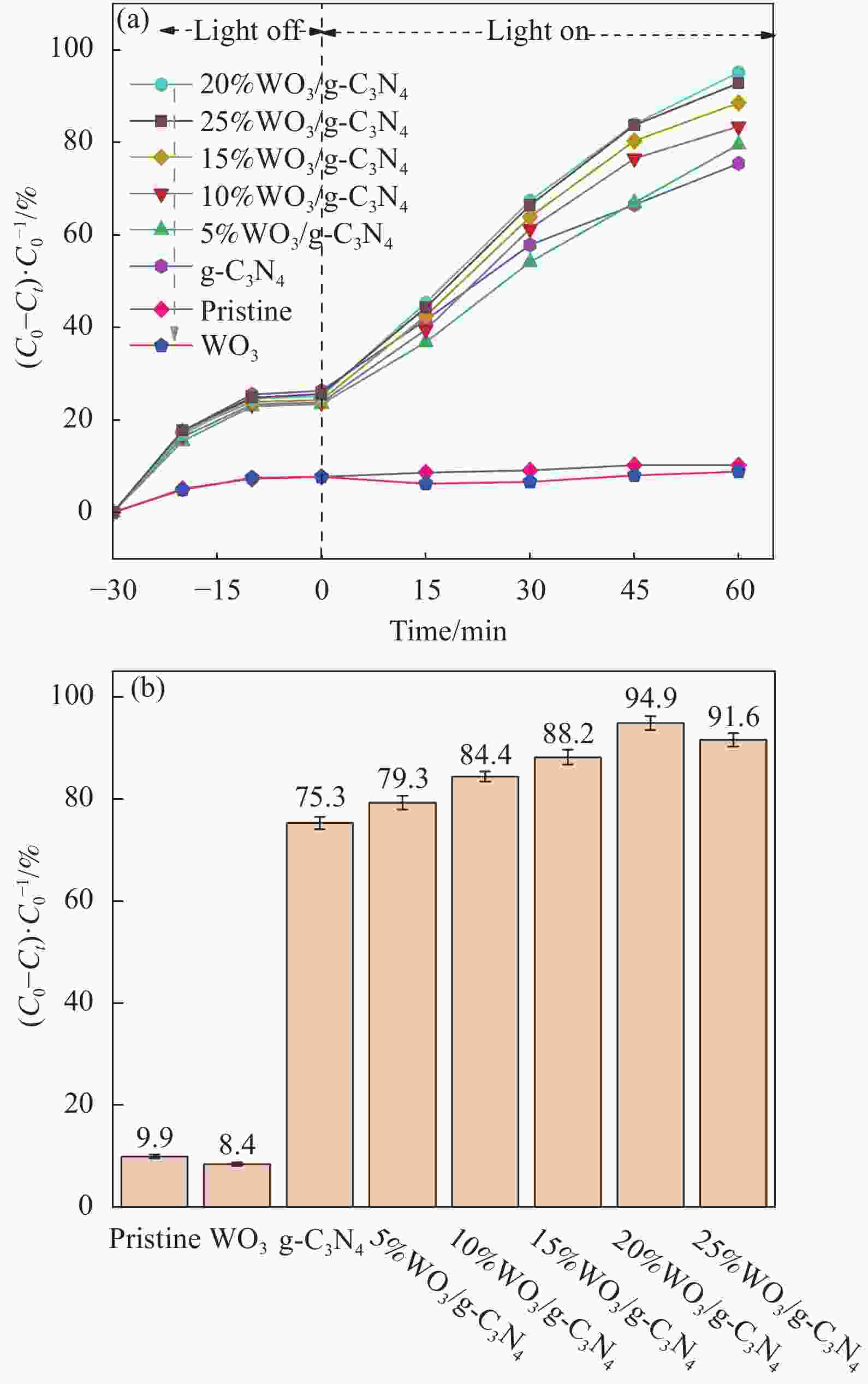
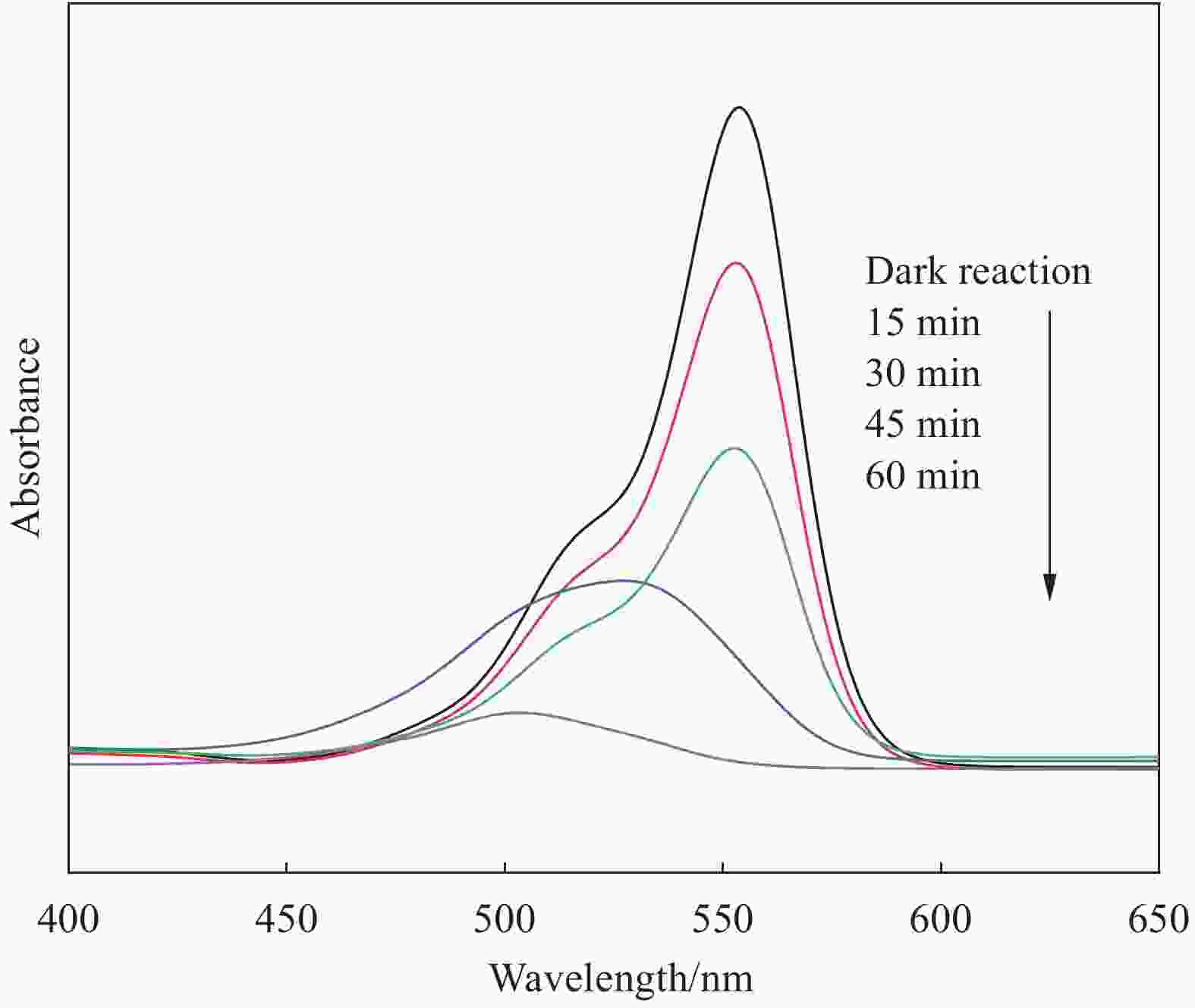
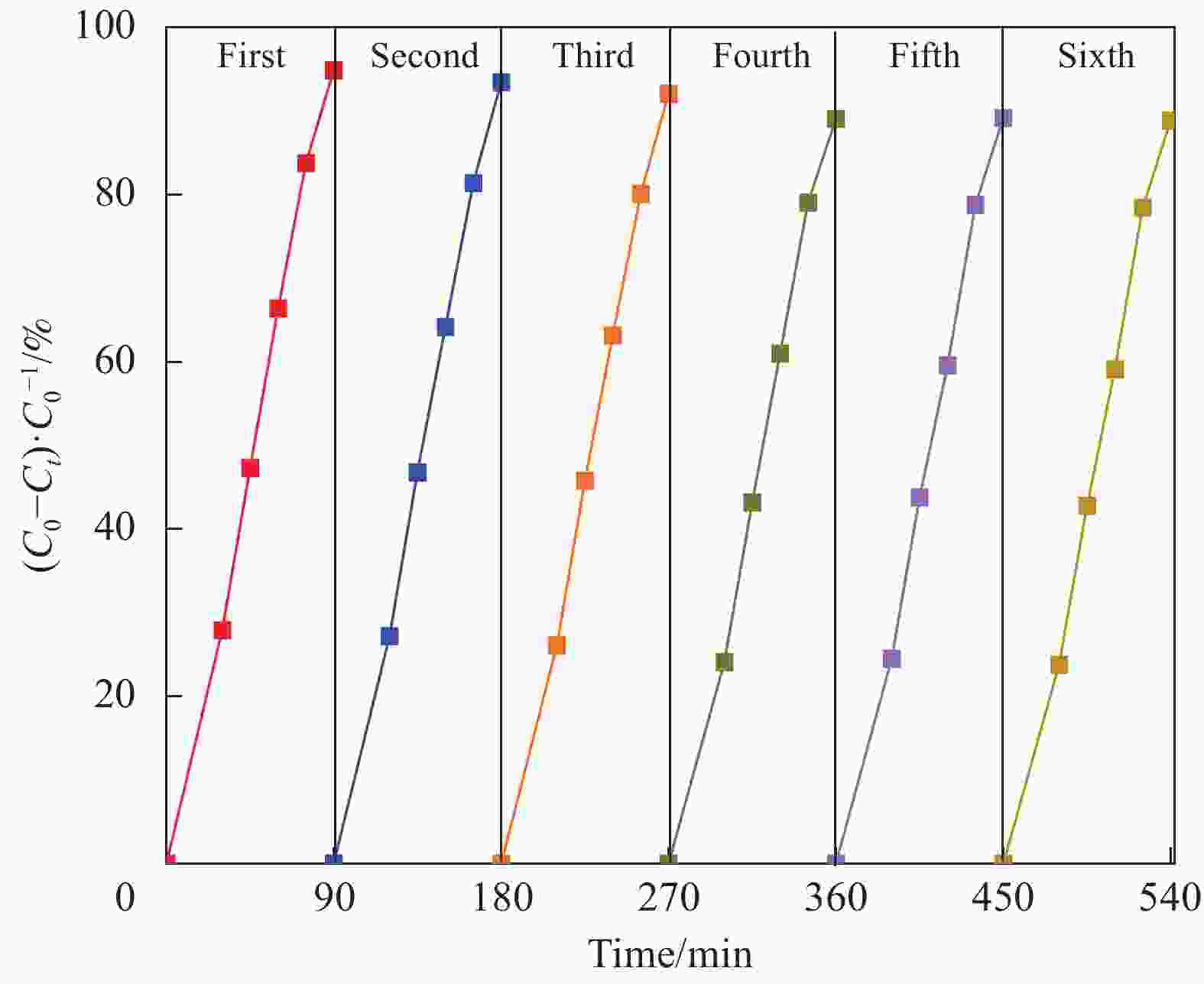
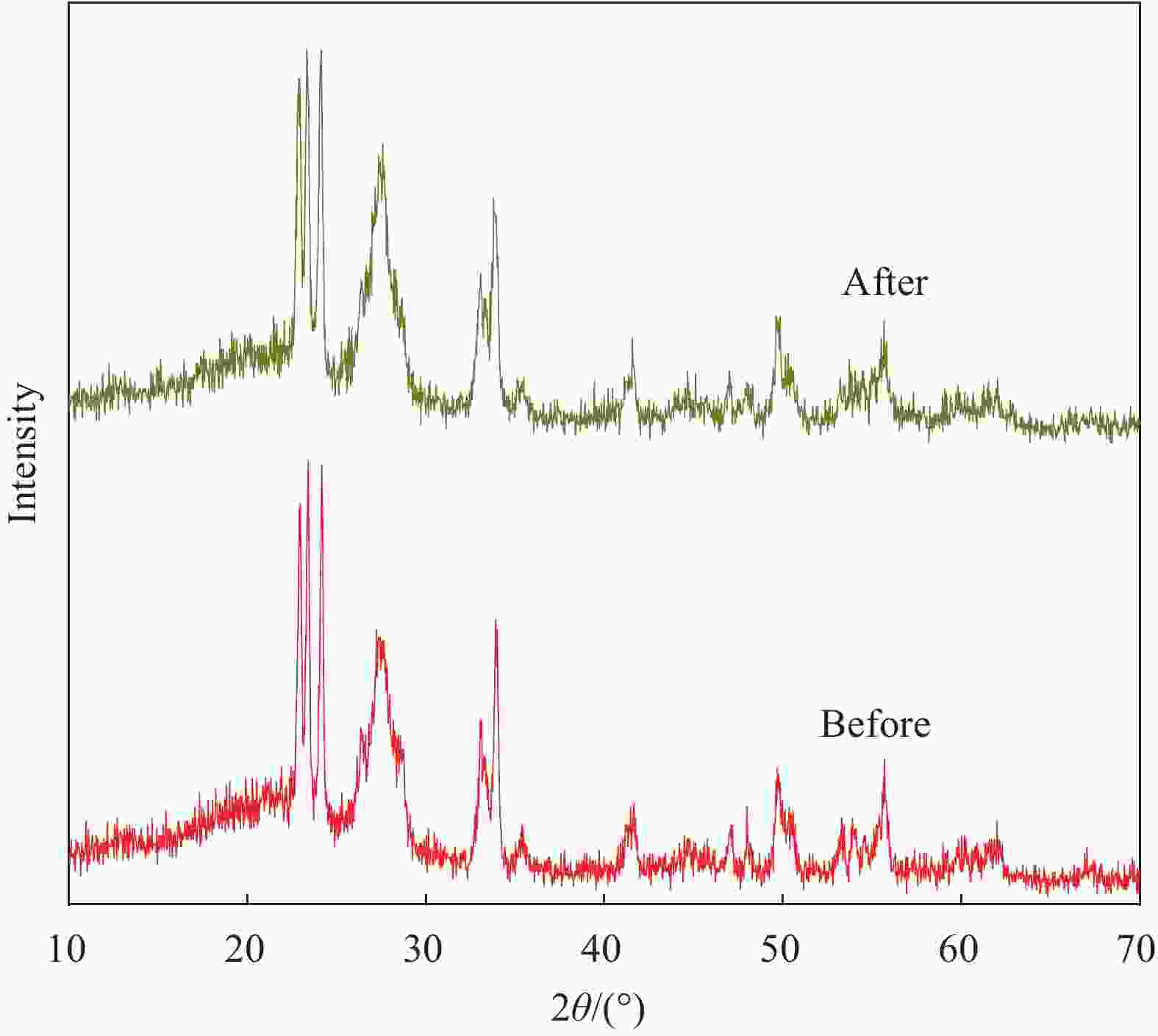
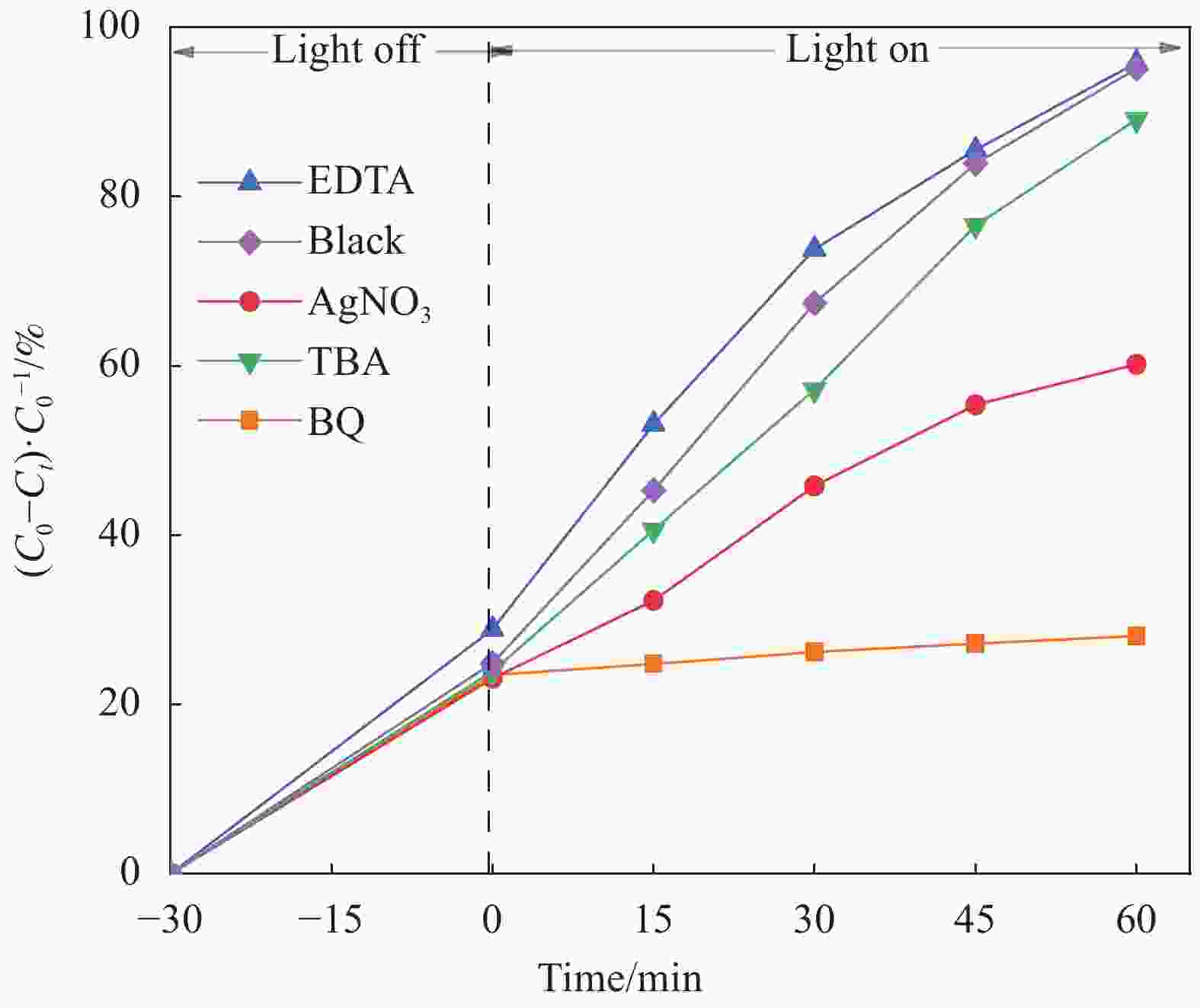
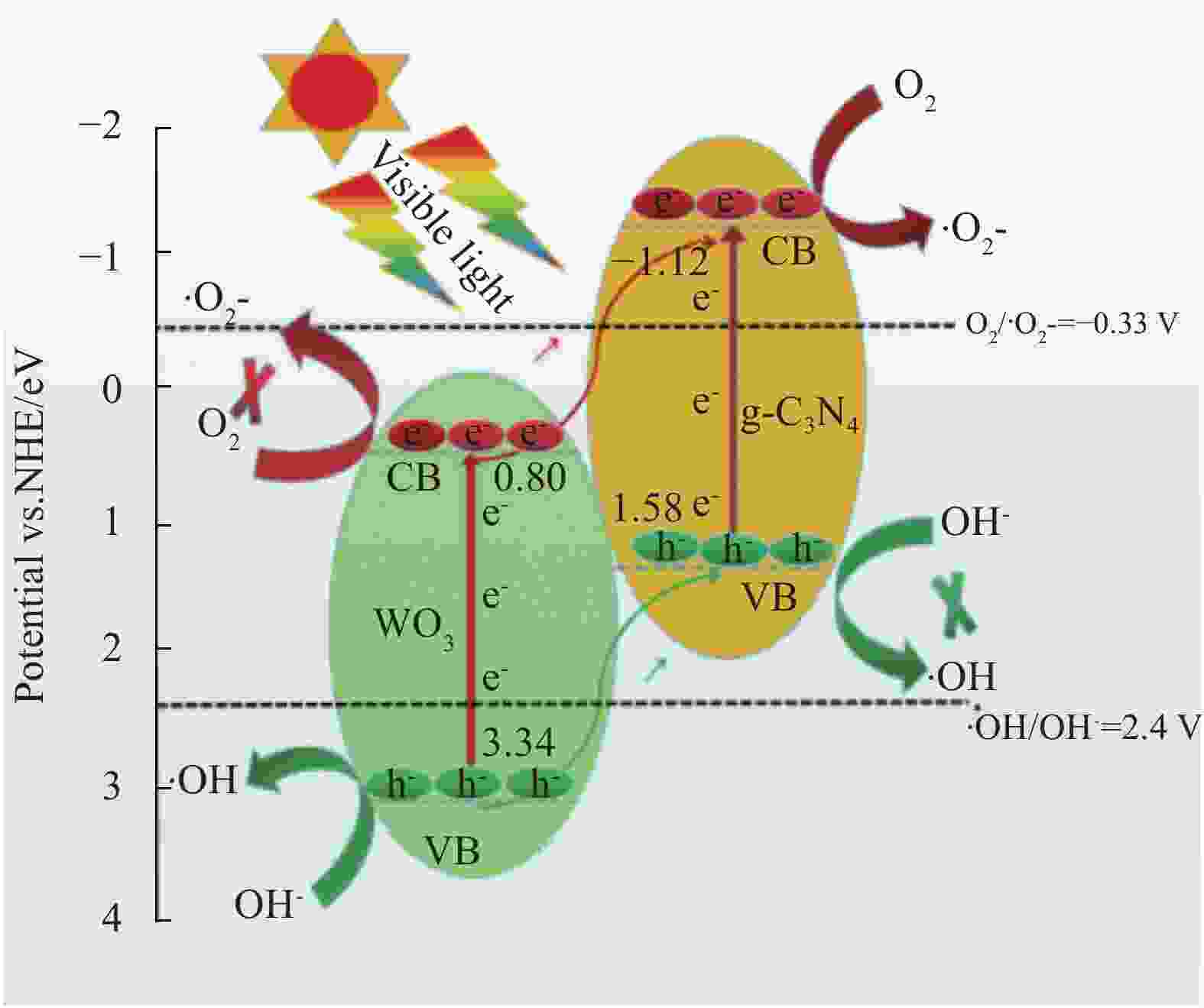
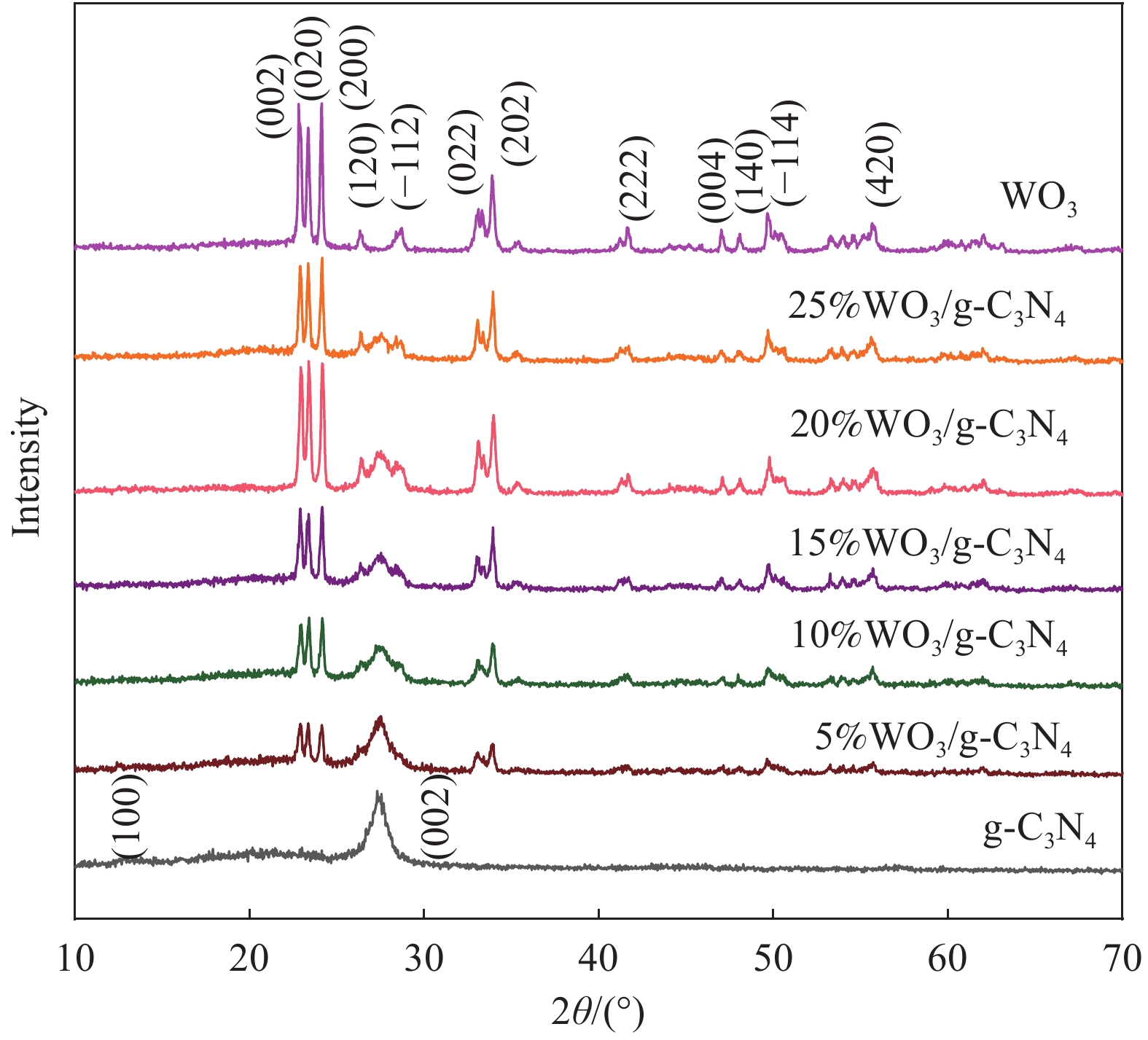
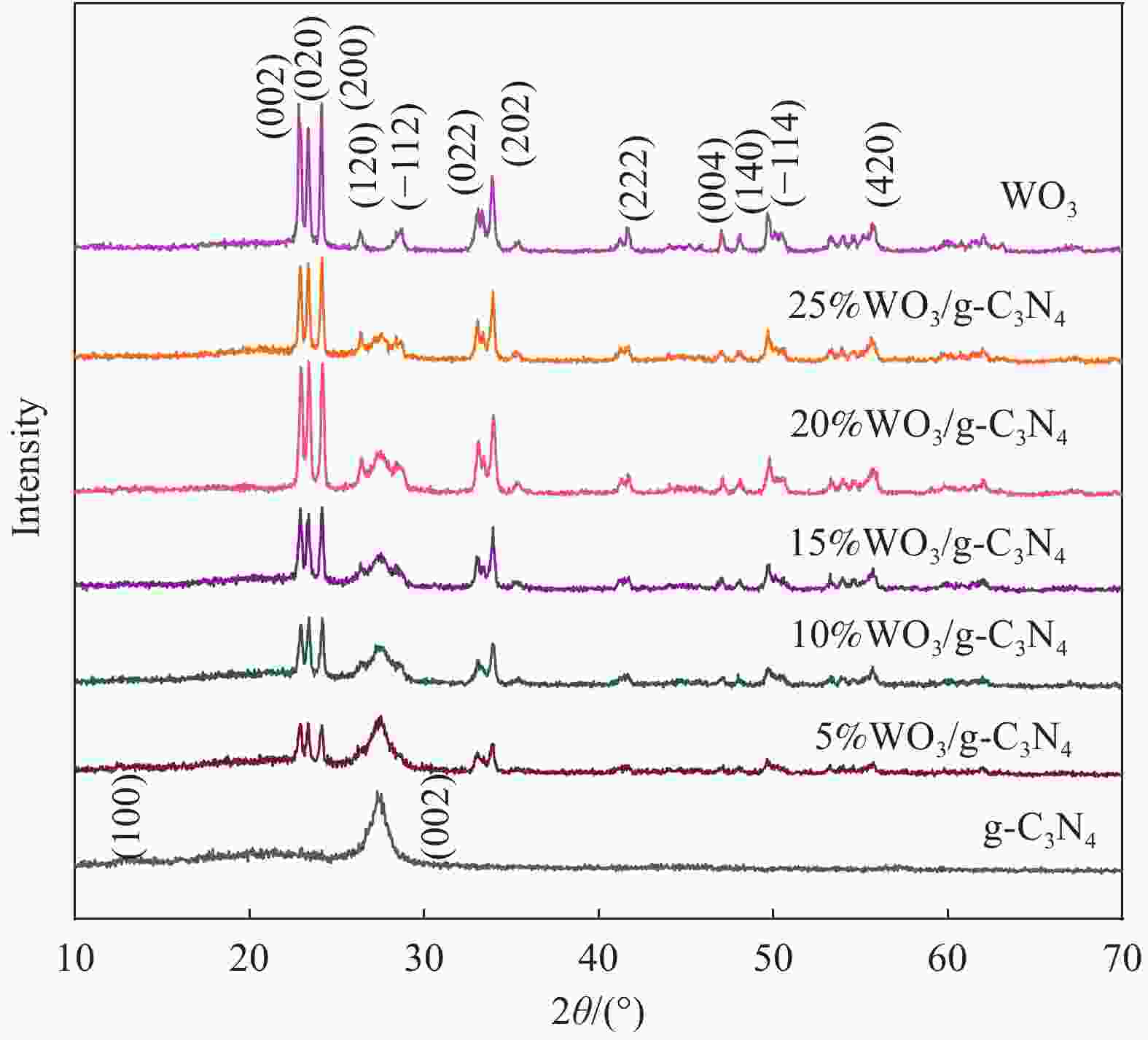
摘要: 将自制层状石墨相氮化碳(g-C3N4)和WO3纳米片均匀混合,经煅烧制备WO3/g-C3N4复合半导体。利用XRD、SEM、TEM、UV-Vis DRS和PL对其进行表征。结果表明,g-C3N4呈现类石墨烯状片层结构,WO3为纳米片状结构,且分散在g-C3N4表面;与WO3复合后,UV-Vis吸收边发生了红移,拓宽了g-C3N4对可见光的响应。以罗丹明B(RhB)为模拟污染物,考察WO3/g-C3N4的光催化降解性能。WO3/g-C3N4质量比为1∶5时,表现出最佳的光催化活性,可见光照60 min后,RhB降解率可达到94.9%。光催化剂具有良好的稳定性,重复使用6次后,RhB的降解率依然达到88.9%。光催化机制研究表明,超氧自由基(·O2−)是光催化降解RhB的主要活性物种。
-
关键词:
- WO3/g-C3N4 /
- 复合半导体 /
- 罗丹明B /
- 光催化降解 /
- 纳米片
Abstract: The WO3/graphite phase carbon nitride (g-C3N4) composites were prepared by mixing self-made layered g-C3N4 with WO3 nanoplates and afterward calcination process, and were characterized by XRD, SEM, TEM, UV-Vis DRS and PL. The results show that g-C3N4 presents graphene-like layered structure, and WO3 indicates nanoplate structure, and scatters on the surfaces of g-C3N4. After compounding with WO3, the absorption edge of UV-Vis spectrum shifts to red, which widens the response of g-C3N4 to visible light. The photocatalytic degradation properties of WO3/g-C3N4 were examined using rodamine B (RhB) as a simulated pollutant. When the mass ratio of WO3/g-C3N4 is 1∶5, the best photocatalytic activity is obtained. After 60 min of visible light irradiation, the degradation rate of RhB can reach 94.9%. The photocatalyst shows good stability, as the photodegradation rate of RhB reaches 88.9% after repeated use of the same photocatalyst for 6 times. The study of photocatalytic mechanism shows that superoxide radical (·O2−) is the main active species for photocatalytic degradation of RhB.-
Key words:
- WO3/g-C3N4 /
- composite semiconductor /
- RhB /
- photocatalytic degradation /
- nanoplate
-
表 1 石墨相氮化碳(g-C3N4)复合材料质量比参数
Table 1. Mass ratio parameters of graphite phases carbon nitride (g-C3N4) composites
Sample Mass ratio of WO3 to g-C3N4 WO3/g 5%WO3/g-C3N4 5∶100 0.005 10%WO3/g-C3N4 10∶100 0.010 15%WO3/g-C3N4 15∶100 0.015 20%WO3/g-C3N4 20∶100 0.020 25%WO3/g-C3N4 25∶100 0.025 -
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature,1972,238:37-38. doi: 10.1038/238037a0 [2] LIU Y, JI H W, ZHOU D F, et al. Controllable synthesis and photocatalytic activity of TiO2 /LaFeO3 micro-nanofibers[J]. Chemical Journal of Chinese Universities,2014,35(1):19-25. [3] WATANBE A, KOTAKE K, KAMATE K, et al. Photoelectrochemical behavior of self-assembled Ag/Co plasmonic nanostructures capped with TiO2[J]. The Journal of Physical Chemistry Letters,2014,5(1):25-29. doi: 10.1021/jz402320p [4] ZHANG L Z, ZHENG Z, YU J C. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity[J]. Chemistry of Materials,2003,15(11):2280-2286. doi: 10.1021/cm0340781 [5] WANG M, JU P, LI J J, et al. Facile synthesis of MoS2/g-C3N4/GO ternary heterojunction with enhanced photocatalytic activity for water splitting[J]. Sustainable Chemistry,2017,5(9):7878-7886. [6] SU Q, SUN J, WANG J Q, et al. Urea-derived graphitic carbon nitride as an efficient hetero geneous catalyst for CO2 conversion into cyclic carbonates[J]. Catalysis Science & Technology,2014,4:1556. doi: 10.1039/c3cy00921a [7] WANG X C, MAEDA K, THOMAS A. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials,2009,8:76-80. doi: 10.1038/nmat2317 [8] IQBAL W, DONG C Y, XING M Y, et al. Eco-friendly one-potsyn thesis of well-adorned mesoporous g-C3N4 with efficiently enhanced visible light photocatalytic activity[J]. Catalysis Science & Technology,2017,7:1726. doi: 10.1039/C7CY00286F [9] TONDA S, KUMAR S, KANDULA S, et al. Fe-doped and mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight[J]. Journal of Materials Chemistry A,2014,2(19):6772. doi: 10.1039/c3ta15358d [10] FU J, TIAN Y, CHANG B, et al. BiOBr-carbon nitride heterojunctions: Synthesis enhanced activity and photocatalytic mechanism[J]. Journal of Materials Chemistry A,2012,22(39):21159-21166. doi: 10.1039/c2jm34778d [11] ADHIKARI S, HE Y K. Heterojunction C3N4/MoO3 microcomposite for highly efficient photocatalytic oxidation of Rhodamine B[J]. Applied Surface Science,2020,511(5):145595. [12] CHENG C, SHI J W, HU Y C, et al. WO3/g-C3N4 composites: One-pot preparation and enhanced photocatalytic H2 production under visible light irradiation[J]. Nanotechnology,2017,28(16):164002. doi: 10.1088/1361-6528/aa651a [13] XU H T, XIAO R, HUANG J R, et al. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production[J]. Chinese Journal of Catalysis,2020,42(1):107-114. [14] CHEN S, HU Y, MENG S, FU X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4/WO3[J]. Applied Catalysis B: Environmental,2014,150:564-573. [15] KE D N, LIU H J, PENG T Y, et al. Preparation and photo catalytic activity of WO3/TiO2 nanocomposite particles[J]. Materials Letters,2008,62(3):447-450. doi: 10.1016/j.matlet.2007.05.060 [16] NOGUEIRA H I S, CAVALEIRO A M V, ROCHA J, et al. Synthesis and characterization of tungsten trioxide powders prepared from tungstic acids[J]. Materials Research Bulletin,2004,39(4):683-693. [17] BALAZSI C, PFEIFER J. Development of tungsten oxide hydrate phasesduring precipitation, room temperature ripening and hydrothermal treatment[J]. Solid State Ionics,2002,151(1):353-358. [18] JANAKY C, RAJESHWAR K, TACCONI N R, et al. Tungsten-based oxide semiconductors for solar hydrogen generation[J]. Catalysis Today,2013,199(1):53-64. [19] DONG F, LI Y H, WANG Z Y, et al. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation[J]. Applied Surface Science,2015,358(1):393-403. [20] SZILAGYI I M, FORIZS B, ROSSELE O, et al. WO3 photocatalysts: Influence of structure and composition[J]. Journal of Catalysis,2012,294:119-127. doi: 10.1016/j.jcat.2012.07.013 [21] ZHOU X J, SHAO C L, YANG S, et al. Heterojunction of g-C3N4/BiOI immobilized on flexible electrospun poly-acrylonitrile nanofibers: Facile preparation and enhanced visible photocatalytic Activity for floating photocatalysis[J]. ACS Sustainable Chemistry & Engineering,2018,6(2):2316-2323. [22] SINGH J, ARORA A, BASU S. Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light[J]. Journal of Alloys and Compounds,2019,808:151734. doi: 10.1016/j.jallcom.2019.151734 [23] HUANG L Y, XU H, LI Y P, et al. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity[J]. Dalton Transactions,2013,42(24):8606-8616. doi: 10.1039/c3dt00115f [24] LIU H, JIN Z T, XU Z Z, et al. Fabrication of ZnIn2 S4-g-C3N4 sheet-on-sheet nanocomposites for efficient visible-light photocatalytic H2-evolution and degradation of organic pollutants[J]. RSC Advances,2015,5(119):97951-97961. doi: 10.1039/C5RA17028A [25] HAI B T, BUI T H, SCHINDRA K R, et al. H2O2-assisted photocatalysis for removal of natural organic matter using nanosheet C3N4-WO3 composite under visible light and the hybrid system with ultrafiltration[J]. Chemical Engineering Journal,2020,399:125733. doi: 10.1016/j.cej.2020.125733 [26] ZHU B C, XIA P F, HO W K, et al. Isoelectric point and adsorption activity of porous g-C3N4[J]. Applied Surface Science,2015,344:188-189. doi: 10.1016/j.apsusc.2015.03.086 [27] LI X X, WAN T, QIU J Y, et al. In-situ photocalorimetry-fluorescence spectroscopy studies of RhB photocatalysis over Z-scheme g-C3N4@Ag@Ag3PO4 nanocomposites: A pseudo-zero-order rather than a first-order process[J]. Applied Catalysis: Environmental,2017,217:591-602. doi: 10.1016/j.apcatb.2017.05.086 -





 下载:
下载: