Preparation of phosphorus-doped graphitic carbon nitride and its application in lithium-sulfur batteries
-
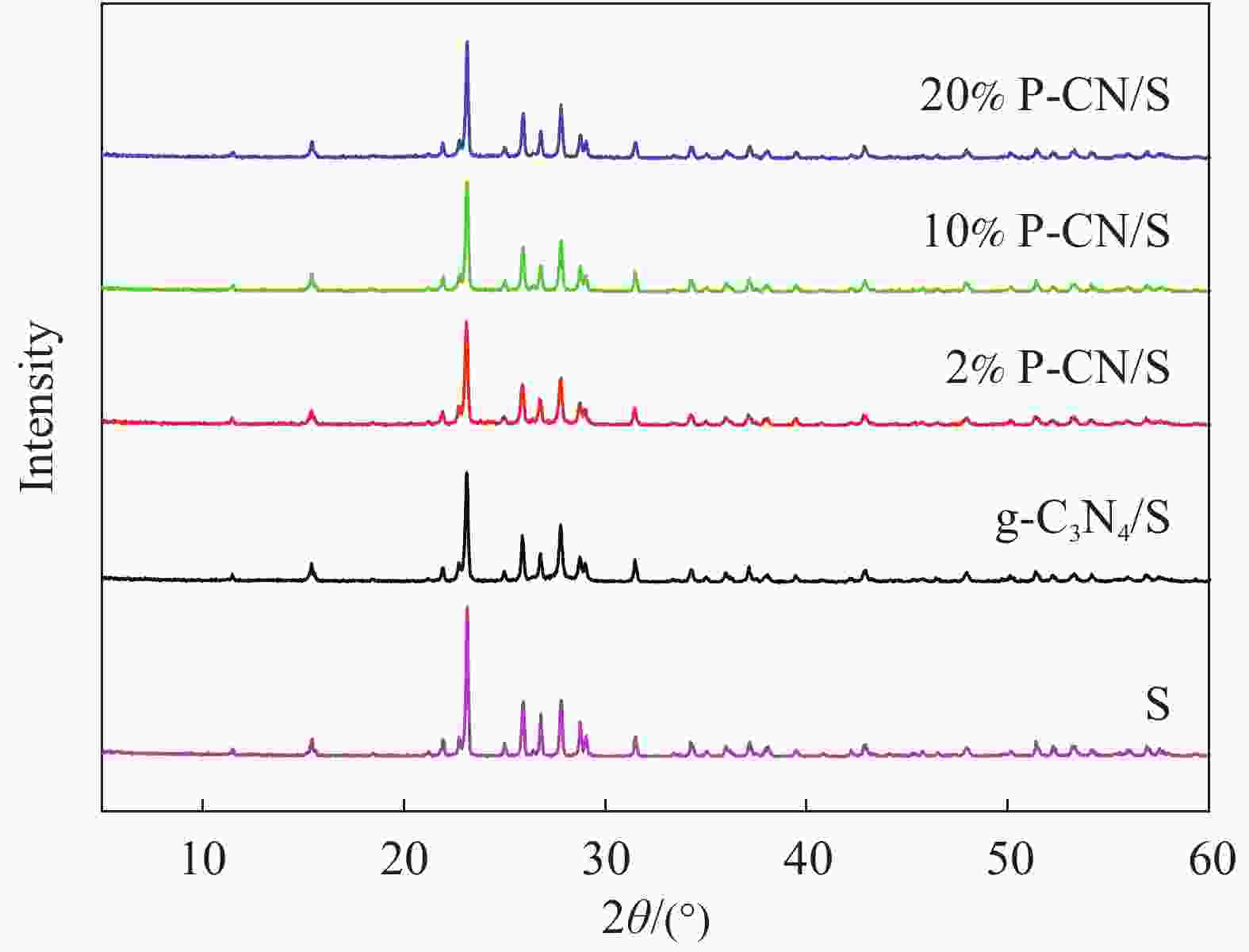
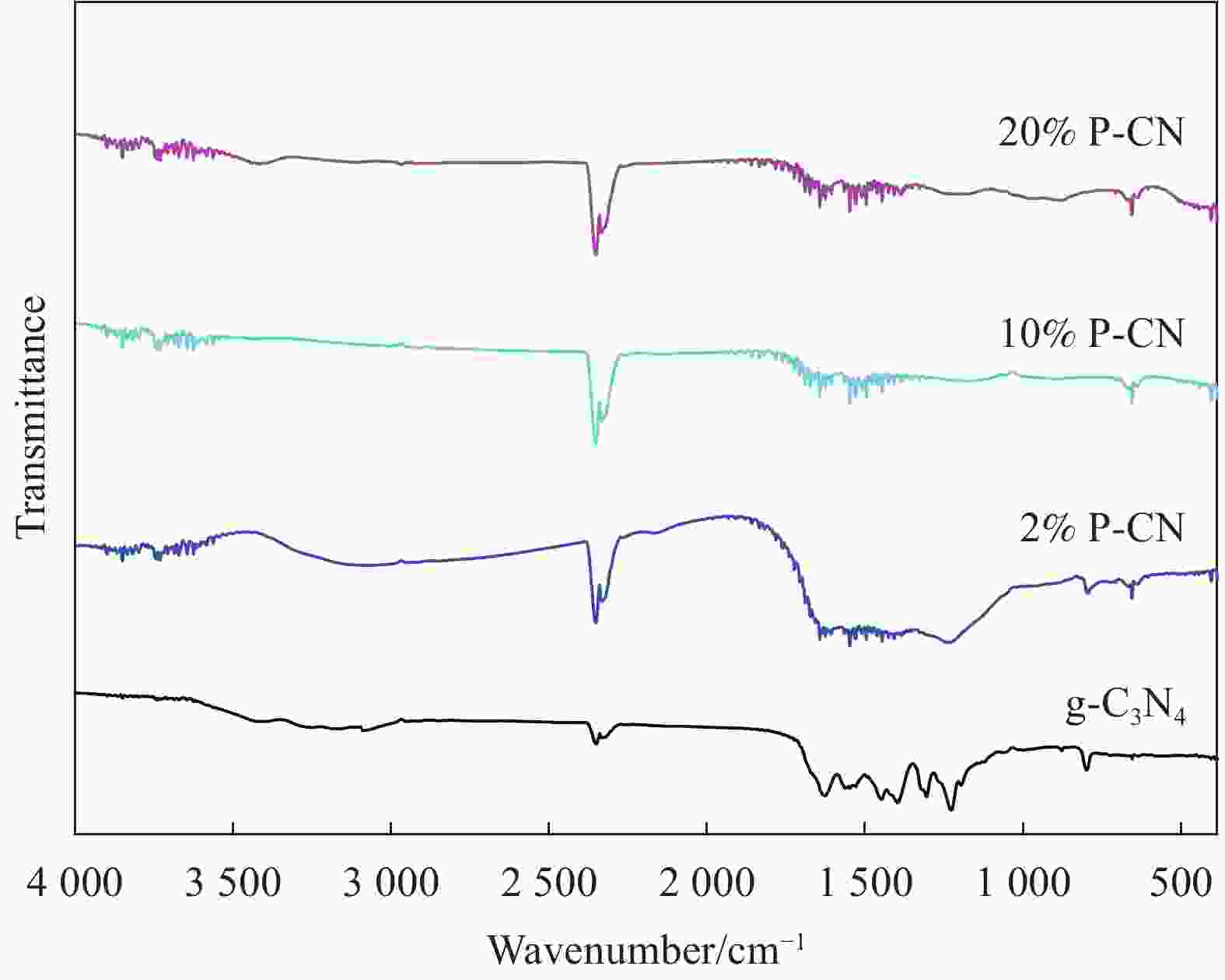
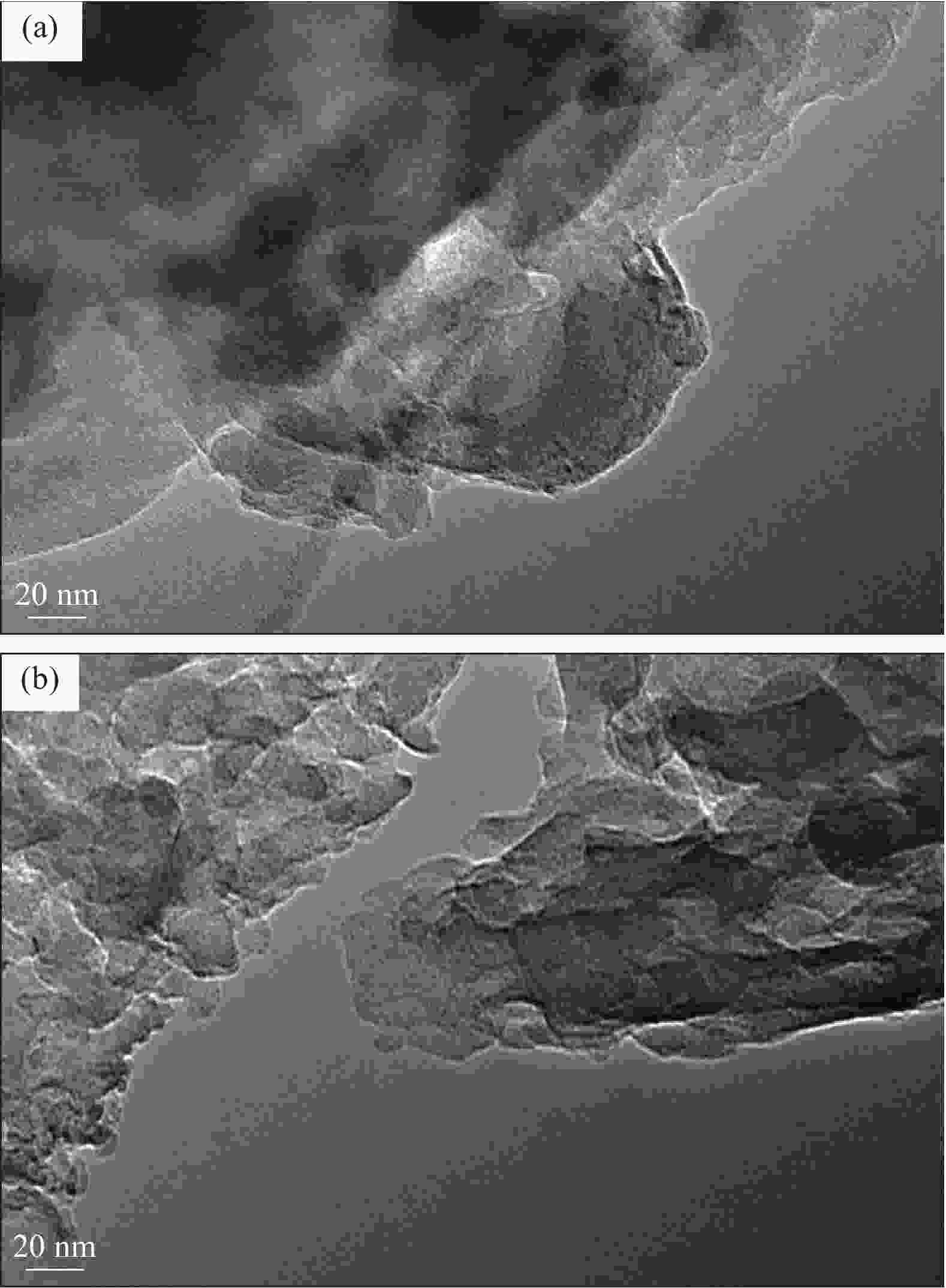
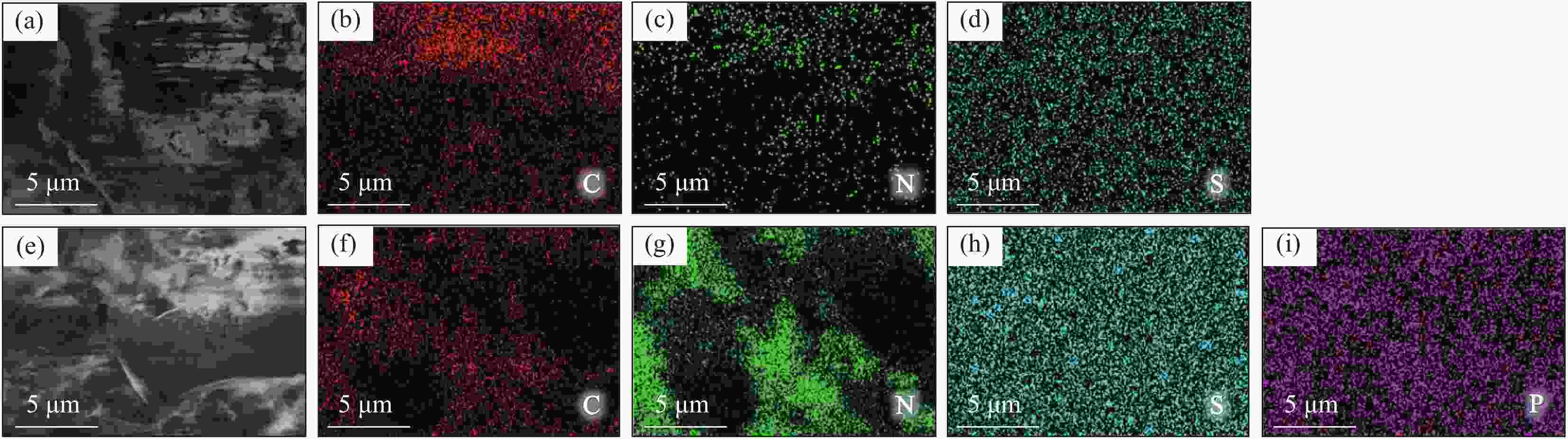
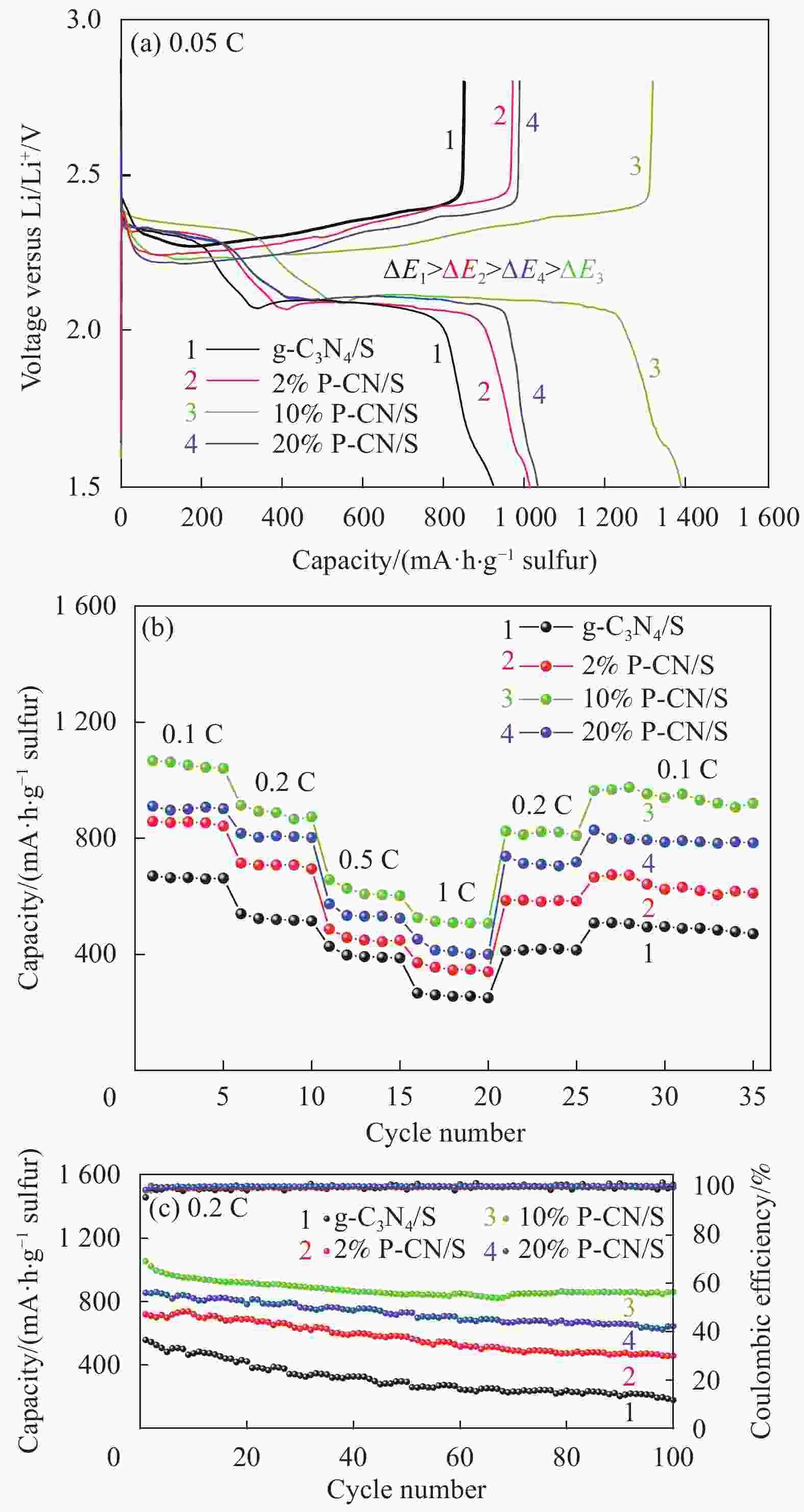
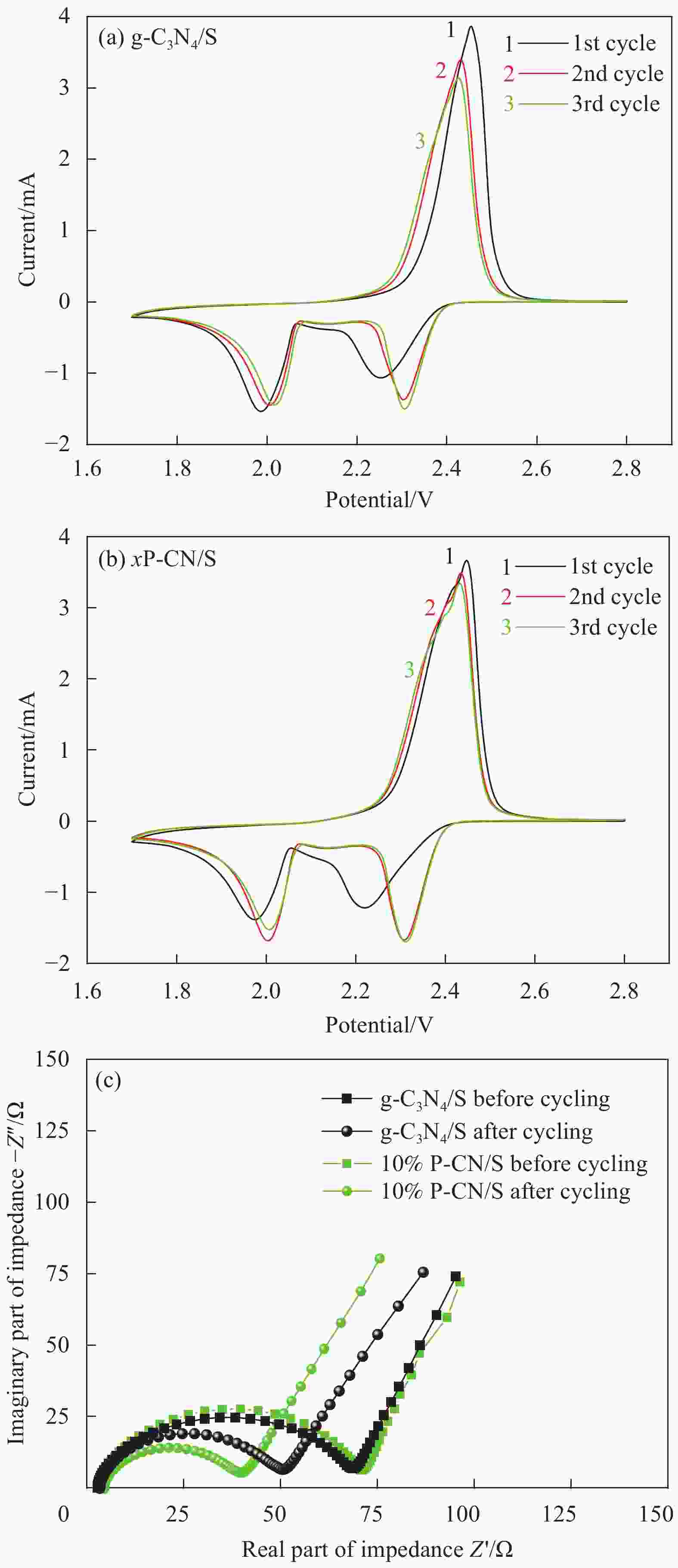
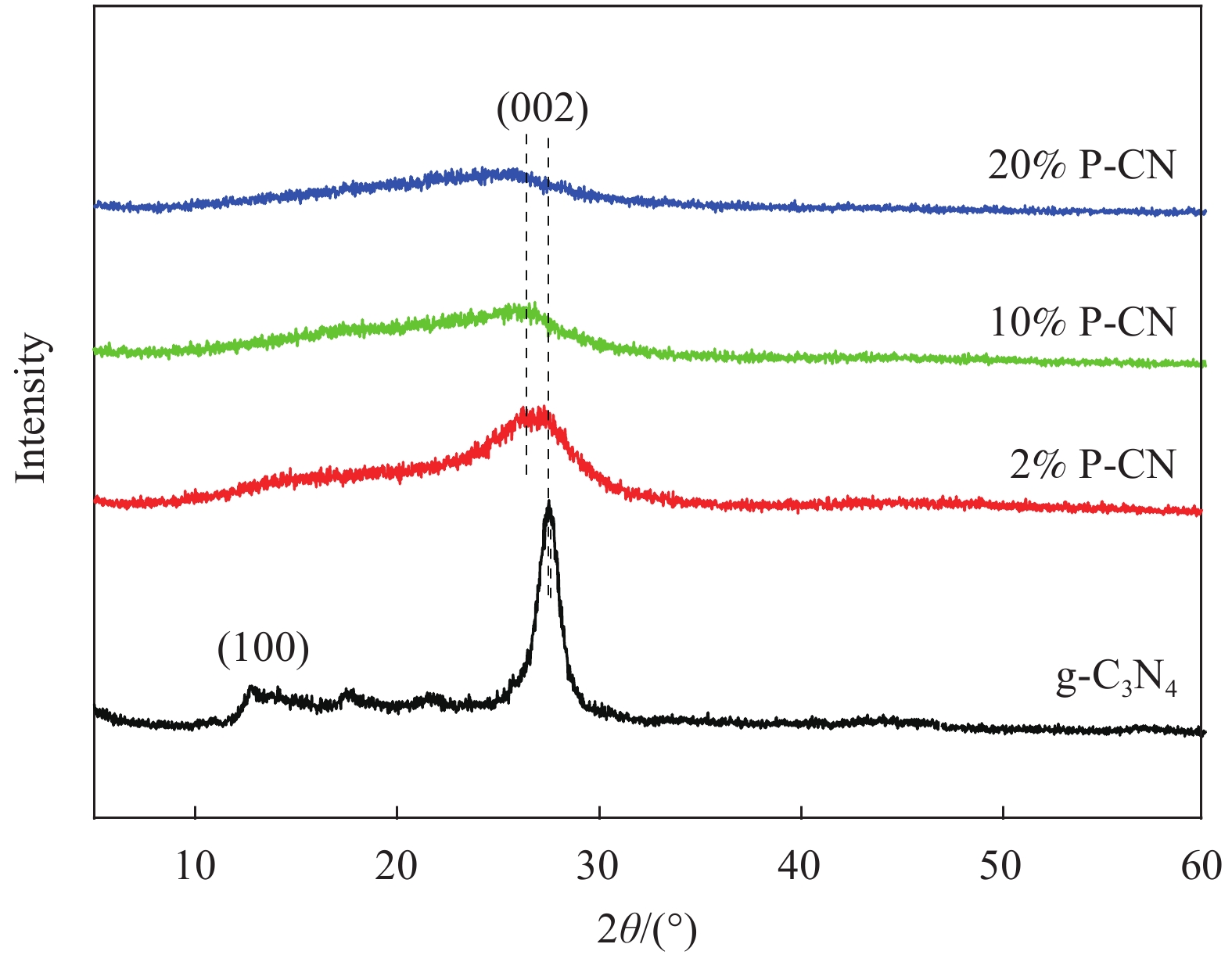
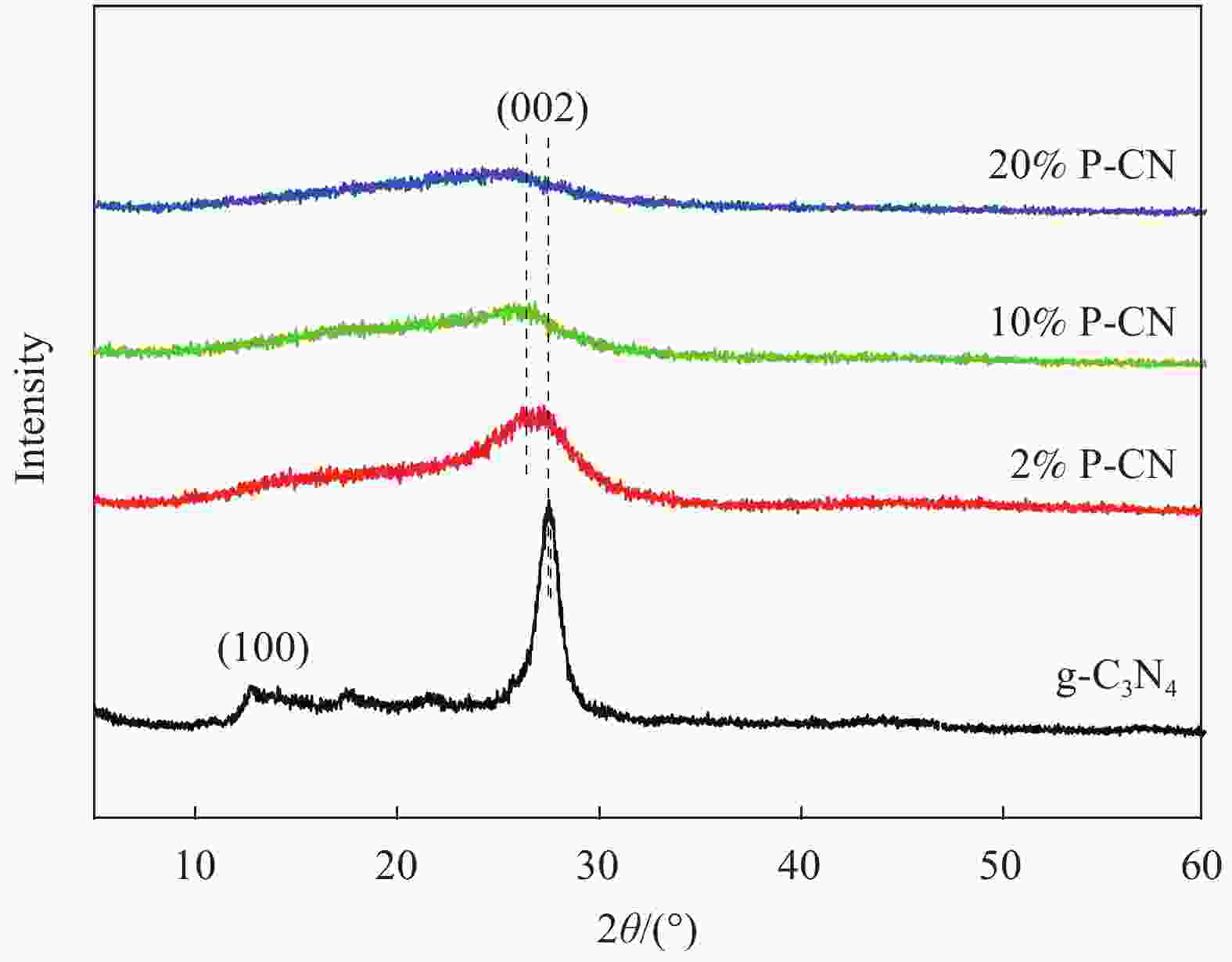
摘要: 通过热缩聚合成法,采用尿素为原料,制备石墨相氮化碳(g-C3N4),以磷酸氢二胺作为磷源,制备不同磷含量的磷掺杂g-C3N4 (xP-CN),研究磷掺杂对xP-CN的微观结构、形貌及xP-CN/S复合材料作为锂硫电池正极材料电化学性能的影响。研究表明,磷掺杂后xP-CN的层间距增大,导电性提高,比表面积变大,10% P-CN的比表面积最大达到101.741 m2·g−1。10% P-CN/S复合材料在0.05 C (1 C=1675 mA·h·g−1)下首次放电比容量达到1383.8 mA·h·g−1,在0.2 C下循环100次后可逆比容量为860.0 mA·h·g−1,而g-C3N4/S复合材料比容量仅为178.3 mA·h·g−1;10% P-CN/S复合材料经过倍率测试后比容量可以回复到0.2 C时的93.6%,表现出良好的循环性能和倍率性能。
-
关键词:
- 锂硫电池 /
- 石墨相氮化碳(g-C3N4) /
- 电极材料 /
- 磷元素 /
- 掺杂
Abstract: Graphite-phase carbon nitride (g-C3N4) was prepared by heat shrinkage polymerization method using urea as raw material, and phosphorus-doped g-C3N4 with different phosphorus content (xP-CN) was prepared by using hydrogen phosphate diamine as a phosphorus source. The effect of doping on the microstructure, morphology, and electrochemical performance of xP-CN/S composites as cathode materials for lithium-sulfur batteries was studied. The studies show that the layer spacing of xP-CN increases after phosphorus doping, the electrical conductivity increases, and the specific surface area becomes larger. The specific surface area of the 10% P-CN reaches 101.741 m2·g−1. The initial discharge specific capacity of the 10% P-CN/S composite at 0.05 C (1 C=1675 mA·h·g−1) reaches 1383.8 mA·h·g−1. The reversible specific capacity after 100 cycles at 0.2 C is 860.0 mA·h·g−1, the reversible specific capacity of g-C3N4/S composite is only 178.3 mA·h·g−1; The specific capacity of 10% P-CN/S composite can be restored to 93.6% at 0.2 C after the rate test, showing good cycle performance and rate performance.-
Key words:
- lithium-sulfur batteries /
- graphitic carbon nitride (g-C3N4) /
- electrode materials /
- phosphorus /
- doping
-
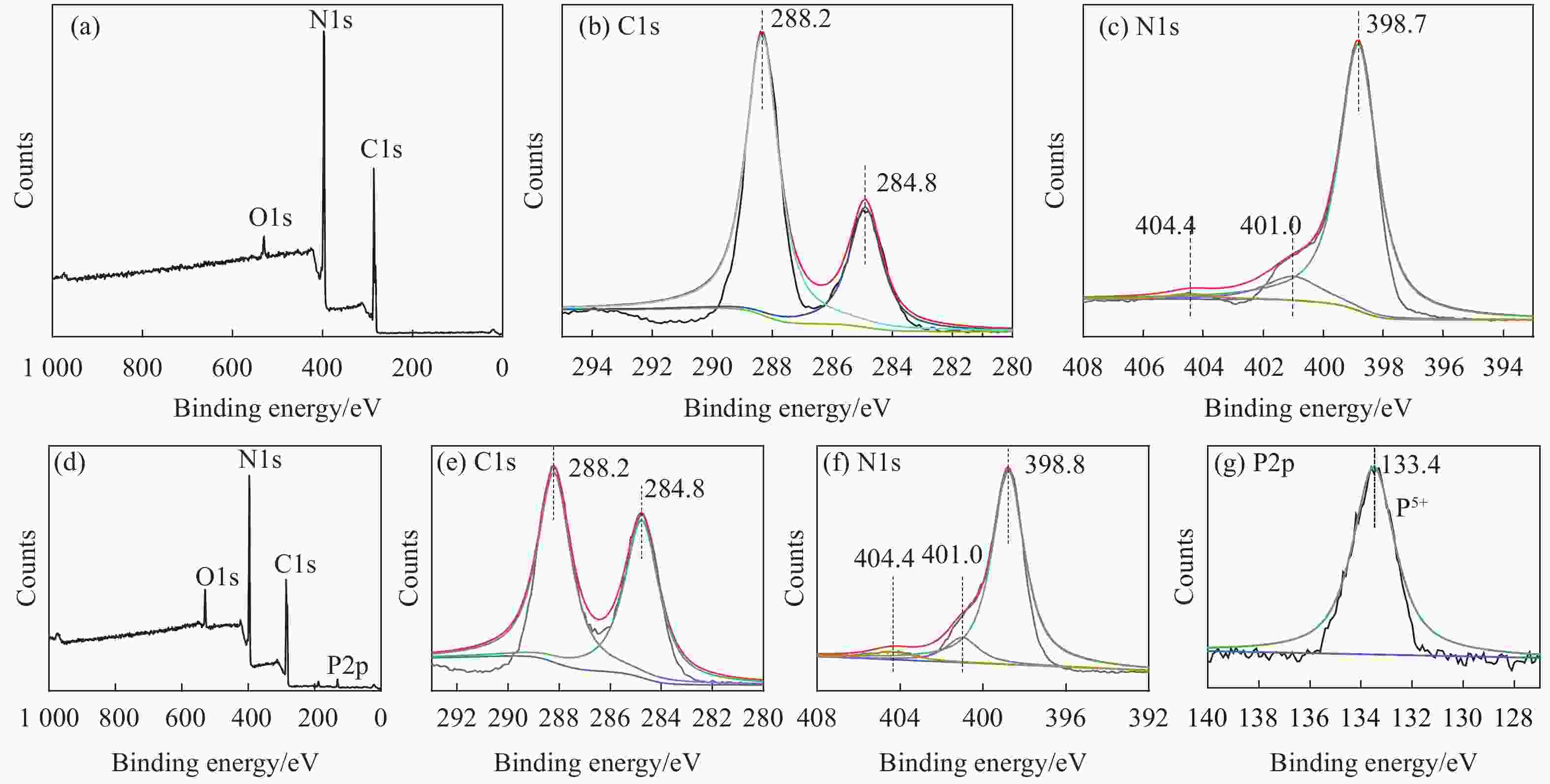
表 1 g-C3N4和xP-CN中P元素含量
Table 1. Element contents of P in g-C3N4 and xP-CN
Line Type P/wt% g-C3N4 K 0 2% P-CN K 1.63 10% P-CN K 8.07 20% P-CN K 19.02 -
[1] PANG Q. Advanced electrodes and electrolytes for long-lived and high-energy-density lithium-sulfur batteries[D]. Waterloo: The University of Waterloo, 2017. [2] YANG X, YU Y, YAN N, et al. 1D oriented cross-linking hierarchical porous carbon fibers as a sulfur immobilizer for high performance lithium-sulfur batteries[J]. Journal of Materials Chemistry A,2016,4(16):5965-5972. doi: 10.1039/C6TA01060A [3] LI L, JACOBS R, GAO P, et al. Origins of large voltage hysteresis in high-energy-density metal fluoride lithium-ion battery conversion electrodes[J]. Journal of the American Chemical Society,2016,138(8):2838-2848. doi: 10.1021/jacs.6b00061 [4] DUNN B, KAMATH H, TARASCON JM. Electrical energy storage for the grid: A battery of choices[J]. Science,2011,334(6058):928-935. doi: 10.1126/science.1212741 [5] NATARAJAN A, STEPHAN A M, CHAN C H, et al. Electrochemical studies on composite gel polymer electrolytes for lithium sulfur-batteries[J]. Journalof Applied Polymer Science,2017,134(11):44594-44602. [6] LI H, MA S, CAI H, et al. Ultra-thin Fe3C nanosheets promote the adsorption and conversion of polysulfides in lithium-sulfur batteries[J]. Energy Storage Materials,2019,18:338-348. [7] LIU J D, CHEN H, CHEN W D, et al. New insight into the “shuttle mechanism” of rechargeable lithium-sulfur batteries[J]. ChemElectroChem,2019,6(10):2782-2787. doi: 10.1002/celc.201900420 [8] 官亦标, 李万隆, 谢潇怡, 等. TiO2/CNTs复合材料涂覆隔膜的制备及在锂硫电池中的应用[J]. 高等学校化学学报, 2019, 40(3):536-541.GUAN Yibiao, LI Wanlong, XIE Xiaoyi, et al. Preparation of TiO2/CNTs composite coated separator and its application in Li-S battery[J]. Chemical Journal of Chinese Universities,2019,40(3):536-541(in Chinese). [9] YUAN Y, TAN G, WEN J, et al. Encapsulating various sulfur allotropes within graphene nanocages for long-lasting lithium storage[J]. Advanced Functional Materials,2018,28(38):1706443. [10] ZHOU Y, ZHOU C, LI Q, et al. Enabling prominent high-rate and cycle performances in one lithium-sulfur battery: Designing permselective gateways for Li+ transportation in holey-CNT/S cathodes[J]. Advanced Materials,2015,27(25):3774-3781. doi: 10.1002/adma.201501082 [11] YU Q, LU Y, LUO R, et al. In situ formation of copper-based hosts embedded within 3D N-doped hierarchically porous carbon networks for ultralong cycle lithium-sulfur batteries[J]. Advanced Functional Materials,2018,28(39):1804520. doi: 10.1002/adfm.201804520 [12] SONG J, YU Z, GORDIN M L, et al. Advanced sulfur cathode enabled by highly crumpled nitrogen-doped graphene sheets for high-energy-density lithium-sulfur batteries[J]. Nano Letters,2016,16(2):864-870. doi: 10.1021/acs.nanolett.5b03217 [13] SU D, CORTIE M, WANG G. Fabrication of N-doped graphene-carbon nanotube hybrids from prussian blue for lithium-sulfur batteries[J]. Advanced Energy Materials,2017,7(8):1602014-1602026. doi: 10.1002/aenm.201602014 [14] PANG Q, NAZAR L F. Long-life and high-areal-capacity Li-S batteries enabled by a light-weight polar host with intrinsic polysulfide adsorption[J]. ACS Nano,2016,10(4):4111-4118. doi: 10.1021/acsnano.5b07347 [15] MENG Z, XIE Y, CAI T, et al. Graphene-like g-C3N4 nanosheets/sulfur as cathode for lithium-sulfur battery[J]. Electrochimica Acta,2016,210:829-836. doi: 10.1016/j.electacta.2016.06.032 [16] 游然, 陈露, 张永平. 原子掺杂改善石墨相氮化碳光催化性能的研究进展[J]. 物理化学进展, 2017, 6(2):84-96. doi: 10.12677/JAPC.2017.62011YOU Ran, CHEN Lu, ZHANG Yongping. Research progress on improving the photocatalysis of graphite-C3N4via O, S and P doping[J]. Journal of Advances in Physical Chemistry,2017,6(2):84-96(in Chinese). doi: 10.12677/JAPC.2017.62011 [17] 石磊. 氮化碳基光催化材料的制备及性能[D]. 哈尔滨: 哈尔滨工业大学, 2016.SHI Lei. Synthesis and property of carbon nitride basedphotocatalytic materials[D]. Harbin: Harbin Institute of Technology, 2016(in Chinese). [18] ZHANG Y, MORI T, YE J, et al. Phosphorus-doped carbon nitride solid: enhanced electrical conductivity and photocurrent generation[J]. Journal of the American Chemical Society,2010,132(18):6294-6295. doi: 10.1021/ja101749y [19] GUO S, DENG Z, LI M, et al. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution[J]. Angewandte Chemie International Edition,2016,55(5):1830-1834. doi: 10.1002/anie.201508505 [20] WANG X, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nature Materials,2008,8:76-80. [21] DUAN J, CHEN S, JARONIEC M, et al. Porous C3N4 nanolayers@N-graphene films as catalyst electrodes for highly efficient hydrogen evolution[J]. ACS Nano,2015,9(1):931-940. doi: 10.1021/nn506701x [22] RONG X, QIU F, RONG J, et al. Synthesis of porous g-C3N4/La and enhanced photocatalytic activity for the degradation of phenol under visible light irradiation[J]. Journal of Solid State Chemistry,2015,230:126-134. doi: 10.1016/j.jssc.2015.07.003 [23] GOETTMANN F, FISCHER A, ANTONIETTI M, et al. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for friedel-crafts reaction of benzene[J]. Angewandte Chemie International Edition,2006,45(27):4467-4471. doi: 10.1002/anie.200600412 [24] 徐赞, 于薛刚, 单妍, 等. 一步法合成磷掺杂石墨相氮化碳及其光催化性能[J]. 无机材料学报, 2017, 32(2):155-162. doi: 10.15541/jim20160256XU Zan, YU Xuegang, SHAN Yan, et al. One-pot synthesis of phosphorus doped g-C3N4 with enhanced visible-light photocatalytic activity[J]. Journal of Inorganic Materials,2017,32(2):155-162(in Chinese). doi: 10.15541/jim20160256 [25] YAN S C, LI Z S, ZOU Z G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine[J]. Langmuir,2009,25(17):10397-10401. [26] ZHANG L, CHEN X, GUAN J, et al. Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity[J]. Materials Research Bulletin,2013,48(9):3485-3491. doi: 10.1016/j.materresbull.2013.05.040 [27] HU S, MA L, YOU J, et al. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts Co-doped with iron and phosphorus[J]. Applied Surface Science,2014,311:164-171. doi: 10.1016/j.apsusc.2014.05.036 [28] HU S, MA L, XIE Y, et al. Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions[J]. Dalton Transactions,2015,44(48):20889-20897. doi: 10.1039/C5DT04035C [29] DAKE L S, BAER D R, FRIEDRICH D M. Auger parameter measurements of phosphorus compounds for characterization of phosphazenes[J]. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films,1989,7(3):1634-1638. [30] MA X, LV Y, XU J, et al. A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: A first-principles study[J]. The Journal of Physical Chemistry C,2012,116(44):23485-23493. doi: 10.1021/jp308334x [31] 刘树和, 刘彬, 赵焱, 等. 香蒲活性炭用于锂硫电池正极材料[J]. 材料导报, 2020, 34(8):8014-8019.LIU Shuhe, LIU Bin, ZHAO Yan, et al. Activated carbon from cattail used for the cathode material of lithium-sulfur batteries[J]. Materials Reports,2020,34(8):8014-8019(in Chinese). [32] PARK J, YU S H, SUNG Y E. Design of structural and functional nanomaterials for lithium-sulfur batteries[J]. Nano Today,2018,18:35-64. doi: 10.1016/j.nantod.2017.12.010 [33] HOU Y, LI J, WEN Z, et al. N-doped graphene/porous g-C3N4 nanosheets supported layered-MoS2 hybrid as robust anode materials for lithium-ion batteries[J]. Nano Energy,2014,8:157-164. doi: 10.1016/j.nanoen.2014.06.003 [34] 杨绍斌, 郭鑫瑶, 董伟, 等. 盐酸活化对石墨相氮化碳(g-C3N4)结构和g-C3N4/S锂硫电池正极复合材料性能的影响[J]. 复合材料学报, 2019, 36(1):254-260.YANG Shaobin, GUO Xinyao, DONG Wei, et al. Effect of hydrochloric acid activation on properties of graphite carbon nitride (g-C3N4) structure and g-C3N4/S cathode composites for lithium-sulfur batteries[J]. Acta Materiae Compositae Sinica,2019,36(1):254-260(in Chinese). [35] MA T Y, RAN J, DAI S, et al. Phosphorus-doped graphitic carbon nitrides grown in situ on carbon-fiber paper: Flexible and reversible oxygen electrodes[J]. Angewandte Chemie International Edition,2015,54(15):4646-4650. doi: 10.1002/anie.201411125 [36] ZHOU Y, ZHANG L, LIU J, et al. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light[J]. Journal of Materials Chemistry A,2015,3(7):3862-3867. doi: 10.1039/C4TA05292G [37] ZHANG X, YANG S, CHEN Y, et al. Effect of phosphorous-doped graphitic carbon nitride on electrochemical properties of lithium-sulfur battery[J]. Ionics,2020,26(4):5491-5501. doi: 10.1007/s11581-020-03728-w [38] ZHENG G, YANG Y, CHA J J, et al. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries[J]. Nano Letters,2011,11(10):4462-4467. doi: 10.1021/nl2027684 [39] LI L, CHEN L, MUKHERJEE S, et al. Phosphorene as a polysulfide immobilizer and catalyst in high-performance lithium-sulfur batteries[J]. Advanced Materials,2017,29(2):1602734. doi: 10.1002/adma.201602734 [40] ZUO P, HUA J, HE M, et al. Facilitating the redox reaction of polysulfides by an electrocatalytic layer-modified separator for lithium-sulfur batteries[J]. Journal of Materials Chemistry A,2017,5(22):10936-10945. doi: 10.1039/C7TA02245J [41] 杨绍斌, 夏英凯, 刘凤霞, 等. 活化剂对焦煤基多孔碳制备的影响及在锂硫电池中的应用[J]. 复合材料学报, 2020, 37(3):716-723.YANG Shaobin, XIA Yingkai, LIU Fengxia, et al. Effect of activator on preparation of coal-based porous carbon and its application in lithium-sulfur battery[J]. Acta Materiae Compositae Sinica,2020,37(3):716-723(in Chinese). -





 下载:
下载: