Preparation of recyclable BiOBr/graphene hydrogel composite and its photodegradation of sodium butyl xanthate
-
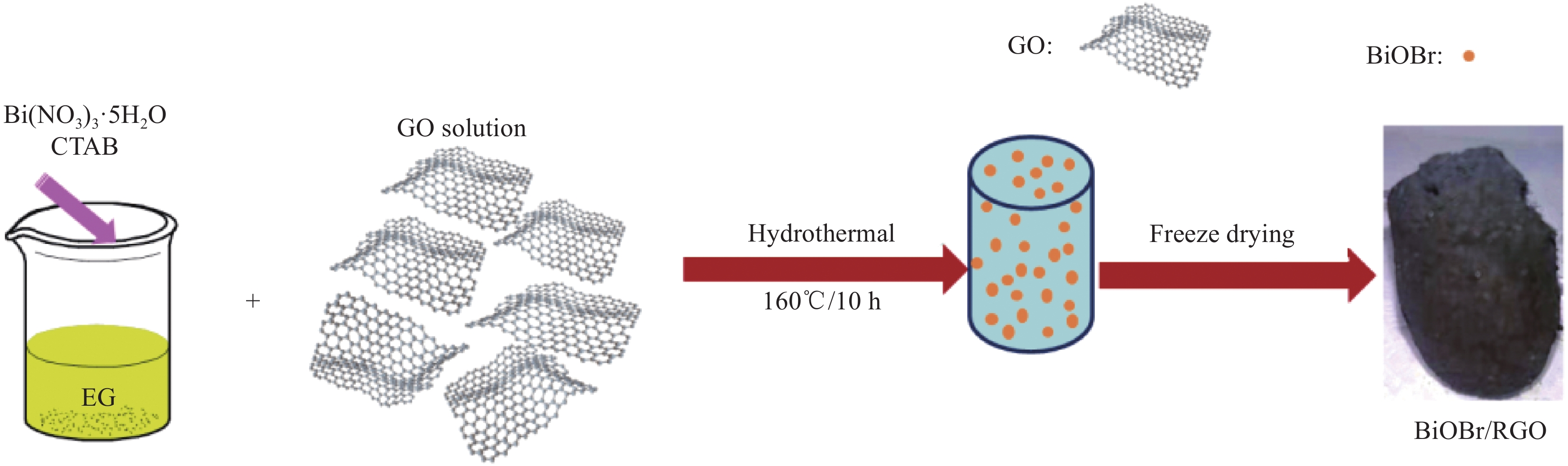
摘要: 本文利用水热法合成BiOBr/石墨烯(BiOBr/RGO)水凝胶复合材料,采用XRD、SEM等手段表征复合材料的组成、形貌特征,并探究了BiOBr/RGO水凝胶复合材料对正丁基钠黄药的降解性能。结果表明,成功制备出有利于回收利用的三维宏观BiOBr/RGO水凝胶复合材料;50 mL浓度为25 mg/L的正丁基钠黄药溶液,降解时间为85 min时,10 mg BiOBr/RGO水凝胶复合材料(BiOBr质量分数为92wt%)对黄药的降解率可达96.69%,而纯BiOBr降解率仅为44.84%。总之,RGO的引入可以提升BiOBr的光催化性能,且宏观材料有利于回收再利用。Abstract: The BiOBr/graphene (BiOBr/RGO) hydrogel composites were prepared via hydrothermal method in this study. The composition and morphology of materials were characterized by XRD and SEM. Photocatalytic properties of the BiOBr/RGO hydrogel composites on sodium n-butyl xanthate were systematically investigated, respectively. The results show that three dimensional macroscopical BiOBr/RGO hydrogel composites are successfully prepared, which is beneficial to recycling. When initial concentration of 50 mL sodium n-butyl xanthate is 25 mg·L−1, degradation time is 85 min, and the dosage of photocatalyst is 10 mg. The degradation efficiency of the BiOBr/RGO hydrogel composite (mass fraction of BiOBr is 92wt%) for sodium n-butyl xanthate can reach 96.69%, and that of BiOBr is merely 44.84%. In all, the introduction of RGO can improve the photocatalytic performance of BiOBr, and macro material is favorable for recycling.
-
Key words:
- BiOBr /
- graphene hydrogel composite /
- photocatalysis /
- sodium butyl xanthate /
- recyclable
-
图 8 BiOBr和BiOBr/RGO水凝胶复合材料对正丁基钠黄药(SBX)的吸附性能对比(SBX溶液体积为50 mL,10 mg样品)
Figure 8. Comparison of adsorption properties of BiOBr and BiOBr/RGO hydrogel composites for sodium n-butyl xanthate (SBX) (Volume of SBX is 50 mL, the dosages of all samples is 10 mg
C0—Initial concentration of SBX solution (25 mg/L)
图 9 BiOBr和BiOBr/RGO水凝胶复合材料在300 W氙灯下对SBX光降解性能(a)及光降解动力学(b)(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)
Figure 9. Photocatalytic activities under 300 W xenon lamp (a) and kinetic curves of photocatalytic degradation (b) for SBX of BiOBr and BiOBr/RGO hydrogel composites (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)
k—First-order kinetics constant
图 12 在300 W氙灯下不同捕获剂对BiOBr/RGO水凝胶复合材料光催化性能的影响(C0为 25 mg/L,SBX体积为50 mL,10 mg样品)
Figure 12. Effect of photodegradation on BiOBr/RGO hydrogel composite in presence of different scavengers under 300 W xenon lamp irradiation (C0 is 25 mg/L, volume of SBX is 50 mL, the dosage of all samples is 10 mg)
EDTA-2Na—Ethylenediaminetetraacetic acid disodium salt
表 1 BiOBr/RGO水凝胶复合材料的名称及含量
Table 1. Name and content of BiOBr/RGO hydrogel composites
Composite name Mass fraction of BiOBr/wt% Mass fraction of RGO/wt% 98wt%BiOBr/RGO 98 2 95wt%BiOBr/RGO 95 5 92wt%BiOBr/RGO 92 8 90wt%BiOBr/RGO 90 10 -
[1] AMROLLAHI A, MASSINAEI M, MOGHADDAM A Z. Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles[J]. Minerals Engineering,2019,134:142-155. doi: 10.1016/j.mineng.2019.01.031 [2] KIANINIA Y, KHALESI M R, SEYEDHAKIMI A, et al. Flotation of mercury from the tailings of the Agh-Darreh gold processing plant, Iran[J]. Journal of the Southern African Institute of Mining & Metallurgy,2017,117(1):83-88. [3] BOUJEMAA D, YASSINE T, RACHID H, et al. Recovery of residual silver-bearing minerals from low-grade tailings by froth flotation: The case of zgounder mine, morocco[J]. Minerals,2018,8(7):273. doi: 10.3390/min8070273 [4] GRANO S R, JOHNSON N W, RALSTON J. Control of the solution interaction of metabisulphite and ethyl xanthate in the flotation of the Hilton ore of Mount Isa Mines Limited, Australia[J]. Minerals Engineering,1997,10(1):17-39. doi: 10.1016/S0892-6875(96)00129-X [5] HIDALGO P, GUTZ I G R. Determination of low concentrations of the flotation reagent ethyl xanthate by sampled DC polarography and flow injection with amperometric detection[J]. Talanta,2001,54(2):403-409. doi: 10.1016/S0039-9140(01)00311-3 [6] VEZIROGLU S, OBERMANN A L, ULLRICH M, et al. Photodeposition of Au nanoclusters for enhanced photocatalytic dye degradation over TiO2 thin film[J]. ACS Applied Materials & Interfaces,2020,12(13):14983-14992. [7] BHATKHANDE D S, PANGARKAR V G, BEENACKERS A A C M. Photocatalytic degradation for environmental applications: A review[J]. Journal of Chemical Technology & Biotechnology,2002,77(1):102-116. [8] TURCHI C S, OLLIS D F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack[J]. Journal of Catalysis,1990,122(1):178-192. doi: 10.1016/0021-9517(90)90269-P [9] LIU Z Y, MIAO Y E, LIU M k, et al. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance[J]. Journal of Colloid and Interface Science,2014,424:49-55. doi: 10.1016/j.jcis.2014.03.009 [10] 李健, 闫龙, 潘盼盼, 等. 活性炭负载ZnS/TiO2光催化剂的制备及其性能研究[J]. 非金属矿, 2019, 42(2):76-79. doi: 10.3969/j.issn.1000-8098.2019.02.021LI J, YAN L, PAN P P, et al. Preparation and properties of ZnS/TiO2 photocatalyst supported by activated carbon[J]. Non-Metallic Mines,2019,42(2):76-79(in Chinese). doi: 10.3969/j.issn.1000-8098.2019.02.021 [11] 周琪, 钟永辉, 陈星, 等. 石墨烯/纳米TiO2复合材料的制备及其光催化性能[J]. 复合材料学报, 2014, 31(2):255-262.ZHOU Q, ZHONG Y H, CHEN X, et al. Preparation of reduced graphene oxide/nano TiO2 composites by two-step hydrothermal method and their photocatalytic properties[J]. Acta Materiae Compositae Sinica,2014,31(2):255-262(in Chinese). [12] 陈苗, 敖卫, 王如意, 等. 氮掺杂TiO2中空复合微球的制备及可见光光催化性能[J]. 复合材料学报, 2015, 32(3):918-923.CHEN M, AO W, WANG R Y, et al. Preparation and visible-light photocatalytic property of nitrogen-doped TiO2 hollow composite microspheres[J]. Acta Materiae Compositae Sinica,2015,32(3):918-923(in Chinese). [13] HIROMI Y, MASARU H, JUNKO M, et al. Photocatalytic degradation of organic compounds diluted in water using visible light-responsive metal ion-implanted TiO2 catalysts: Fe ion-implanted TiO2[J]. Catalysis Today,2003,84(3):191-196. [14] QU X, LIU M, LI L, et al. BiOBr flakes decoration and structural modification for CdTe/TiO2 spheres: Towards water decontamination under simulated light irradiation[J]. Materials Science in Semiconductor Processing,2019,93:331-338. doi: 10.1016/j.mssp.2019.01.006 [15] JIANG G, LI X, WEI Z, et al. Growth of N-doped BiOBr nanosheets on carbon fibers for photocatalytic degradation of organic pollutants under visible light irradiation[J]. Powder Technology,2014,260:84-89. doi: 10.1016/j.powtec.2014.04.005 [16] TIAN H, LI J, GE M, et al. Removal of bisphenol A by mesoporous BiOBr under simulated solar light irradiation[J]. Catalysis Science & Technology,2012,2(11):23-51. [17] FU J, TIAN Y, CHANG B, et al. BiOBr-carbon nitride heterojunctions: Synthesis, enhanced activity and photocatalytic mechanism[J]. Journal of Materials Chemistry,2012,22(39):21159-21166. doi: 10.1039/c2jm34778d [18] DENG W, PAN F, BATCHELOR B, et al. Mesoporous TiO2-BiOBr microspheres with tailorable adsorption capacities for photodegradation of organic water pollutants: Probing adsorption-photocatalysis synergy by combining experiments and kinetic modeling[J]. Environmental Science: Water Research & Technology,2019,5(4):769-781. [19] ZHANG L, WANG W, SUN S, et al. Elimination of BPA endocrine disruptor by magnetic BiOBr@SiO2@Fe3O4 photocatalyst[J]. Applied Catalysis B: Environmental,2014,148-149:164-169. doi: 10.1016/j.apcatb.2013.10.053 [20] SONG X C, ZHENG Y F, YIN H Y, et al. The solvothermal synthesis and enhanced photocatalytic activity of Zn2+ doped BiOBr hierarchical nanostructures[J]. New Journal of Chemistry,2016,40(1):130-135. doi: 10.1039/C5NJ01282A [21] LIU W, CAI J, LI Z. Self-assembly of semiconductor nanoparticles/reduced graphene oxide (RGO) composite aerogels for enhanced photocatalytic performance and facile recycling in aqueous photocatalysis[J]. ACS Sustainable Chemistry & Engineering,2015,3(2):277-282. [22] HE Y R, LI S C, LI X L, et al. Graphene (rGO) hydrogel: A promising material for facile removal of uranium from aqueous solution[J]. Chemical Engineering Journal,2018,338:333-340. doi: 10.1016/j.cej.2018.01.037 [23] PRASITTHIKUN T, WU X, SATO T, et al. Synthesis and photocatalytic activity of visible-light responsive BiOBr/GO composites[J]. Key Engineering Materials,2017,751:807-812. doi: 10.4028/www.scientific.net/KEM.751.807 [24] 杨旭宇, 王贤保, 李静, 等. 氧化石墨烯的可控还原及结构表征[J]. 高等学校化学学报, 2012, 33(9):1902-1907. doi: 10.3969/j.issn.0251-0790.2012.09.005YANG X Y, WANG X B, LI J, et al. Controllable reduction and structural characterizations of graphene oxides[J]. Chemical Journal of Chinese Universities Chinese,2012,33(9):1902-1907(in Chinese). doi: 10.3969/j.issn.0251-0790.2012.09.005 [25] CHEN Y, GE H, WEI L, et al. Reduction degree of reduced graphene oxide (RGO) dependence of photocatalytic hydrogen evolution performance over RGO/ZnIn2S4 nanocomposites[J]. Catalysis Science & Technology,2013,3(7):1712-1717. [26] LI X, WANG L, ZHANG L, et al. A facile route to the synthesis of magnetically separable BiOBr/NiFe2O4 composites with enhanced photocatalytic performance[J]. Applied Surface Science,2017,419(15):586-594. [27] 盘雨凝, 帅欢, 李男, 等. 纳米TiO2/硅藻土复合材料制备及亚甲基蓝降解[J]. 非金属矿, 2019, 42(5):98-100.PAN Y N, SHUAI H, LI N, et al. Study on preparation of TiO2/diatomite composite materials and its photocatalytic of methylene blue[J]. Non-Metallic Mines,2019,42(5):98-100(in Chinese). [28] CHEN F, AN W, LIU L, et al. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy[J]. Applied Catalysis B: Environmental,2017,217:65-80. doi: 10.1016/j.apcatb.2017.05.078 -






 下载:
下载: