Preparation, catalytic property and antibacterial property of Ag@Fe3O4 core-shell composite nanomaterials
-
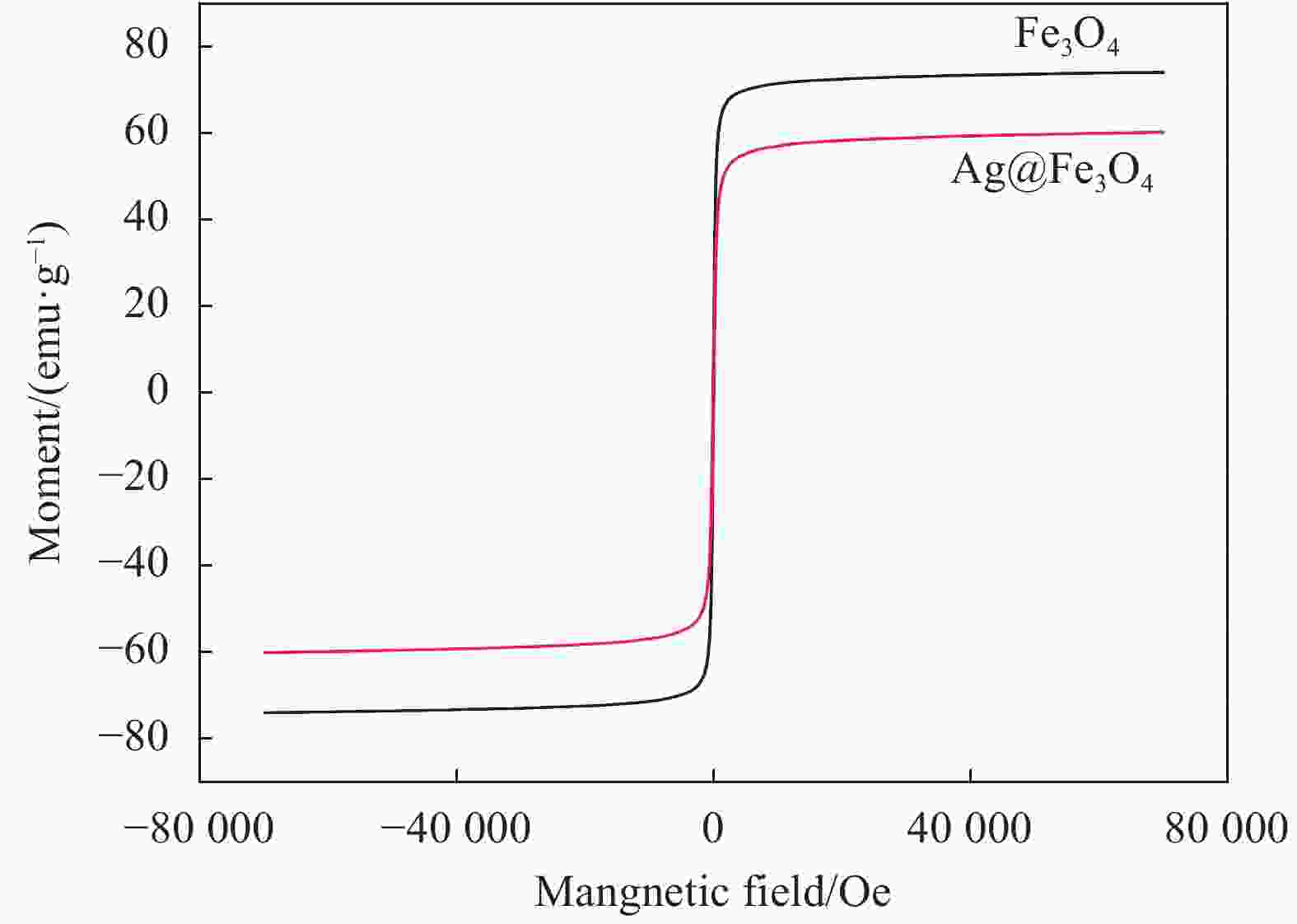
摘要: 采用“一锅法”制备纳米核-壳结构的Ag@Fe3O4复合材料。利用TEM、XRD、UV-vis DRS、振动探针式磁强计(VSM)对Ag@Fe3O4复合材料进行表征。以甲基橙为目标污染物,研究Ag@Fe3O4复合材料在过量NaBH4介质中加氢还原的催化活性,并探讨其催化机制;以单质Ag和Fe3O4作参比,研究Ag@Fe3O4复合材料对金黄色葡萄球菌和大肠杆菌的抑菌性能。结果表明,在10 min内,Ag@Fe3O4复合材料对甲基橙的加氢还原催化率为98%以上,且活性Ag转移电子至甲基橙的N=N键,使其断裂还原成对氨基苯磺酸钠和对二氨基苯;抑菌实验表明,Ag@Fe3O4复合材料比单质Ag具有更强的抑菌活性,并对细胞壁中含有更薄的磷脂双分子层的大肠杆菌更为敏感。Abstract: The Ag@Fe3O4 composites with nano core-shell structure were successfully prepared by a one-pot method. The resulting Ag@Fe3O4 composites were characterized by TEM, XRD, UV-vis DRS and vibrating sample magnetometry (VSM). The catalytic performance and mechanism of Ag@Fe3O4 composites were investigated by photometrically monitoring the reduction of methyl orange in the presence of excess of NaBH4. Furthermore, the antibacterial of Ag@Fe3O4 composites against Staphylococcus aureus (S.aureus) and Escherichia coli (E.coli) was studied by the paper diffusion experiment using Ag and Fe3O4 as the references. The results indicate that over 98% of methyl orange is catalytically degraded within 10 min. This superior catalytic activity may be resulted from transferring electron to N=N bond by Ag, thereby causing N=N fracture and generating sodium 4-aminobenzenesulfonate and p-diaminobenzene. Antibacterial experiment shows that Ag@Fe3O4 composites more excellent bacteriostatic activity than Ag and is more sensitive to E.coli than to S.aureus. The main reason can be ascribed to the fact that phospholipid bilayer in cell wall of E.coli is thinner than that of S.aureus.
-
Key words:
- composite materials /
- Ag /
- catalytic /
- inhibition /
- the Core-shell type /
- Fe3O4
-
图 7 不同浓度的水、SNP、Fe3O4和Ag@Fe3O4复合材料对大肠杆菌(E. coli)和金黄色葡萄球菌(S. aureus)的滤纸片扩散照片
Figure 7. Inhibition zones of as-synthesized water, SNP, Fe3O4e and Ag@Fe3O4 composite with different concentrations against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus)
O—Water; A—SNP; B—Fe3O4; C—Ag@Fe3O4; a1, b1—10 µg/mL; a2, b2—20 µg/mL; a3, b3—30 µg/mL; a4, b4—40 µg/mL
表 1 实验所用材料、剂量和不同材料对甲基橙的催化效率
Table 1. Table information of experimental material, dosage and catalytic efficiency of different materials for methyl orange
Sample Concentration Volume/mL Catalytic efficiency R/% Methyl orange 0.25 mmol/L 1 — NaBH4 0.02 mol/L 2 — Fe3O4 0.5 mg/mL 0.2 0 Ag 0.5 mg/mL 0.2 46 Ag@Fe3O4 0.5 mg/mL 0.2 98 Note: R=(C0–C)/C0×100%, C0—Initial concentration, C—Residual concentration. -
[1] SONG S Y, LIU R X, ZHANG Y, et al. Colloidal noble-metal and bimetallic alloy nanocrystals: A general synthetic method and their catalytic hydrogenation properties[J]. Chemistry: A European Journal,2010,16(21):6251-6256. doi: 10.1002/chem.200903279 [2] PRADHAN N, ANLI P. Catalytic reduction of aromatic nitro compounds by coinage metal nanoparticles[J]. Langmuir,2001,17(5):1800-1802. doi: 10.1021/la000862d [3] MEI L, LU Z T, ZHANG X G. Polymer-Ag nanocomposites with enhanced antimicrobial activity against bacterial infection[J]. ACS Applied Materials & Interfaces,2014,6(18):15813-15821. doi: 10.1021/am502886m [4] CONG Y, XIA T, ZOU M, et al. Mussel-inspired polydopamine coating as aversatile platform for synthesizing polystyrene/Ag nanocomposite particles with enhanced antibacterial activities[J]. Journal of Materials Chemistry B,2014,2(22):3450-3461. [5] DENG H, MCSHAN D, ZHANG Y, et al. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics[J]. Environmental Science & Technology,2016,50(16):8840-8848. [6] ZHOU W H, JIA Z J, XIONG P, et al. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications[J]. ACS Applied Materials & Interfaces,2017,9(31):25830-25846. doi: 10.1021/acsami.7b06757 [7] ZHENG K Y, MAGDIEL I, SETYA W, et al. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin[J]. ACS Nano,2016,10(8):7934-7942. doi: 10.1021/acsnano.6b03862 [8] HAMIDREZA A, EHSAN N K, SAMANEH K, et al. Multifunctional thin-film nanofiltration membrane incorporated with reduced graphene oxide@TiO2@Ag nanocomposites for high desalination performance, dye retention, and antibacterial properties[J]. ACS Applied Materials & Interfaces,2019,11(26):23535-23545. doi: 10.1021/acsami.9b03557 [9] DAVIT J, WU Y T, WANG D. Preparation and self-assembly of dendronized janus Fe3O4-Pt and Fe3O4-Au heterodimers[J]. ACS Nano,2017,11(8):7958-7966. doi: 10.1021/acsnano.7b02485 [10] OLENA I C, JUSTYNA J S, EERSON C, et al. Fourier transform infrared and Raman spectroscopy studies on magnetite/Ag/antibiotic nanocomposites[J]. Applied Surface Science,2016,364:400-409. doi: 10.1016/j.apsusc.2015.12.149 [11] JIANG Z J, LIU C Y, SUN L W, et al. Catalytic properties of silver nanoparticles supported on silica spheres[J]. Journal of Physical Chemistry B,2005,109(5):1730-1735. doi: 10.1021/jp046032g [12] ZHANG Y X, DING H L, LIU Y Y, et al. Facile one-step synthesis of plasmonic/magnetic core/shell nanostructures and their multifunctionality[J]. Journal of Materials Chemistry,2012,22(21):10779-10786. doi: 10.1039/c2jm16293h [13] PRADAN N, PAL A, PA T, et al. Silver nanoparticle catalyzed reduction of aromatic nitro compounds[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2002,196(2-3):247-257. [14] 石振武, 郭少波, 薛群虎. 纳米复合材料Ag@TiO2@SiO2制备、光催化及其抑菌性能[J]. 无机材料学报, 2016, 31(5):466-472.SHI Z W, GUO S B, XUE Q H. Preparation, photocatalytic property and antibacterial property of Ag@TiO2@SiO2 composite nanomaterials[J]. Journal of Inorganic Materials,2016,31(5):466-472(in Chinese). [15] CHEN B, JIAO X L. Size-controlled and size-designed synthesis of nano/submicrometer Ag particles[J]. Crystal Growth & Design,2010,10(8):3379-3386. [16] SAKIR M, ONSES M S. Solid substrates decorated with Ag nanostructures for the catalytic degradation of methyl orange[J]. Results in Physics,2019,12:1133-1143. doi: 10.1016/j.rinp.2018.12.084 [17] XU Z C, HOU Y L, SUN S H. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties[J]. Journal of the American Chemical Society,2007,129(28):8698-8699. doi: 10.1021/ja073057v [18] AMANULLA M F, KULANDAIVELU B J, MORUKATTU G, et al. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria[J]. Nanomedicine: Nanotechnology, Biology, and Medicine,2010,6(1):103-109. doi: 10.1016/j.nano.2009.04.006 [19] PADMANABH J, ZHOU Y, TEVHIDE O A, et al. Quantitative SERS-based detection using Ag-Fe3O4 nanocomposites with an internal reference[J]. Journal of Materials Chemistry C,2014,2(46):9964-9968. doi: 10.1039/C4TC01550A [20] ANANDA S, GRISESER F, ASHOKKUMAR M. Sonochemical Synthesis of Au-Ag core-shell bimetallic nanoparticles[J]. Journal of Physical Chemistry C,2008,112(39):15102-1510. doi: 10.1021/jp806960r [21] LI S H, WANG E B, TIAN C G, et al. Jingle-bell-shaped ferrite hollow sphere with a noble metal core: Simple synthesis and their magnetic and antibacterial properties[J]. Journal of Solid State Chemistry,2008,181(7):1650-1658. [22] 中华人民共和国工业和信息化部. 对氨基苯磺酸钠: HG/T 2746—2010[S]. 北京: 化工出版社, 2011.Ministry of Industry and Information Technology of the People’s Republic of China. Sodium sulphanilate: HG/T 2746—2010[S]. Beijing: Chemical Industrial Press, 2011(in Chinese). [23] JIANG W Q, ZHOU Y F, ZHANG Y L, et al. Superparamagnetic Ag-Fe3O4 core-shell nanospheres: Fabrication, characterization and application as reusable nanocatalysts[J]. Dalton Transactions,2012,41(15):4594-4601. doi: 10.1039/c2dt12307j [24] LI P, LI J, WU C Z, et al. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles[J]. Nanotechnology,2005,77(16):1912-1917. [25] 郭少波, 马剑琪, 兰阿峰, 等. 核-壳纳米Ag@ZrO2复合材料的制备及其抑菌性能研究[J]. 复合材料学报, 2016, 33(4):821-826.GUO S B, MA J Q, LAN A F, et al. Preparation and its antibacterial properties of core-shell nano Ag@ZrO2 composite[J]. Acta Materiae Compositae Sinica,2016,33(4):821-826(in Chinese). -






 下载:
下载: