A novel Co3O4 and WC co-doped β-PbO2 electrode for zinc electrowinning: Deposition behavior and electrochemical properties
-
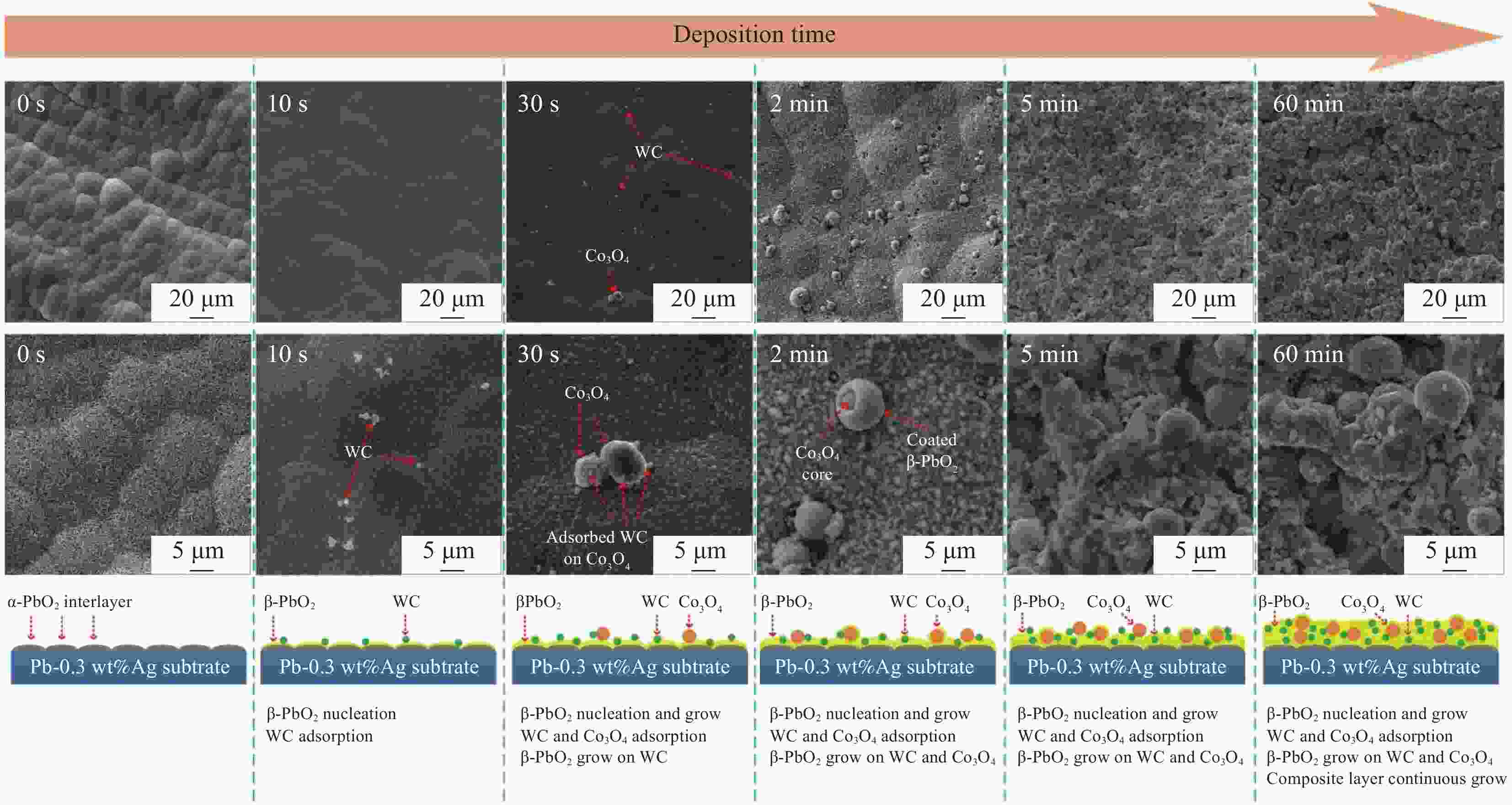
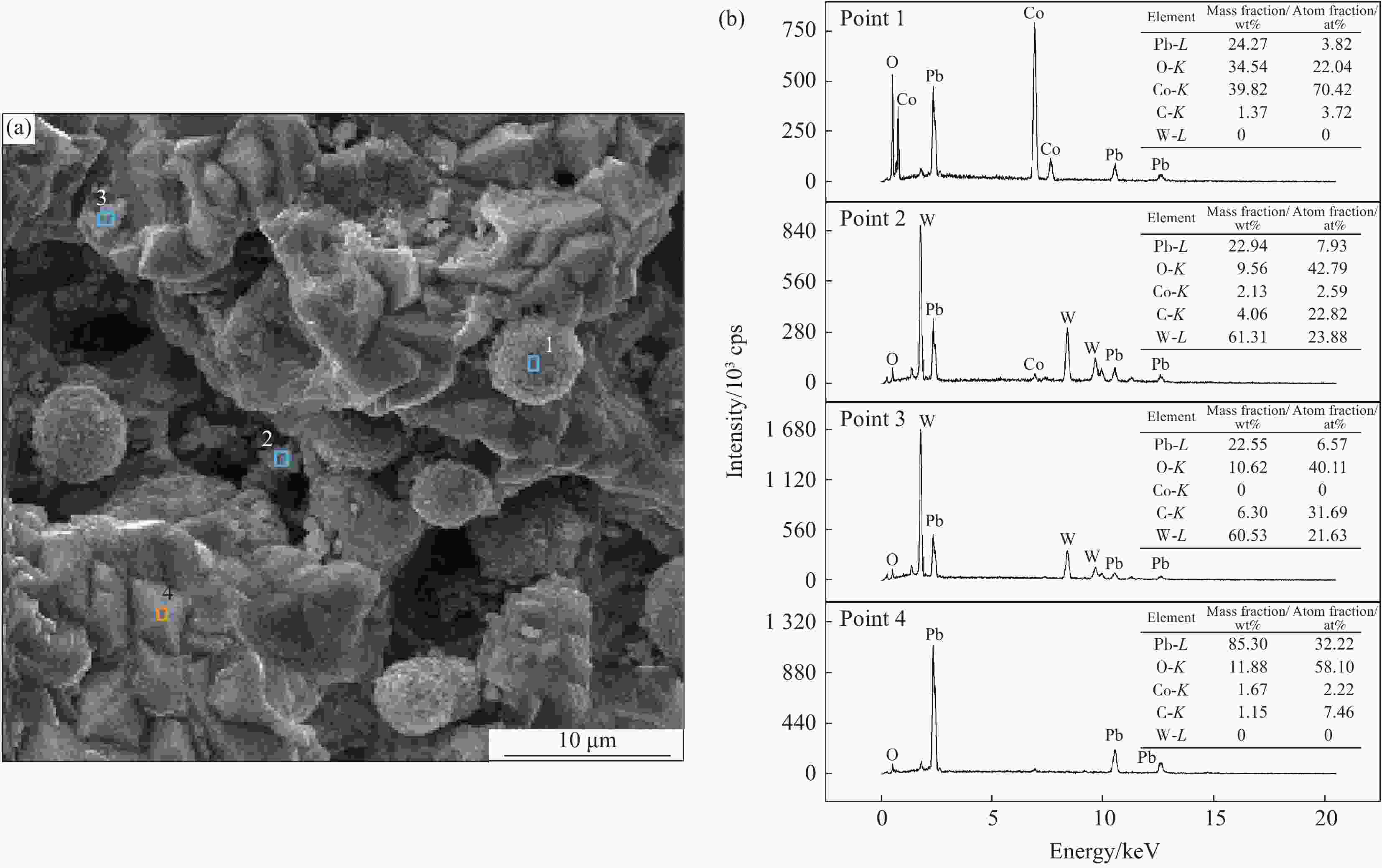
摘要: 采用复合电沉积技术在Pb-0.3wt%Ag/α-PbO2基体上合成了WC和Co3O4颗粒共沉积的β-PbO2复合沉积层。沉积行为研究发现,WC颗粒先于Co3O4颗粒吸附于基体上,将WC颗粒与Co3O4颗粒共沉积是一种抑制当Co3O4颗粒单独共沉积于β-PbO2沉积层时发生团聚情况的有效方法。电极性能研究发现,WC或Co3O4颗粒的共沉积均会提高复合阳极的析氧电催化活性,此外,WC颗粒还有助于提高复合阳极的显微硬度和在Zn电解沉积溶液中的耐腐蚀性能。Co3O4颗粒的共沉积不利于β-PbO2相的生长,WC颗粒的共沉积对β-PbO2相的生长影响不大,两种颗粒同时共沉积有助于抑制酸性镀液中α-PbO2相的生长。Abstract: In this study, the WC and Co3O4 particles co-doped β-PbO2 composite coatings were synthesized on a Pb-0.3wt%Ag/α-PbO2 substrate using composite electrodeposition. The study of deposition behavior has found that WC particles are adsorbed on the substrate before Co3O4 particles, and co-deposition of WC particles and Co3O4 particles is an effective way to inhibit the agglomeration of Co3O4 particles when single co-deposited into β-PbO2 matrix. Electrode performance studies have found that co-deposition of WC or Co3O4 particles can both improve the electrocatalytic activity of oxygen evolution of the composite anode. In addition, WC particles can also help to improve the microhardness of the composite anode and the corrosion resistance in the Zn electrodeposition solution. The co-deposition of Co3O4 particles is not conducive to the growth of the β-PbO2 phase. The co-deposition of WC particles has little effect on the growth of the β-PbO2 phase. The simultaneous co-deposition of two particles helps to inhibit the growth of the α-PbO2 phase in the acid plating solution.
-
Key words:
- PbO2 /
- WC /
- Co3O4 /
- Zn electrowinning /
- deposition
-
表 1 不同复合电极的沉积层厚度和表面显微硬度
Table 1. Surface microhardness and resistivity of different composite electrodes
Composite
electrodeCoating
thickness/μmSurface
microhardness (HV)β-PbO2 117.5 738.5 β-PbO2-WC 128.9 821.9 β-PbO2-Co3O4 136.4 679.3 β-PbO2-WC-Co3O4 132.4 762 表 2 不同电极在Zn电解沉积模拟溶液中析氧反应(OER)的动力学参数和过电位
Table 2. Kinetic parameters and overpotential for oxygen evolution reaction (OER) of different electrodes in a simulation Zn electrowinning solution
Electrode type Oxygen evolution overpotential η/V a b i0/(A·cm−2) 300 A·m−2 400 A·m−2 500 A·m−2 600 A·m−2 β-PbO2 0.934 0.963 0.986 1.004 1.289 0.233 2.94×10−6 β-PbO2-WC 0.845 0.860 0.871 0.881 1.025 0.118 2.06×10−9 β-PbO2-Co3O4 0.588 0.600 0.609 0.617 0.733 0.095 1.92×10−8 β-PbO2-WC-Co3O4 0.608 0.642 0.668 0.689 1.018 0.269 1.64×10−4 Notes: a—Tafel intercept; b—Tafel slope; i0—Exchange current density. 表 3 不同电极在Zn电解沉积模拟溶液中的腐蚀电位和腐蚀电流密度
Table 3. Corrosion potentials and corrosion current densities for different β-PbO2 coating electrodes in a simulation Zn electrowinning solution
Electrode type Ecorr(vs SCE)/V icorr/(10−5A·cm−2) β-PbO2 1.312 19.8 β-PbO2-WC 1.314 5.68 β-PbO2-Co3O4 1.298 8.02 β-PbO2-WC-Co3O4 1.37 9.84 Notes: Ecorr—Corrosion potential; icorr—Corrosion current. -
[1] JIN L, HUANG H, FEI Y, et al. Polymer anode used in hydrometallurgy: Anodic behaviour of PANI/CeO2/WC anode from sulfate electrolytes[J]. Hydrometallurgy,2018,176:201-207. doi: 10.1016/j.hydromet.2018.01.020 [2] CHEN B, WANG S, LIU J, et al. Corrosion resistance mechanism of a novel porous Ti/Sn-Sb-RuOx/β-PbO2 anode for zinc electrowinning[J]. Corrosion Science,2018,144:136-144. doi: 10.1016/j.corsci.2018.08.049 [3] YANG H, LIU H, GUO Z, et al. Electrochemical behavior of rolled Pb-0.8%Ag anodes[J]. Hydrometallurgy,2013,140:144-150. doi: 10.1016/j.hydromet.2013.10.003 [4] BODE H. Lead-acid batteries[M]. New York: Wiley, 1977. [5] DEVILLIERS D, DINH T, MAHÉ E, et al. Electroanalytical investigations on electrodeposited lead dioxide[J]. Journal of Electroanalytical Chemistry,2004,573(2):227-239. doi: 10.1016/j.jelechem.2004.07.008 [6] CHEN B, GUO Z, HUANG H, et al. Effect of the current density on electrodepositing alpha-lead dioxide coating on aluminum substrate[J]. Acta Metallurgica Sinica,2009,22:373-382. [7] HE S, XU R, HU G, et al. Study on the electrosynthesis of Pb-0.3%Ag/α-PbO2 composite inert anode materials[J]. Electrochemistry,2015,83(11):974-978. doi: 10.5796/electrochemistry.83.974 [8] HAO X, WUQI G, WU J, et al. Preparation and characterization of titanium-based PbO2 electrodes modified by ethylene glycol[J]. RSC Advances,2016,6(9):7610-7617. doi: 10.1039/C5RA21195F [9] PAVLOV D, MONAHOV B. Temperature dependence of the oxygen evolution reaction on the Pb/PbO2 electrode[J]. Journal of the Electrochemical Society,1998,145(1):70-77. doi: 10.1149/1.1838213 [10] JOVIĆ B M, LAČNJEVAC U Č, JOVIĆ V D, et al. Kinetics of the oxygen evolution reaction on NiSn electrodes in alkaline solutions[J]. Journal of Electroanalytical Chemistry,2015,754:100-108. doi: 10.1016/j.jelechem.2015.07.013 [11] SURENDRANATH Y, KANAN M W, GNOCERA D. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH[J]. Journal of the American Chemical Society,2010,132(46):16501-16509. doi: 10.1021/ja106102b [12] LI J, SHU C, HU A, et al. Tuning oxygen non-stoichiometric surface via defect engineering to promote the catalysis activity of Co3O4 in Li-O2 batteries[J]. Chemical Engineering Journal,2020,381:122678-122688. doi: 10.1016/j.cej.2019.122678 [13] GAO R, SHANG Z, ZHENG L, et al. Enhancing the catalytic activity of Co3O4 nanosheets for Li-O2 batteries by the incoporation of oxygen vacancy with hydrazine hydrate reduction[J]. Inorganic Chemistry,2019,58:4989-4996. doi: 10.1021/acs.inorgchem.9b00007 [14] WANG X, CHEN X, GAO L, et al. One-dimensional arrays of Co3O4 nanoparticles: Synthesis, characterization, and optical and electrochemical properties[J]. Journal of Physical Chemistry B,2004,108(42):16401-16404. doi: 10.1021/jp048016p [15] YAN Q, LI X, ZHAO Q, et al. Shape-controlled fabrication of the porous Co3O4 nanoflower clusters for efficient catalytic oxidation of gaseous toluene[J]. Journal of Hazardous Materials,2012,209-210:385-391. doi: 10.1016/j.jhazmat.2012.01.039 [16] XU M, FEI W, ZHAO M, et al. Molten hydroxides synthesis of hierarchical cobalt oxide nanostructure and its application as anode material for lithium ion batteries[J]. Electrochimica Acta,2011,56(13):4876-4881. doi: 10.1016/j.electacta.2011.03.027 [17] WANG Z, WEI F, SUI Y, et al. A novel core-shell polyhedron Co3O4/MnCo2O4.5 as electrode materials for supercapacitors[J]. Ceramics International,2019,45:12558-12562. doi: 10.1016/j.ceramint.2019.03.010 [18] XIONG L, TENG Y, WU Y. Large-scale synthesis of aligned Co3O4 nanowalls on nickel foam and their electrochemical performance for Li-ion batteries[J]. Ceramics International, 2014, 40(10): 15561-15568. [19] WANG F, LU C C, QIN Y, et al. Solid state coalescence growth and electrochemical performance of plate-like Co3O4 mesocrystals as anode materials for lithium-ion batteries[J]. Journal of Power Sources,2013,235(4):67-73. doi: 10.1016/j.jpowsour.2013.01.190 [20] DAN Y, LU H, LIU X, et al. Ti/PbO2+nano-Co3O4 composite electrode material for electrocatalysis of O2 evolution in alkaline solution[J]. International Journal of Hydrogen Energy,2011,36(3):1949-1954. [21] BERTONCELLO R, FURLANETTO F, GUERRIERO P, et al. Electrodeposited composite electrode materials: Effect of the concentration of the electrocatalytic dispersed phase on the electrode activity[J]. Electrochimica Acta,1999,44(23):4061-4068. doi: 10.1016/S0013-4686(99)00165-6 [22] HE S, XU R, GUO Y, et al. Fabrication and nucleation study of β-PbO2-Co3O4 OER energy-saving electrode[J]. SN Applied Sciences,2019,1(3):219-227. doi: 10.1007/s42452-019-0228-7 [23] LEVY R B, BOUDART M. Platinum-like behavior of tungsten carbide in surface catalysis[J]. Science,1973,181:547-549. doi: 10.1126/science.181.4099.547 [24] MCINTYRE D R, BURSTEIN G T, VOSSEN A. Effect of carbon monoxide on the electrooxidation of hydrogen by tungsten carbide[J]. Journal of Power Sources,2002,107(1):67-73. doi: 10.1016/S0378-7753(01)00987-9 [25] ALIDOKHT S A, YUE S, CHROMIK R R. Effect of WC morphology on dry sliding wear behavior of cold-sprayed Ni-WC composite coatings[J]. Surface & Coatings Technology,2019,357:849-863. [26] NAMINI A S, AHMADI Z, BABAPOOR A, et al. Microstructure and thermomechanical characteristics of spark plasma sintered TiC ceramics doped with nano-sized WC[J]. Ceramics International,2019,45:2153-2160. doi: 10.1016/j.ceramint.2018.10.125 [27] HE L, TAN Y, WANG X, et al. Tribological properties of WC and CeO2 particles reinforced in-situ synthesized NiAl matrix composite coatings at elevated temperature[J]. Surface & Coatings Technology,2014,244:123-130. [28] HAMID Z A, GHAYAD I M, IBRAHIM K M. Electrodepo-sition and characterization of chromium-tungsten carbide composite coatings from a trivalent chromium bath[J]. Surface and Interface Analysis,2005,37:573-579. doi: 10.1002/sia.2052 [29] BOONYONGMANEERAT Y, SAENGKIETTIYUT K, SAENAPITAK S, et al. Effects of WC addition on structure and hardness of electrodeposited Ni-W[J]. Surface & Coatings Technology,2009,203:3590-3594. [30] HUIXI L, ZHEN C, QIANG Y, et al. Effects of tungsten carbide on the electrocatalytic activity of PbO2-WC composite inert anodes during zinc electrowinning[J]. Journal of The Electrochemical Society,2017,164:H1064-H1071. doi: 10.1149/2.0791714jes [31] LIU J H, LIU F H, XU J, et al. Effect of current density on interface structure and performance of CF/β-PbO2 electrodes during zinc electrowinning[J]. Ceramics International,2020,46(2):2403-2408. doi: 10.1016/j.ceramint.2019.09.233 [32] MARSALEK R. Particle size and zeta potential of ZnO[J]. Apcbee Procedia,2014,9:13-17. [33] TANG X, ZHENG H, TENG H, et al. An alternative method for preparation of polyaluminum chloride coagulant using fresh aluminum hydroxide gels: Characterization and coagulation performance[J]. Chemical Engineering Research & Design,2015,104:208-217. [34] SUSAN D F, BARMAK K, MARDER A R. Electrodeposited NiAl particle composite coatings[J]. Thin Solid Films,1997,307(1-2):133-140. doi: 10.1016/S0040-6090(98)80004-7 [35] KEDWARD E C, WRIGHT K W, TENNETT A A B. The development of electrodeposited composites for use as wear control coatings on aero engines[J]. Tribology,1974,7(5):221-227. [36] GUGLIELMI N. Kinetics of the deposition of inert particles from electrolytic baths[J]. Journal of the Electrochemical Society,1972,119(8):1009-1012. doi: 10.1149/1.2404383 [37] MUSIANI M, FURLANETTO F, GUERRIERO P. Electrochemical deposition and properties of PbO2+Co3O4 composites[J]. Journal of Electroanalytical Chemistry,1997,440:131-138. [38] LI Z, YU X Y, PAIK U. Facile preparation of porous Co3O4 nanosheets for high-performance lithium ion batteries and oxygen evolution reaction[J]. Journal of Power Sources,2016,310:41-46. doi: 10.1016/j.jpowsour.2016.01.105 [39] SONG X, YANG T, DU H, et al. New binary Mn and Cr mixed oxide electrocatalysts for the oxygen evolution reaction[J]. Journal of Electroanalytical Chemistry,2016,760:59-63. doi: 10.1016/j.jelechem.2015.11.044 [40] ABREU-SEPULVEDA M, TRINH P, MALKHANDI S, et al. Investigation of oxygen evolution reaction at LaRuO3, La3.5Ru4O13, and La2RuO5 [J]. Electrochimica Acta,2015,180:401-408. doi: 10.1016/j.electacta.2015.08.067 -






 下载:
下载: