Preparation of nano-Fe3O4@tea waste/calcium alginate magnetic composited bead and it’s adsorption characteristics and mechanisms for methylene blue from aqueous solution
-
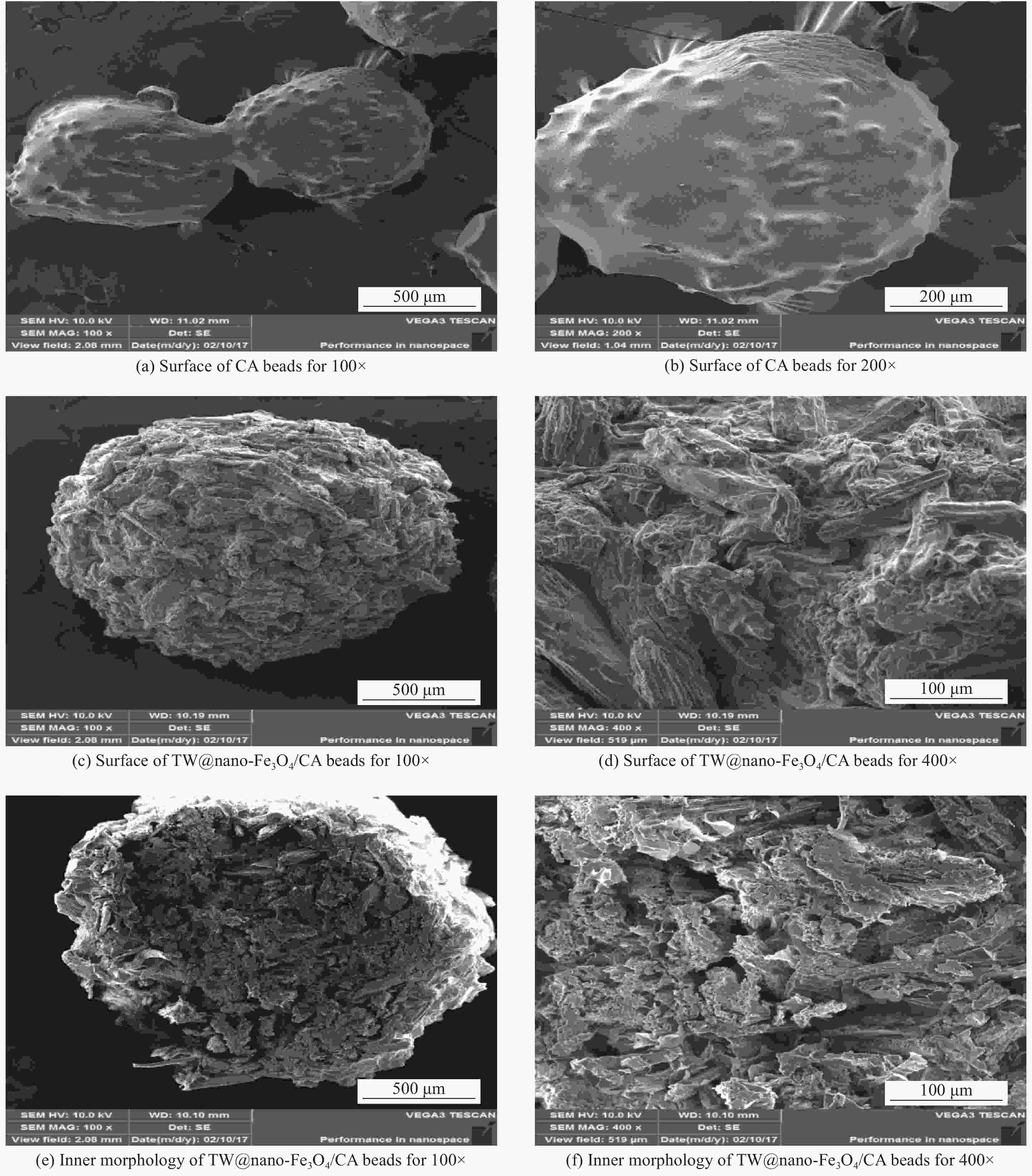
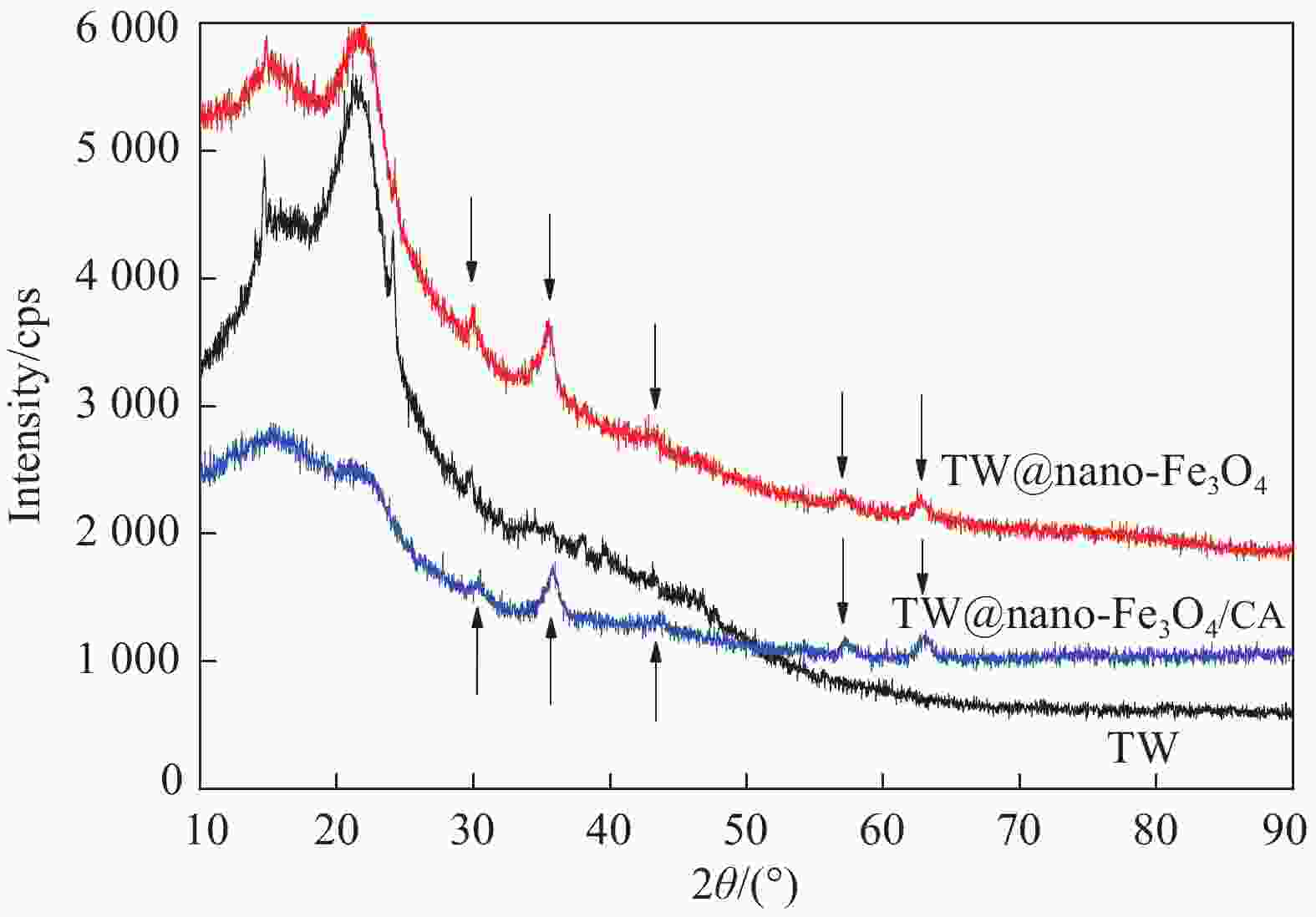
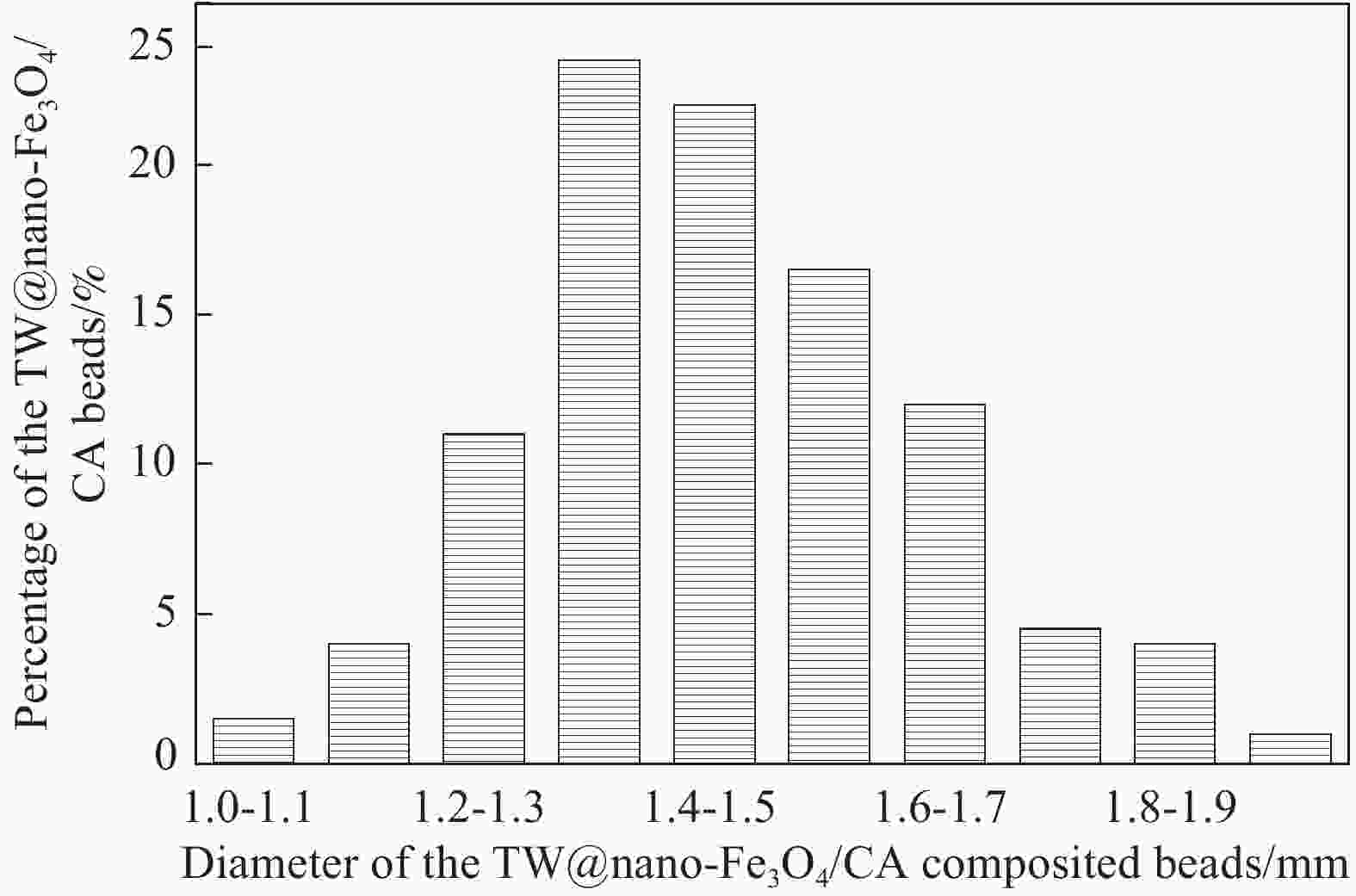
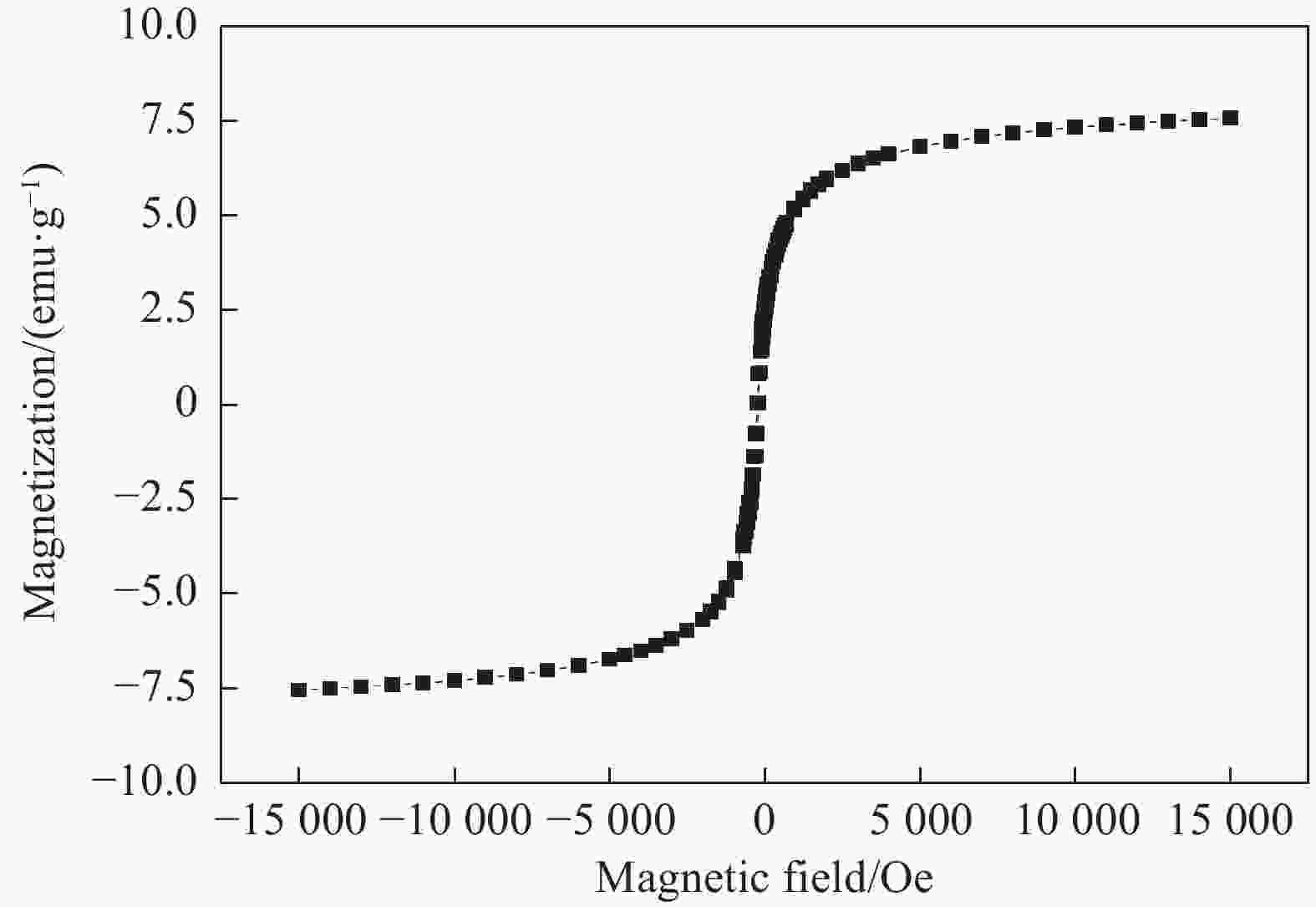
摘要: 采用离子共沉淀技术在茶渣(Tea waste, TW)表面沉积纳米Fe3O4粒子(TW@nano-Fe3O4),用溶胶凝胶法制备茶渣@纳米Fe3O4/海藻酸钙(TW@nano-Fe3O4/CA)磁性复合微球,通过SEM、XPS、XRD、振动样品磁强计(VSM)及万能试验机对材料结构和性能进行了表征与测试,并研究了其对水溶液中亚甲基蓝(Methylene blue, MB)的吸附性能与机制。结果表明,TW@nano-Fe3O4/CA复合微球磁性响应明显,粒径为1.2~1.7 mm。微球表面粗糙、褶皱,内部为疏松多孔道结构。随TW@nano-Fe3O4含量增加,微球粒径增加,磁响应增强,但对MB的吸附量缓慢下降;TW@nano-Fe3O4/CA微球对MB的吸附动力学数据与准二级动力学方程拟合较好,等温吸附过程符合Langmuir模型,对MB的吸附过程是自发性和熵减小的放热过程。在303 K下,质量配比为TW@nano-Fe3O4∶CA=4∶1的复合微球对MB的Langmuir最大吸附量为272.5 mg·g−1,比TW提高86.7%,并具有良好的再生与循环使用性能。Abstract: Nano-Fe3O4 particles were deposited on the surface of tea waste (TW) by co-precipitation method to form tea waste@nano-Fe3O4 magnetic composited material, and then spherical tea waste@nano-Fe3O4/calcium algnate (TW@nano-Fe3O4/CA) magnetic beads were prepared by sol-gel approach. The magnetic composites were characterized by SEM, XPS, XRD, vibrating sample magnetometer (VSM) and universal testing machine. The adsorption properties and mechanism of methylene blue (MB) from aqueous solution onto the beads were studied. The results show that the TW@nano-Fe3O4/CA beads possess a good magnetic response, with the diamters ranges from 1.2 mm to 1.7 mm. The composited beads display a rough and folded surface morphology and porous inner structure. The diameters and magnetic response of the beads increase while the adsorption abilities of MB decrease with the increasing content of TW@nano-Fe3O4 in the beads. The adsorption kinetics followed is second order, and the adsorption isotherm data are well fitted to Langmuir model. The adsorption process of MB onto the beads is spontaneous, entropy decreased and exothermic process. The Langmuir maximum adsorption capacity of MB onto the beads with 80% mass fraction of TW@nano-Fe3O4 is found to be 272.5 mg·g−1 at 303 K, which is increased by 86.7% than TW. The adsorbent shows satisfactory regeneration and recycling utiliziation performance.
-
Key words:
- tea waste /
- sodium alginate /
- beads /
- magnetic separation /
- adsorption /
- methylene blue
-
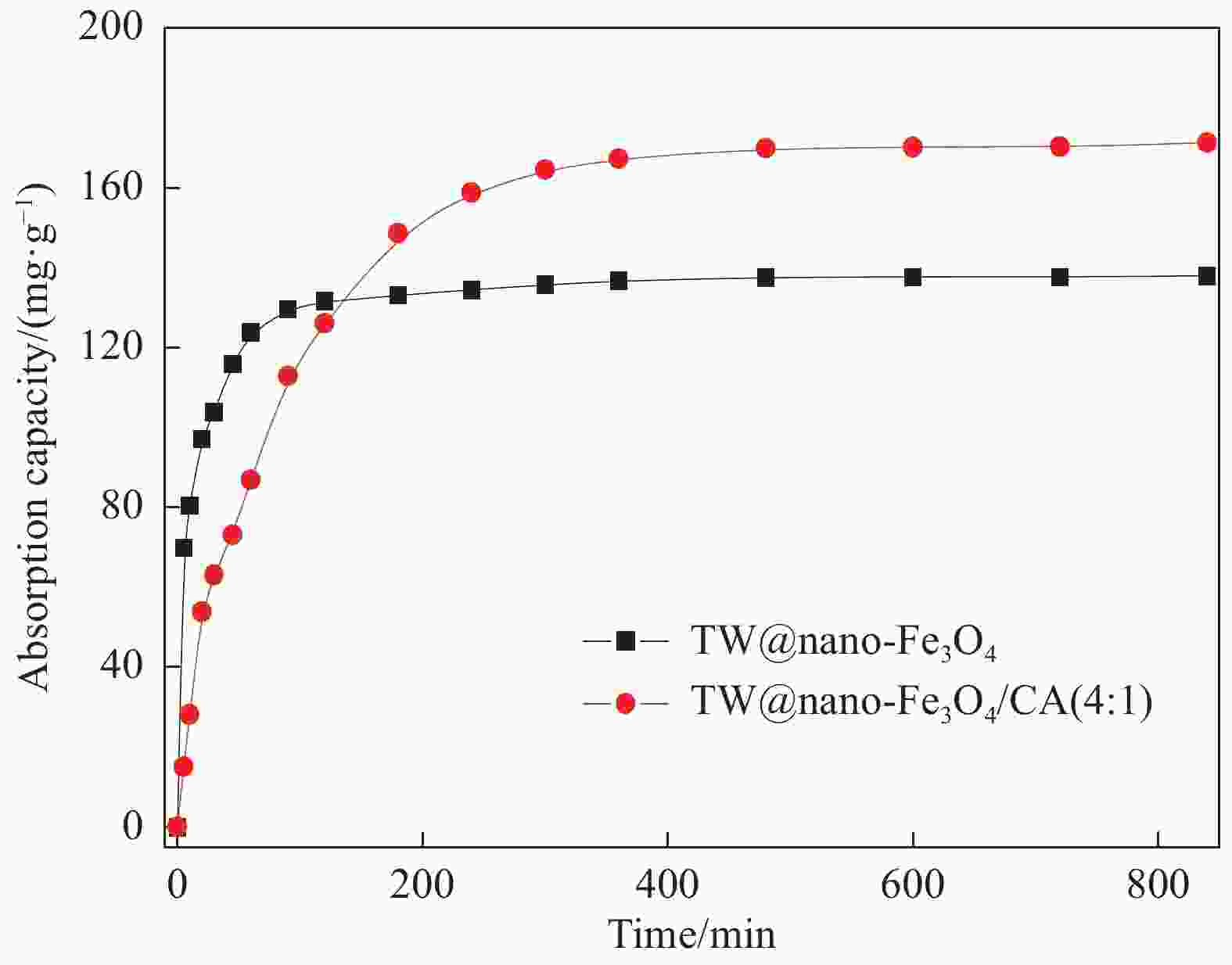
表 1 TW@nano-Fe3O4/CA(4∶1)复合微球吸附MB的动力学模型拟合参数(C0=200 mg·L−1)
Table 1. Parameters of kinetic adsorption models for MB adsorption onto TW@nano-Fe3O4/CA(4∶1) beads ( C0=200 mg·L−1)
Model Parameter Value Qe(exp)/(mg·g−1) 171.3 Pesudo-
first-
orderk1/min−1 8.0×10−3 Qe(cal)/(mg·g−1) 123.8 R2 0.9542 Pesudo-
second-
orderk2/(g·mg−1·min−1) 1.0×10−4 Qe(cal)/(mg·g−1) 185.2 R2 0.9985 Notes: k1, k2—Psudo-first-order kinetic constant and Psudo-second-order kinetic constant, respectively; Qe(cal)—Calculation amount of MB removed per unit mass of adsorbent; Qe(exp)—Experimental amount of MB removed per unit mass of adsorbent. 表 2 TW、TW@nano-Fe3O4及TW@nano-Fe3O4/CA(4∶1)对MB的吸附等温式拟合结果
Table 2. Isothermal parameters for the adsorption of MB onto TW, TW@nano-Fe3O4 and TW@nano-Fe3O4/CA(4∶1) beads
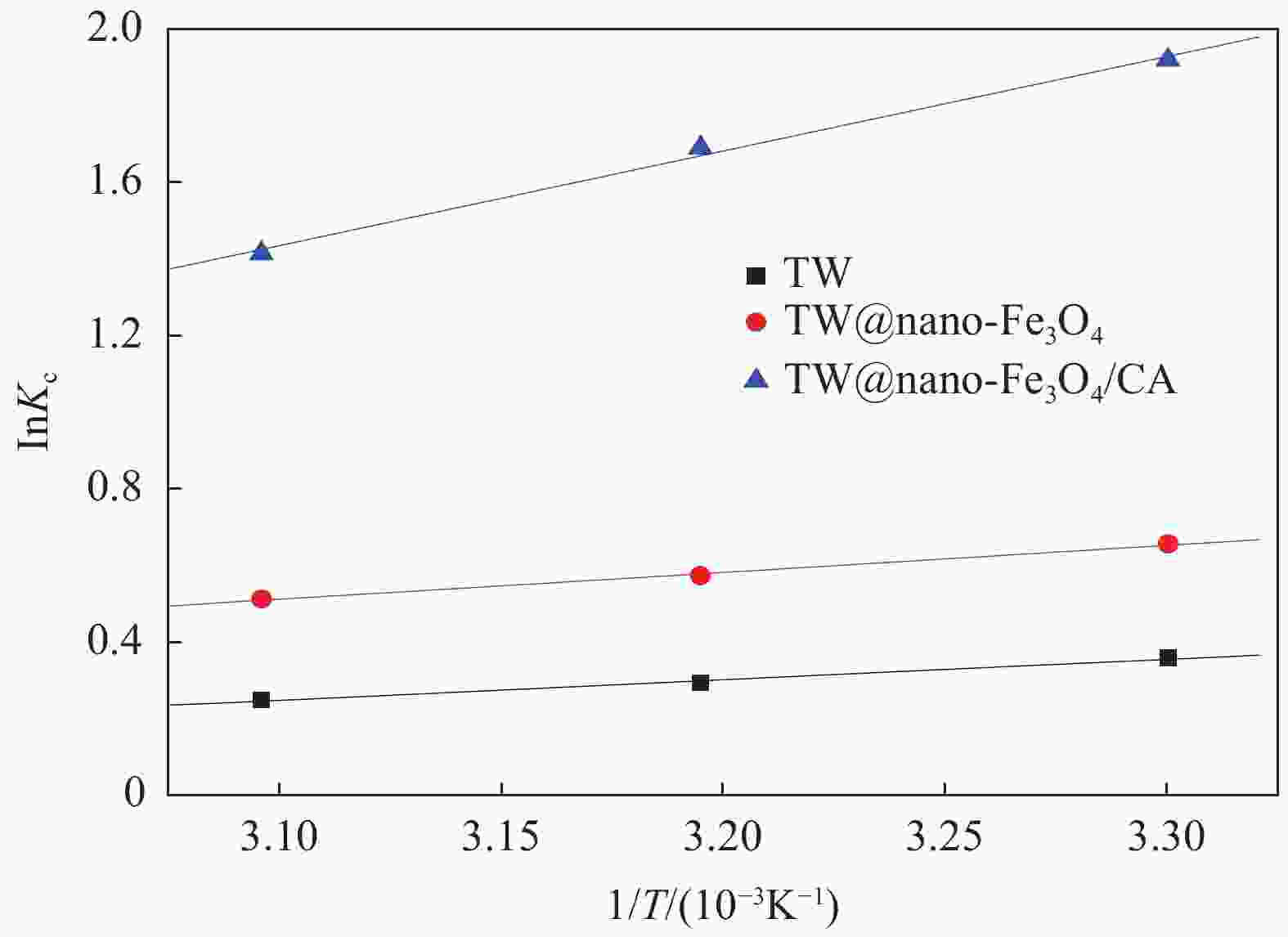
Adsorbent Model Parameter T/K 303 313 323 TW Langmuir Qm/(mg·g−1) 146.0 145.3 143.3 KL×10−2/(L·mg−1) 5.2 4.4 4.2 R2 0.9995 0.9992 0.9989 TW@nano-Fe3O4 Langmuir Qm/(mg·g−1) 160.5 154.8 152.0 KL×10−2/(L·mg−1) 9.1 8.1 7.4 R2 0.9993 0.9996 0.9998 TW@nano-Fe3O4/CA Langmuir Qm/(mg·g−1) 272.5 266.0 261.8 KL×10−2/(L·mg−1) 5.1 4.8 4.2 R2 0.9949 0.9971 0.9952 Fredudlich KF/(L·mg−1) 26.0 25.6 24.3 n 2.0 2.1 2.1 R2 0.9089 0.9482 0.9718 Notes: Qm—Langmuir adsorption maximum; KL—Langmuir coefficient of distribution of the adsorption; KF—Freundlich coefficient of distribution of the adsorption; n—Empirical constant related to temperature and system. 表 3 TW、TW@nano-Fe3O4及TW@nano-Fe3O4/CA(4∶1)复合微球吸附MB的热力学参数分析
Table 3. Values of thermodynamic parameters for the adsorption of MB onto TW, TW@nano-Fe3O4 andTW@nano-Fe3O4/CA(4∶1) beads
Adsorbent T/K ∆Gθ/(kJ·mol−1) ∆Hθ/(kJ·mol−1) ∆Sθ/(J·mol−1·K−1) R2 TW 303 −0.9 313 −0.8 −4.4 −11.7 0.9943 323 −0.7 TW@nano-Fe3O4 303 −1.6 313 −1.5 −5.9 −13.9 0.9960 323 −1.4 TW@nano-Fe3O4/CA
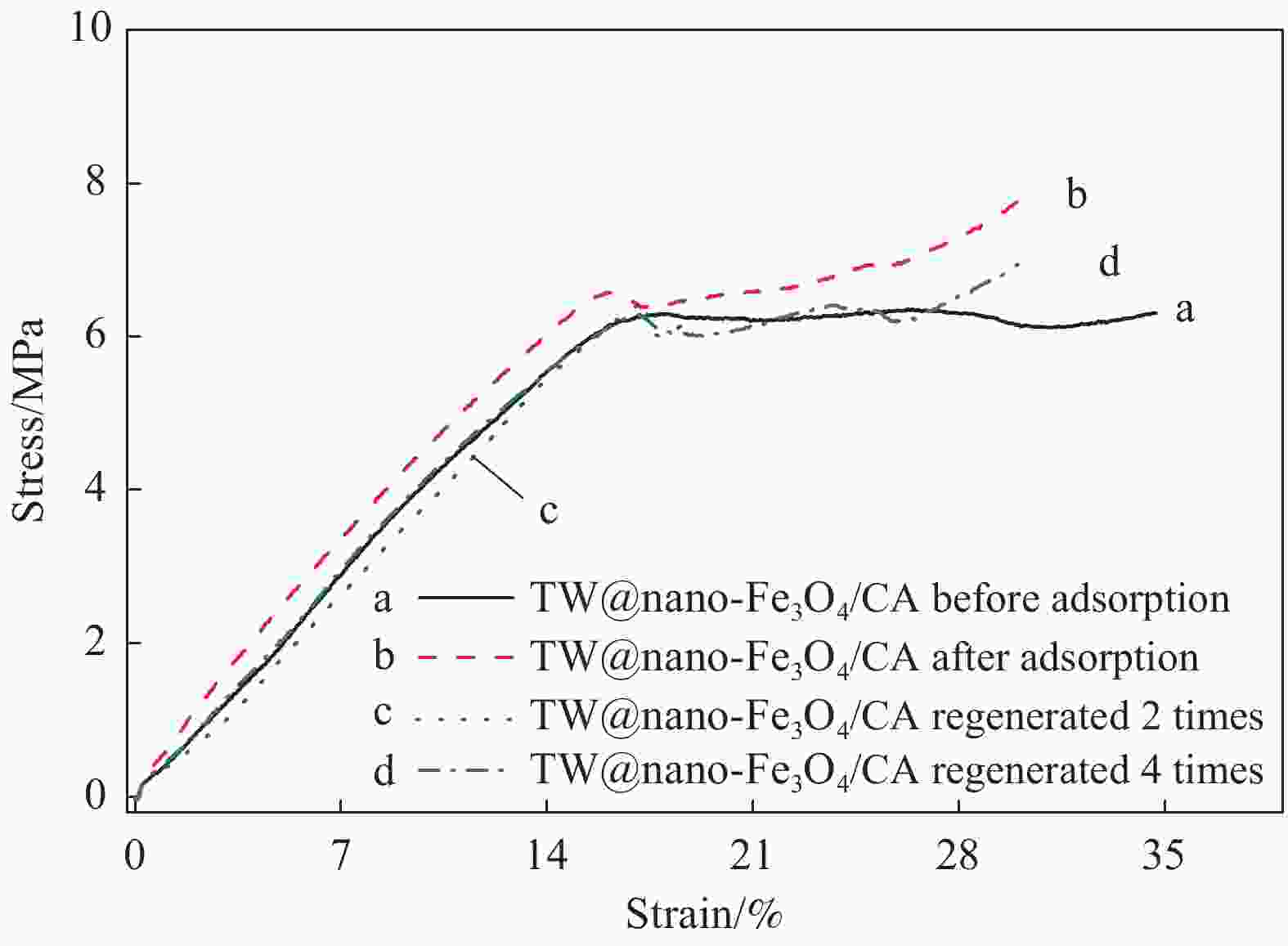
beads303 −4.8 313 −4.4 −20.5 −51.7 0.9952 323 −3.8 Notes: ∆Gθ—Gibbs free energy variation of the adsorption process; ∆Hθ—Enthalpy change of the adsorption process; ∆Sθ—Entropy change of the adsorption process.; R2—Linear correlation coefficient. 表 4 不同处理方法后TW@nano-Fe3O4/CA(4∶1)复合微球的力学性能
Table 4. Mechanical properties of TW@nano-Fe3O4/CA(4∶1) composites with different treatments
TW@nano-Fe3O4/CA beads Compressive strength/MPa Compression modulus/MPa Before adsorption of MB 6.2±0.5 41.1±4.7 After adsorption of MB 6.5±0.4 42.2±4.6 Regeneration for 2 times 6.3±0.3 44.7±3.4 Regeneration for 4 times 6.2±0.2 42.2±3.1 -
[1] DAWOOD S, SEN T K, PHAN C. Performance and dynamic modelling of biochar and kaolin packed bed adsorption column for aqueous phase methylene blue(MB) dye removal[J]. Environmental Technology,2019,40(28):3762-3772. doi: 10.1080/09593330.2018.1491065 [2] LIU P, ZHANG L. Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents[J]. Separation & Purification Technology,2007,58(1):32-39. [3] NASUHA N, HAMEED B H, DIN A T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue[J]. Journal of Hazardous Materials,2010,175(1-3):126-132. doi: 10.1016/j.jhazmat.2009.09.138 [4] DIAS J M, ALVIM-FERRAZ M C, ALMEIDA M F, et al. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review[J]. Journal of Environmental Management,2007,85(4):833-846. doi: 10.1016/j.jenvman.2007.07.031 [5] MONDAL S. Methods of dye removal from dye house effluents: An overview[J]. Environmental Engineering Science,2008,25(3):383-396. doi: 10.1089/ees.2007.0049 [6] 龚新怀, 陈良壁. 茶粉/聚丙烯复合材料自然老化性能[J]. 复合材料学报, 2016, 33(7):1437-1445.GONG X H, CHEN L B. Performances of tea dust/polypropylene composites under natural weathering[J]. Acta Materiae Compositae Sinica,2016,33(7):1437-1445(in Chinese). [7] 许咏梅, 芦炜杰, 吕威雄. 中国茶叶出口贸易对中国茶产业发展影响的实证分析[J]. 茶叶, 2019, 45(4):185-192. doi: 10.3969/j.issn.0577-8921.2019.04.002XU Y M, LU W J, LV W X. An empirical analysis of the impact of China′s tea export trade on the development of China’s tea industry[J]. Journal of Tea,2019,45(4):185-192(in Chinese). doi: 10.3969/j.issn.0577-8921.2019.04.002 [8] 谢月琴, 罗君谊, 陈婷, 等. 茶渣资源再利用研究概况[J]. 中国农学通报, 2019, 35(33):141-145. doi: 10.11924/j.issn.1000-6850.casb18060083XIE Y Q, LUO J Y, CHEN T, et al. Reuse of tea residue resources: A review[J]. Chinese Agricultural Science Bulletin,2019,35(33):141-145(in Chinese). doi: 10.11924/j.issn.1000-6850.casb18060083 [9] FOROUGHA-DAHR M, ABOLGHASEMI H, ESMAILI M, et al. Adsorption characteristics of congo red from aqueous solution onto tea waste[J]. Chemical Engineering Communications,2015,202(2):181-193. doi: 10.1080/00986445.2013.836633 [10] BABAEI A A, KHATAEE A, AHMADPOUR E, et al. Optimization of cationic dye adsorption on activated spent tea: Equilibrium, kinetics, thermodynamic and artificial neural network modeling[J]. Korean Journal of Chemical Engineering,2016,33(4):1352-1361. doi: 10.1007/s11814-014-0334-6 [11] ZHANG Y, LI X L, LI Y F. Influence of solution chemistry on heavy metals removal by bioadsorbent tea waste modified by poly (vinyl alcohol)[J]. Desalination & Water Treatment,2015,53(8):2134-2143. [12] ROCHER V, BEE A, SIAUGUE J M, et al. Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin[J]. Journal of Hazardous Materials,2010,178(1-3):434-439. doi: 10.1016/j.jhazmat.2010.01.100 [13] AUTA M, HAMEED B H. Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes[J]. Colloids and Surfaces B: Biointerfaces,2013,105:199-206. doi: 10.1016/j.colsurfb.2012.12.021 [14] CAI H M, CHEN G J, PENG C Y, et al. Enhanced removal of fluoride by tea waste supported hydrous aluminium oxide nanoparticles: Anionic polyacrylamide mediated aluminium assembly and adsorption mechanism[J]. RSC Advances,2015,5(37):29266-29275. doi: 10.1039/C5RA01560J [15] ZHANG Y, LI X L, LI Y F. Influence of solution chemistry on heavy metals removal by bioadsorbent tea waste modified by poly (vinyl alcohol)[J]. Desalination and Water Treatment,2015,53(8):2134-2143. doi: 10.1080/19443994.2013.861775 [16] ŠILLEROVA H, KOMAREK M, LIU C. Biosorbent encapsulation in calcium alginate: Effects of process variables on Cr(VI) removal from solutions[J]. International Journal of Biological Macromolecules,2015,80:260-270. doi: 10.1016/j.ijbiomac.2015.06.032 [17] WANG B, GAO B, ZIMMERMAN A R, et al. Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties[J]. Journal of Environmental Mangement,2018,209(1):105-111. [18] 于长江, 王苗, 董心雨, 等. 海藻酸钙@Fe3O4/生物碳磁性复合材料的制备及其对Co(Ⅱ)的吸附性能和机制[J]. 复合材料学报, 2018, 35(6):1549-1557.YU C J, WANG M, DONG X Y, et al. Preparation and characterization of calcium alginate@Fe3O4/biochar magnetic microsphere and its adso[J]. Acta Materiae Compositae Sinica,2018,35(6):1549-1557(in Chinese). [19] ROCHER V, SIAUGUE J M, CABUIL V, et al. Removal of organic dyes by magnetic alginate beads[J]. Water Research,2008,42(4):1290-1298. [20] 陈铧耀, 周新华, 周红军, 等. 毒死蜱/壳聚糖改性凹凸棒土/海藻酸钠微球的制备与缓释性能[J]. 化工进展, 2017, 36(3):1033-1040.CHEN H Y, ZHOU X H, ZHOU H J, et al. Preparation and slow-release performance of chlorpyrifos/chitosan modified attapulgite/ sodium alginate microspheres[J]. Chemical industry and Engineering Progress,2017,36(3):1033-1040(in Chinese). [21] 胡平, 常恬, 陈震宇, 等. 纳米Fe3O4磁性颗粒表面改性及其在医学和环保领域的应用[J]. 化工学报, 2017, 68(7):2641-2652.HU P, CHANG K, CHEN Z Y, et al. Surface modification and application in biomedicine and environmental protection of magnetic Fe3O4 nanoparticles[J]. Journal of Chemical Industry and Engineering,2017,68(7):2641-2652(in Chinese). [22] 邢敏, 雷西萍, 韩丁, 等. Fe3O4/高岭土磁性复合材料对Cu2+的吸附性能[J]. 复合材料学报, 2019, 36(9):2204-2211.XING M, LEI X P, HAN D, et al. Adsorption properties of Fe3O4/Kaolin magnetic composites for Cu2+[J]. Acta Materiae Compositae Sinica,2019,36(9):2204-2211(in Chinese). [23] PANNERSELVAM P, MORAD N, TAN K A. Magnetic nanoparticle(Fe3O4) impregnated onto tea waste for the removal of nickel(II) from aqueous solution[J]. Journal of Hazardous Materials,2011,186(1):160-168. doi: 10.1016/j.jhazmat.2010.10.102 [24] 龚新怀, 辛梅华, 李明春, 等. 磁性响应茶渣制备及其对水溶液中亚甲基蓝的吸附[J]. 化工进展, 2019, 38(2):1113-1121.GONG X H, XIN M H, LI M C, et al. Preparation of magnetically responsive tea waste and it's adsorption of methylene blue from aqueous[J]. Chemical Industry and Engineering Progress,2019,38(2):1113-1121(in Chinese). [25] ANNADURAI G, JUANG R S, LE D J, et al. Factorial design analysis for adsorption of dye on activated carbon beads incorporated with calcium alginate[J]. Advances in Environmental Research,2002,6(2):191-198. doi: 10.1016/S1093-0191(01)00050-8 [26] ARAVINDHAN R, FATHIMA N N, RAO J R, et al. Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads[J]. Colloids & Surfaces A Physicochemical & Engineering Aspects,2007,299(1-3):232-238. [27] MADRAKIAN T, AFKHAMI A, AHMADI M. Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2012,99:102-109. doi: 10.1016/j.saa.2012.09.025 [28] GOSWAMI M, BORAH L, MAHANTA D, et al. Equilibrium modeling, kinetic and thermodynamic studies on the adsorption of Cr(VI) using activated carbon derived from matured tea leaves[J]. Journal of Porous Materials,2014,21(6):1025-1034. doi: 10.1007/s10934-014-9852-1 [29] 姚时, 张鸣帅, 李林璇, 等. 茶渣负载纳米四氧化三铁复合材料制备及其对亚甲基蓝的吸附机理[J]. 环境化学, 2018, 37(1):96-107. doi: 10.7524/j.issn.0254-6108.2017050401YAO S, ZHANG M S, LI L X, et al. Preparation of tea waste-nano Fe3O4 composite and its removal mechanism of methylene blue from aqueous solution[J]. Environmental Cheistry,2018,37(1):96-107(in Chinese). doi: 10.7524/j.issn.0254-6108.2017050401 [30] LIN Y B, FUGETSU B, TERUI N, et al. Removal of organic compounds by alginate gel beads with entrapped activated carbon[J]. Journal of Hazardous Materials,2005,120(1-3):237-241. doi: 10.1016/j.jhazmat.2005.01.010 [31] ÜNLU N, ERSOZ M. Removal of heavy metal ions by using dithiocarbamated sporopollenin[J]. Separation & Purification Technology,2007,52(3):461-469. -






 下载:
下载: