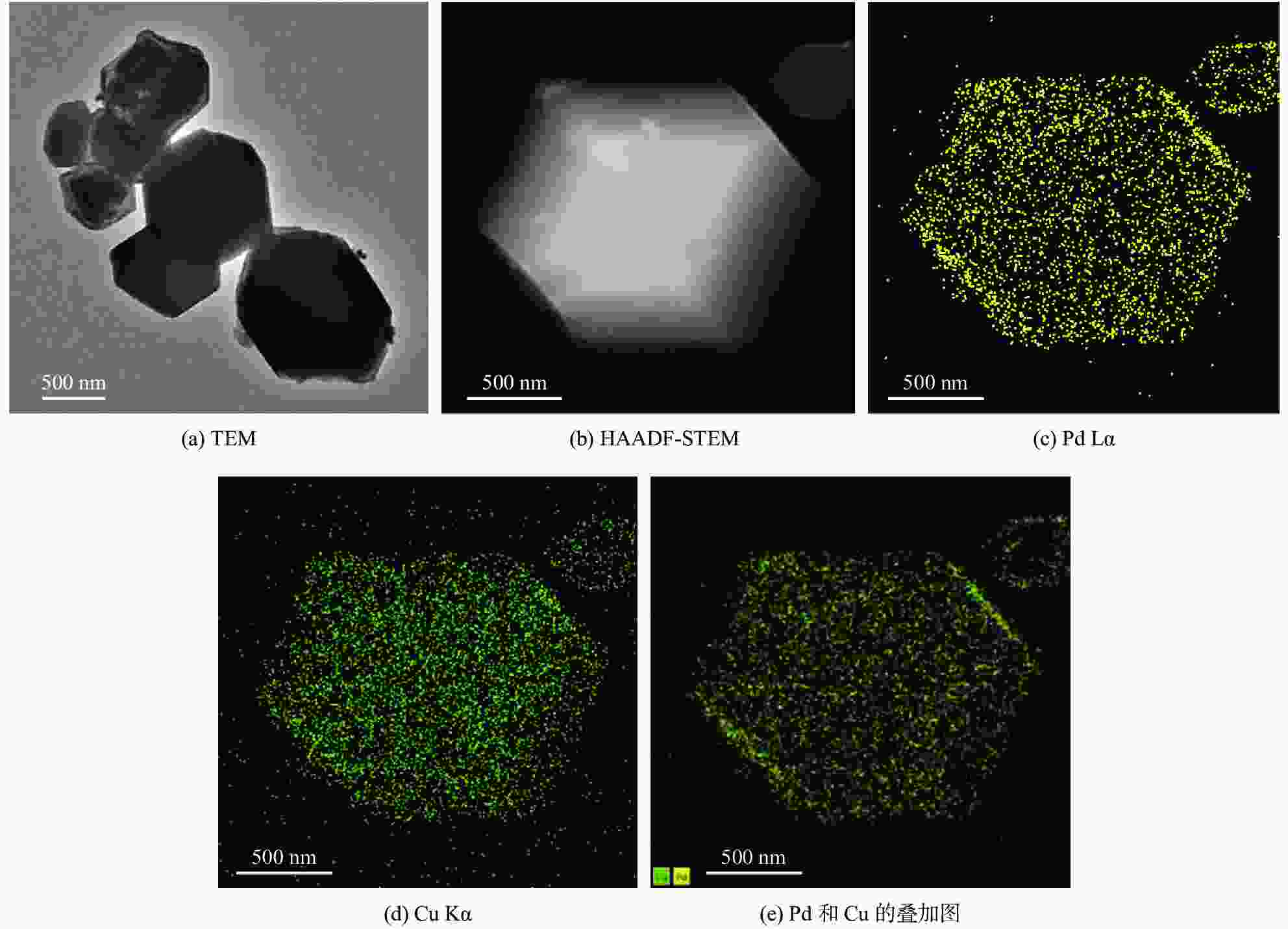
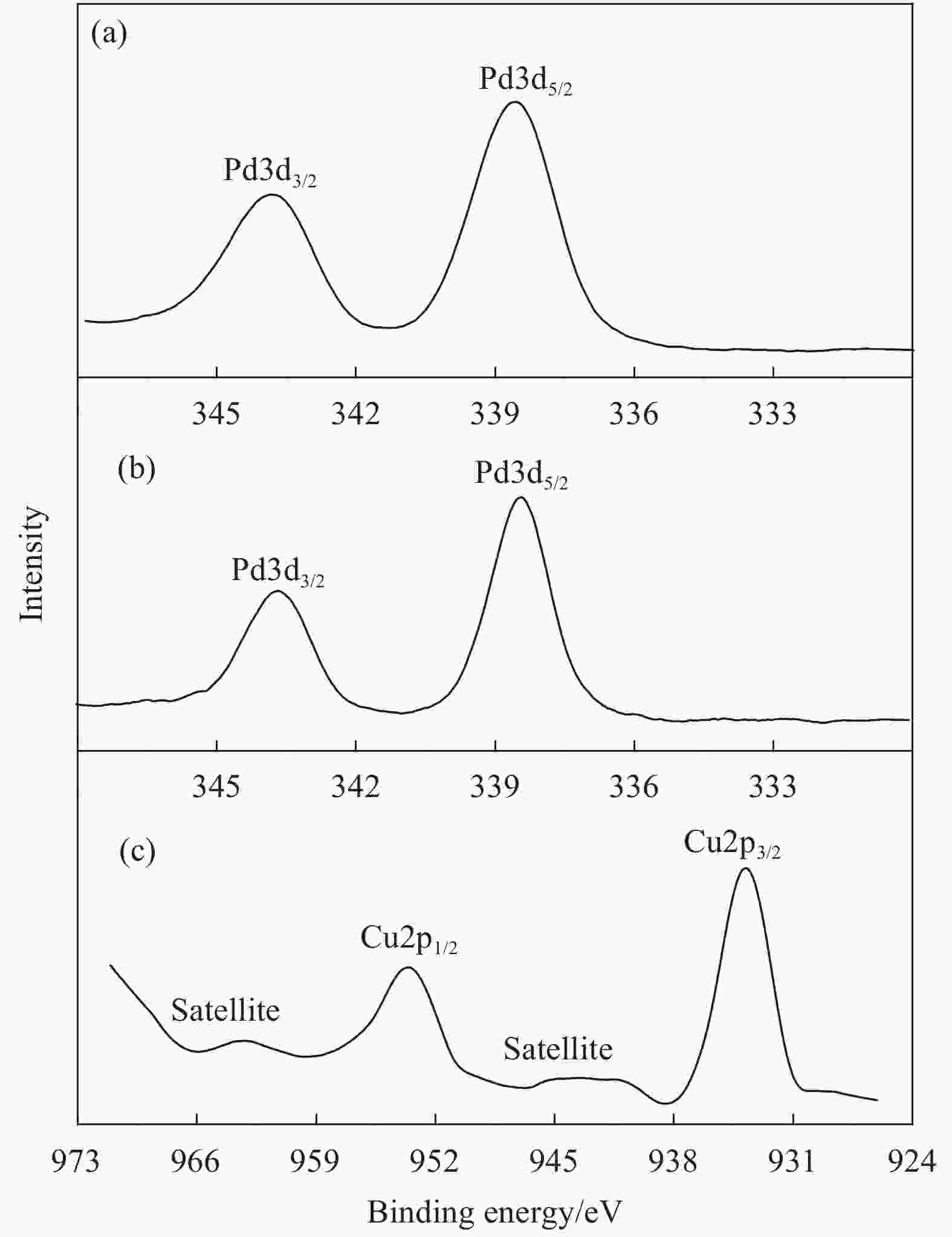
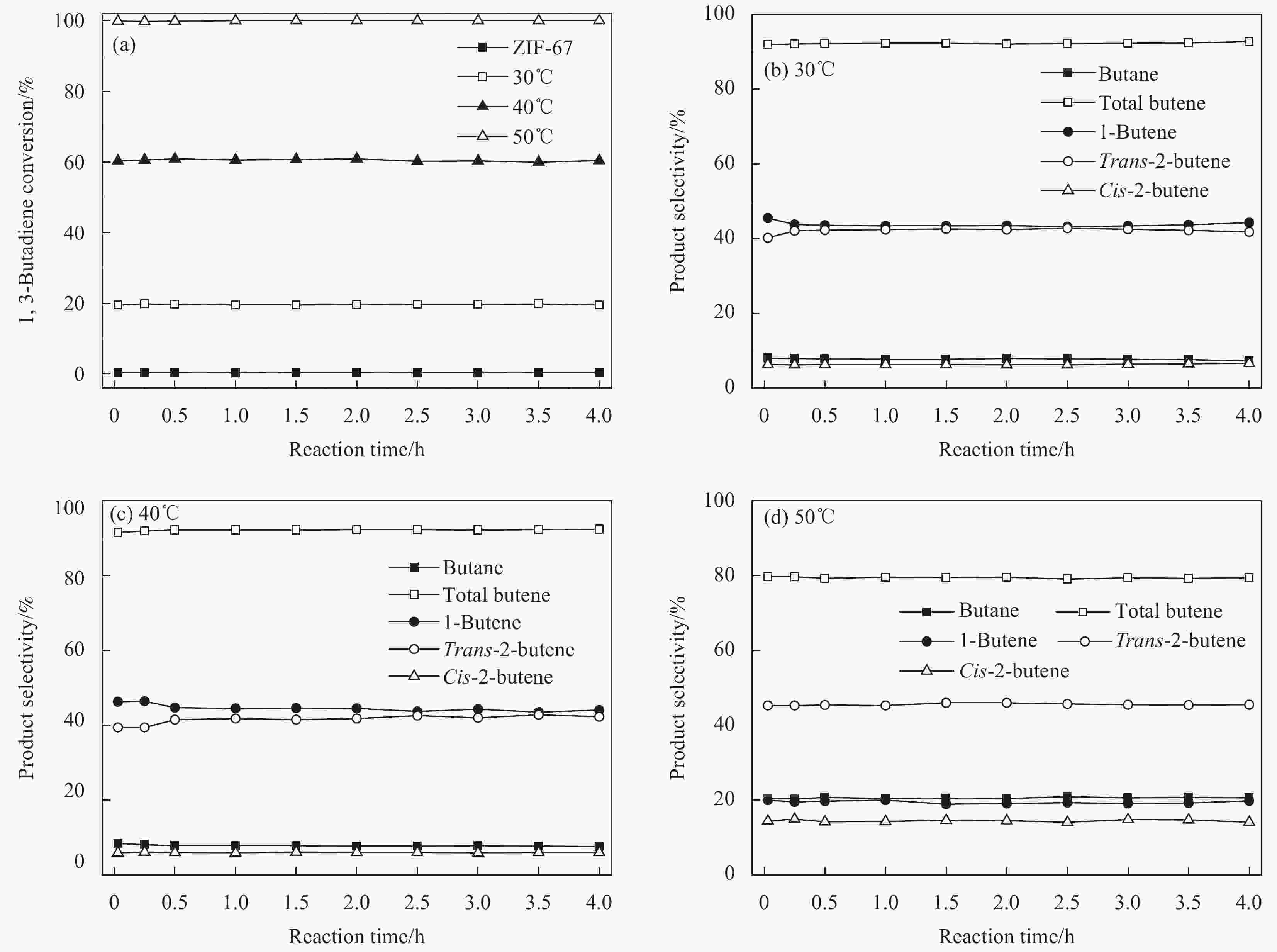
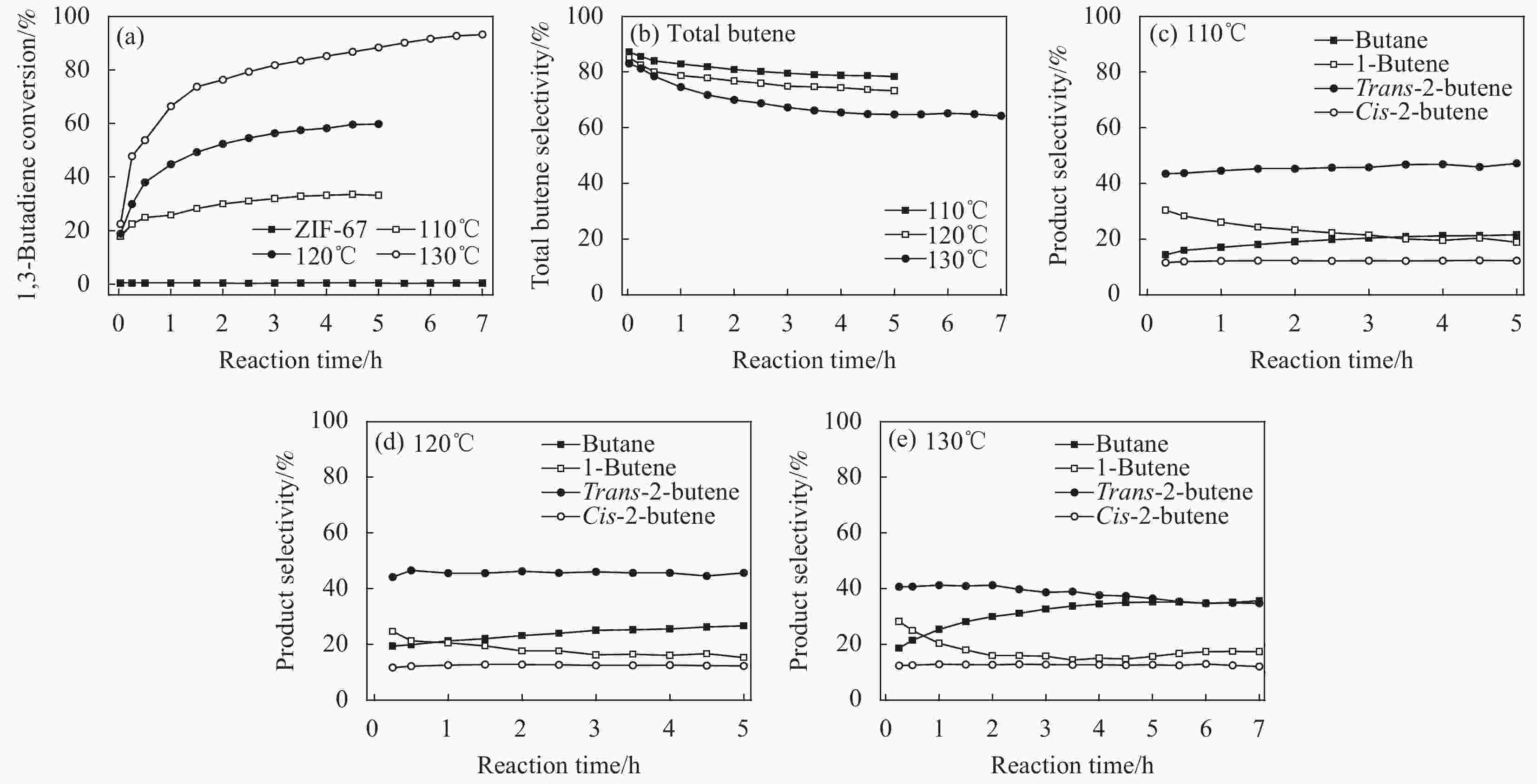
ZIF-67 supported Pd nanoparticles and Pd–Cu nanoparticles for selective hydrogenation of 1,3-butadiene
-
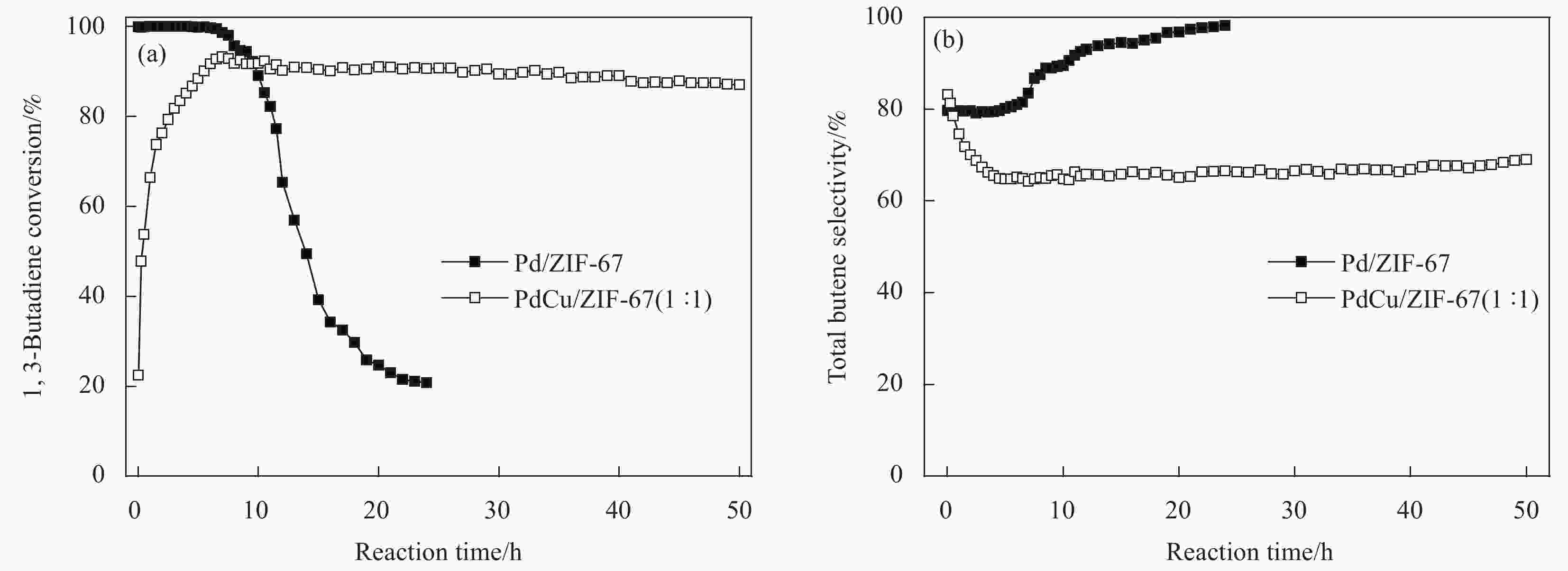
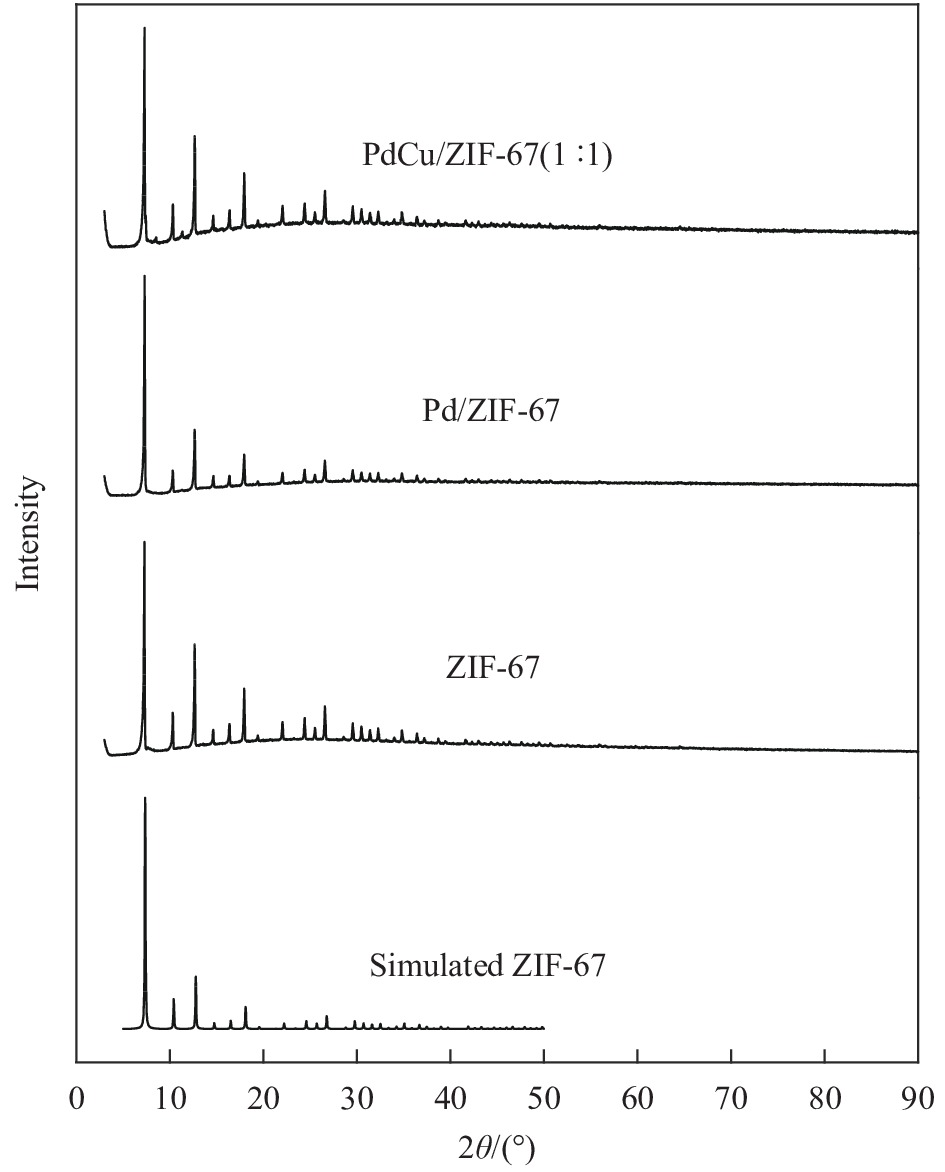
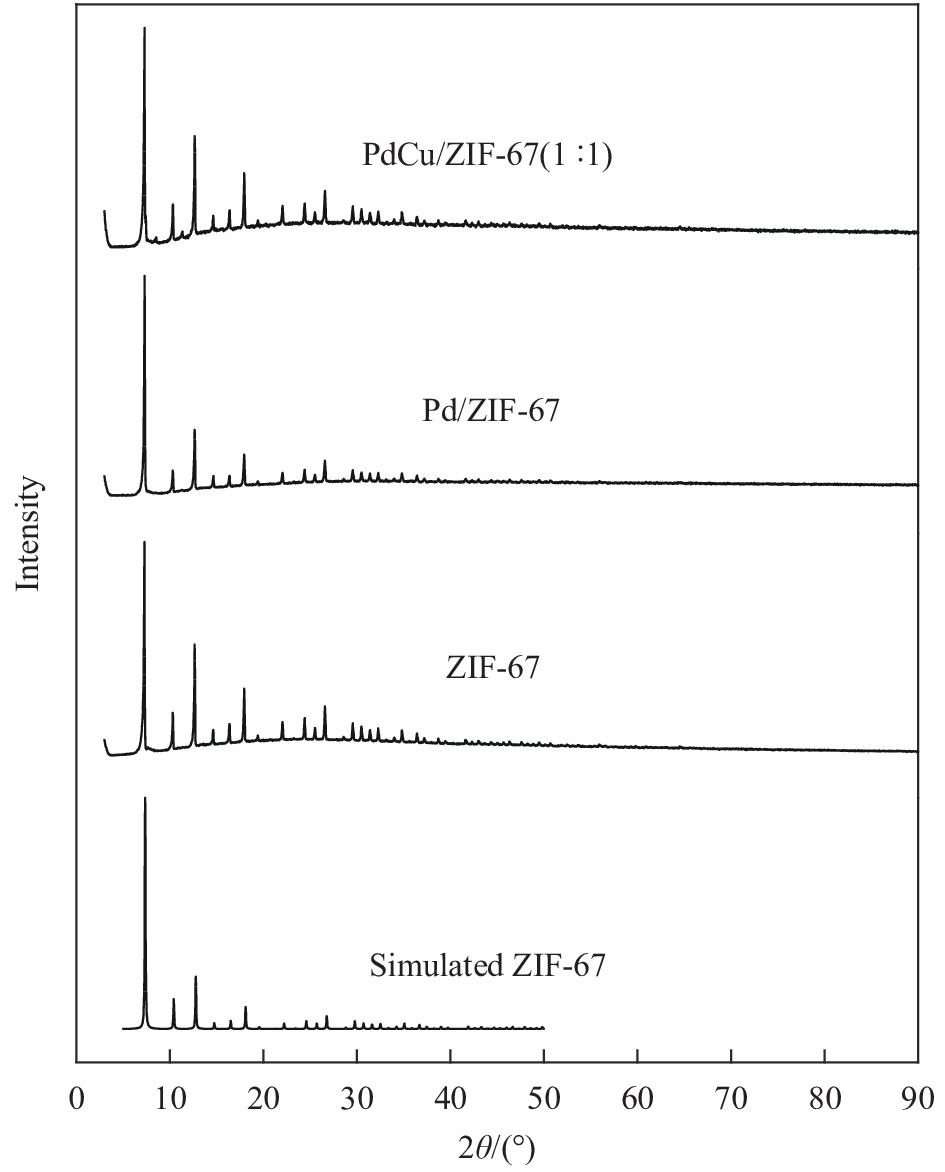
摘要: 1,3-丁二烯选择性加氢是石油化工中有效脱除1,3-丁二烯的方法。选用金属有机骨架ZIF-67作为载体,通过浸渍和H2还原法控制合成了Pd/ZIF-67和PdCu/ZIF-67复合纳米催化剂。采用XRD、氮气低温物理吸附、TEM、EDS、XPS等方法对Pd/ZIF-67和PdCu/ZIF-67进行了系统的物理化学性质表征,并在石英微型固定床上探索了其在1,3-丁二烯加氢反应中的催化活性、选择性和稳定性。结果表明,Pd/ZIF-67和PdCu/ZIF-67(1∶1)中Pd和Cu分别以Pd2+和Cu2+存在,Pd纳米粒子和Pd–Cu纳米粒子高度分散在ZIF-67上。由于金属Pd–Cu和载体ZIF-67之间的相互作用以及Pd和Cu双金属间的几何效应,PdCu/ZIF-67(1∶1)的催化活性低于Pd/ZIF-67。Pd/ZIF-67在50℃催化1, 3-丁二烯加氢时,1,3-丁二烯的转化率和总丁烯的选择性分别为99.9%和79.6%。PdCu/ZIF-67(1∶1)在130℃催化反应7 h后1,3-丁二烯的转化率和总丁烯的选择性分别为93.2%和64.3%。提高Cu的含量,PdCu/ZIF-67对于1,3-丁二烯的转化率降低,而对总丁烯的选择性提高。PdCu/ZIF-67(1∶1)比Pd/ZIF-67具有更高的稳定性,可以连续运行50 h,催化活性和丁烯选择性几乎保持不变。研究结果为新型高效加氢催化剂的设计制备提供了启示和参考。Abstract: Selective hydrogenation of 1,3-butadiene is an effective strategy to remove 1,3-butadiene in the petrochemical industry. ZIF-67 supported monometallic Pd (Pd/ZIF-67) and bimetallic Pd–Cu catalysts (PdCu/ZIF-67) with different Pd:Cu molar ratios (1∶3–3∶1) were synthesized by impregnation and hydrogen reduction. The prepared Pd/ZIF-67 and PdCu/ZIF-67 catalysts were characterized using XRD, N2 adsorption-desorption analysis, TEM, EDS, XPS and ICP-AES. The catalytic performance of supported Pd/ZIF-67 and PdCu/ZIF-67 catalysts were studied in the selective hydrogenation of 1,3-butadiene on the fixed-bed flow quartz reactor under atmospheric pressure. The XPS studies at Pd3d levels and Cu2p levels reveal that Pd and Cu particles on the surface of the ZIF-67 support are in a +2 valence state. TEM and EDS display that Pd nanoparticles and Pd–Cu nanoparticles are uniformly dispersed on ZIF-67. The experiment results show that the catalytic activity of PdCu/ZIF-67(1∶1) is lower than that of Pd/ZIF-67 due to strong interaction between Pd–Cu and ZIF-67 support and the geometric effects, i.e., dilution of blocking of a fraction of the palladium surface by copper. The 1,3-butadiene conversion and butene selectivity reach 99.9% and 79.6% for Pd/ZIF-67 at 50℃, respectively. For the PdCu/ZIF-67(1∶1) catalyst, the 1,3-butadiene conversion and butene selectivity are 93.2% and 64.3% at 130℃ within 7 h, respectively. The hydrogenation activity of PdCu/ZIF-67 catalyst decrease with increasing of Cu content, while the butene selectivity increase. PdCu/ZIF-67(1∶1) show higher stability than Pd/ZIF-67, the conversion of 1,3-butadiene and butene selectivity almost remain the same after continuous run for 50 h at 130℃. The results provide a reference for the design of new high efficiency 1,3-butadiene hydrogenation catalyst.
-
Key words:
- catalyst /
- catalysis /
- hydrogenation /
- nanoparticles /
- bimetallic /
- metal-organic frameworks /
- 1,3-butadiene
-
表 1 Pd和Cu的实际负载量
Table 1. The mass fraction of Pd and Cu
Entry Catalyst wPd/wt% wCu/wt% Pd∶Cu
molar ratio1 PdCu/ZIF-67(3∶1) 3.71 0.72 3∶1 2 PdCu/ZIF-67(2∶1) 3.56 1.16 2∶1 3 PdCu/ZIF-67(1∶1) 3.32 2.05 1∶1 4 PdCu/ZIF-67(1∶2) 3.32 3.72 1∶2 5 PdCu/ZIF-67(1∶3) 2.98 5.26 1∶3 6 Pd/ZIF-67 3.30 — — 表 2 ZIF-67、Pd/ZIF-67和PdCu/ZIF-67(1∶1)的物理织构性质
Table 2. The textural properties of ZIF-67, Pd/ZIF-67 and PdCu/ZIF-67(1∶1)
Sample BET/
(m2·g−1)Mean pore
diameter/nmVolume/
(cm3·g−1)ZIF-67 5915.6 1.7 2.6 Pd/ZIF-67 2036.2 1.5 0.7 PdCu/ZIF-67(1:1) 813.8 1.5 0.3 表 3 不同的Pd基催化剂催化1,3-丁二烯加氢时的转化率和总丁烯的选择性
Table 3. The 1,3-butadiene conversion and total butene selectivity for selective hydrogenation of 1,3-butadiene over different Pd-based catalysts
Entry Catalyst Temperature/°C Conversion/% Total butene selectivity/% Ref. 1 PdCu/ZIF-67(1:1) 130 93.2 64.3 This work 2 PdCuG–DBT-423 150 39 4 [53] 3 Au(2)Pd(1)/MIL-101(Cr) 60 98.8 95.7 [1] 4 Ni1Pd1/ZnO 35 98.8 88.9 [14] 5 Ni1Pd1/SiO2 35 44.0 41.3 [14] 6 PdAu–ZnO-n 40 92 23 [54] 7 PdAu–ZnO-t 40 99 32 [54] 8 Pd/ZIF-67 50 100 79.6 This work 9 PdG–DBT-423 150 25 1 [53] 10 fresh-Pd/g-Al2O3 35 100 51.2 [55] 11 H2–Pd/g-Al2O3 35 100 8.7 [55] Notes: ZIF—Zeolite imidazolate framework; MIL—Materials of institut lavoisier; DBT—Dibenzothiophene. -
[1] LIU L, ZHOU X, GUO L, et al. Bimetallic Au–Pd alloy nanoparticles supported on MIL-101(Cr) as highly efficient catalysts for selective hydrogenation of 1,3-butadiene[J]. RSC Advances,2020,10(55):33417-33427. doi: 10.1039/D0RA06432G [2] YI H, XIA Y, YAN H, et al. Coating Pd/Al2O3 catalysts with FeOx enhances both activity and selectivity in 1,3-butadiene hydrogenation[J]. Chinese Journal of Catalysis,2017,38(9):1581-1587. doi: 10.1016/S1872-2067(17)62768-2 [3] LIU L, TAI X, ZHOU X, et al. Au-Pt bimetallic nanoparticle catalysts supported on UiO-67 for selective 1,3-butadiene hydrogenation[J]. Journal of the Taiwan Institute of Chemical Engineers,2020,114:220-227. doi: 10.1016/j.jtice.2020.09.025 [4] GUO Y, YANG J, ZHUANG J, et al. Selectively catalytic hydrogenation of styrene-butadiene rubber over Pd/g-C3N4 catalyst[J]. Applied Catalysis A: General,2020,589:117312-117320. doi: 10.1016/j.apcata.2019.117312 [5] ZHANG Z C, ZHANG X, YU Q Y, et al. Pd cluster nanowires as highly efficient catalysts for selective hydrogenation reactions[J]. Chemistry-A European Journal,2012,18(9):2639-2645. doi: 10.1002/chem.201102903 [6] DECAROLIS D, LEZCANO-GONZALEZ I, GIANOLIO D, et al. Effect of particle size and support type on Pd catalysts for 1,3-butadiene hydrogenation[J]. Topics in Catalysis,2018,61:162-174. doi: 10.1007/s11244-018-0887-4 [7] LU F, SUN D, JIANG X. Plant-mediated synthesis of AgPd/c-Al2O3 catalysts for selective hydrogenation of 1,3-butadiene at low temperature[J]. New Journal of Chemistry,2019,43(35):13891-13897. doi: 10.1039/C9NJ01733J [8] YI H, DU H, HU Y, et al. Precisely controlled porous alumina overcoating on Pd catalyst by atomic layer deposition: Enhanced selectivity and durability in hydrogenation of 1,3-butadiene[J]. ACS Catalysis,2015,5(5):2735-2739. doi: 10.1021/acscatal.5b00129 [9] YAN H, CHENG H, YI H, et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene[J]. Journal of the American Chemical Society,2015,137(33):10484-10487. doi: 10.1021/jacs.5b06485 [10] HOU R, YU W, POROSOFF M D, et al. Selective hydrogenation of 1,3-butadiene on Pd–Ni bimetallic catalyst: From model surfaces to supported catalysts[J]. Journal of Catalysis,2014,316:1-10. doi: 10.1016/j.jcat.2014.04.015 [11] PATTAMAKOMSAN K, EHRET E, MORFIN F, et al. Selective hydrogenation of 1,3-butadiene over Pd and Pd–Sn catalysts supported on different phases of alumina[J]. Catalysis Today,2011,164(1):28-33. doi: 10.1016/j.cattod.2010.10.013 [12] LIU L, ZHOU X, LIU L, et al. Heterogeneous bimetallic Cu–Ni nanoparticle-supported catalysts in the selective oxidation of benzyl alcohol to benzaldehyde[J]. Catalysts,2019,9(6):538-555. doi: 10.3390/catal9060538 [13] KIM T W, KIM M, KIM S K K, et al. Remarkably fast low-temperature hydrogen storage into aromatic benzyltoluenes over MgO-supported Ru nanoparticles with homolytic and heterolytic H2 adsorption[J]. Applied Catalysis B: Environmental,2021,286:119889-119901. doi: 10.1016/j.apcatb.2021.119889 [14] HUANG J, ODOOM-WUBAH T, JING X, et al. Plant-mediated synthesis of zinc oxide supported nickel-palladium alloy catalyst for the selective hydrogenation of 1,3-butadiene[J]. ChemCatChem,2017,9(5):870-881. doi: 10.1002/cctc.201601178 [15] 叶晓栋, 齐国栋, 徐君, 等. Au负载SBA-15分子筛上葡萄糖氧化反应[J]. 高等学校化学学报, 2020, 41(5):960-966. doi: 10.7503/cjcu20200070YE Xiaodong, QI Guodong, XU Jun, et al. Glucose oxidation on Au-supported SBA-15 molecular sieve[J]. Chemical Journal Of Chinese Universities,2020,41(5):960-966(in Chinese). doi: 10.7503/cjcu20200070 [16] 孙思齐, 王影, 孙传胤, 等. 碗状双亲型 ZSM-5分子筛负载金纳米粒子的制备及催化性能[J]. 高等学校化学学报, 2019, 40(12):2436-2442. doi: 10.7503/cjcu20190415SUN Siqi, WANG Ying, SUN Chuanyin, et al. Preparation and catalytic bowl-shaped amphiphilic ZSM-5 zeolites supported gold nanoparticles[J]. Chemical Journal of Chinese Universities,2019,40(12):2436-2442(in Chinese). doi: 10.7503/cjcu20190415 [17] DENG P, HONG W, CHENG Z, et al. Facile fabrication of nickel/porous g-C3N4 by using carbon dot as template for enhanced photocatalytic hydrogen production[J]. International Journal of Hydrogen Energy,2020,45(58):33543-33551. doi: 10.1016/j.ijhydene.2020.09.115 [18] SALAMA R S, MANNAA M A, ALTASS H M, et al. Palladium supported on mixed-metal–organic framework (Co–Mn-MOF-74) for efficient catalytic oxidation of CO[J]. RSC Advances,2021,11(8):4318-4326. doi: 10.1039/D0RA09970H [19] RAPTOPOULOU C P. Metal-organic frameworks: Synthetic methods and potential applications[J]. Materials,2021,14(2):310-341. doi: 10.3390/ma14020310 [20] TO T, TRAN C B, NGUYEN N T H, et al. An efficient access to β-ketosulfones via β-sulfonylvinylamines: Metal-organic framework catalysis for the direct C—S coupling of sodium sulfinates with oxime acetates[J]. RSC Advances,2018,8(31):17477-17485. doi: 10.1039/C8RA02389A [21] NOORI Y, AKHBARI K. Post-synthetic ion-exchange process in nanoporous metal-organic frameworks: An effective way for modulating their structures and properties[J]. RSC Advances,2017,7(4):1782-1808. doi: 10.1039/C6RA24958B [22] LIU L, TAI X, ZHOU X, et al. Bimetallic Au-Ni alloy nanoparticles in a metal-organic framework (MIL-101) as efficient heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde[J]. Journal of Alloys Compounds,2019,790:326-336. doi: 10.1016/j.jallcom.2019.03.186 [23] LIU L, ZHOU X, YAN Y, et al. Bimetallic gold-silver nanoparticles supported on zeolitic imidazolate framework-8 as highly active heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde[J]. Polymers,2018,10(10):1089-1104. doi: 10.3390/polym10101089 [24] GUO Z, XIAO C, MALIGAL-GANESH R V. Pt nanoclusters confined within metal-organic framework cavities for chemoselective cinnamaldehyde hydrogenation[J]. ACS Catalysis,2014,4(5):1340-1348. doi: 10.1021/cs400982n [25] LI X, GUO Z, XIAO C, et al. Tandem catalysis by palladium nanoclusters encapsulated in metal-organic frameworks[J]. ACS Catalysis,2014,4(10):3490-3497. doi: 10.1021/cs5006635 [26] YOSHIMARU S, SADAKIYO M, MAEDA N, et al. Support effffect of metal-organic frameworks on ethanol production through acetic acid hydrogenation[J]. ACS Applied Materials & Interfaces,2021,13(17):19992-20001. [27] LIU H, CHANG L, CHEN L, et al. Nanocomposites of platinum/metal-organic frameworks coated with metal-organic frameworks with remarkably enhanced chemoselectivity for cinnamaldehyde hydrogenation[J]. ChemCatChem,2016,8(5):946-951. doi: 10.1002/cctc.201501256 [28] HU W, YUAN K, SONG T, et al. Highly effective and selective catalysts for cinnamaldehyde hydrogenation by hydrophobic hybrids of metal-organic frameworks, metal nanoparticles and micro- & mesoporous polymers[J]. Angewandte Chemie,2018,57(20):5708-5713. doi: 10.1002/anie.201801289 [29] CHEN J, LIU R, GUO Y, et al. Selective hydrogenation of biomass-based 5-hydroxymethylfurfural over catalyst of palladium immobilized on amine-functionalized metal-organic frameworks[J]. ACS Catalysis,2015,5(2):722-733. doi: 10.1021/cs5012926 [30] ZHANG W, SHI W, JI W, et al. Microenvironment of MOF channel coordination with Pt NPs for selective hydrogenation of unsaturated aldehydes[J]. ACS Catalysis,2020,10(10):5805-5813. doi: 10.1021/acscatal.0c00682 [31] ERTAS I E, GULCAN M, BULUT A, et al. Metal-organic framework (MIL-101) stabilized ruthenium nanoparticles: Highly effificient catalytic material in the phenol hydrogenation[J]. Microporous and Mesoporous Materials,2016,226:94-103. doi: 10.1016/j.micromeso.2015.12.048 [32] FENG J, LI M, ZHONG Y, et al. Hydrogenation of levulinic acid to γ-valerolactone over Pd@UiO-66-NH2 with high metal dispersion and excellent reusability[J]. Microporous and Mesoporous Materials,2019,294:109858-109866. [33] LUO S, ZENG Z, ZENG G, et al. Metal organic frameworks as robust host of Pd nanoparticles in heterogeneous catalysis: Synthesis, application, and prospect[J]. ACS Applied Materials & Interfaces,2019,11(36):32579-32598. [34] OTTO T, JARENWATTANANON N N, GLOGGLER S, et al. Effects of multivariate linker substitution, metal binding, and reactor conditions on the catalytic activity of a Pd-functionalized MOF for olefin hydrogenation[J]. Applied Catalysis A: General,2014,488:248-255. doi: 10.1016/j.apcata.2014.10.012 [35] LI X L, GOH T W, LI L, et al. Controlling catalytic properties of Pd nanoclusters through their chemical environment at the atomic level using isoreticular metal-organic frameworks[J]. ACS Catalysis,2016,6(6):3461-3468. doi: 10.1021/acscatal.6b00397 [36] JIANG Y, ZHANG X, DAI X P, et al. In situ synthesis of core-shell Pt-Cu frame@metal-organic frameworks as multifunctional catalysts for hydrogenation reaction[J]. Chemistry of Materials,2017,29(15):6336-6345. doi: 10.1021/acs.chemmater.7b01636 [37] JIANG Y, LIU G, WU S, et al. Enhanced performance of well-dispersed Co species incorporated on porous carbon derived from metal-organic frameworks in 1,3-butadiene hydrogenation[J]. Microporous and Mesoporous Materials,2019,288:109557-109565. doi: 10.1016/j.micromeso.2019.06.019 [38] WEN H, ZHANG S, YU T, et al. ZIF-67-based catalysts for oxygen evolution reaction[J]. Nanoscale,2021,13(28):12058-12087. doi: 10.1039/D1NR01669E [39] BIBI S, PERVAIZ E, ALI M. Synthesis and applications of metal oxide derivatives of ZIF-67: A mini-review[J]. Chemical Papers,2021,75:2253-2275. doi: 10.1007/s11696-020-01473-y [40] CHU C, RAO S, MA Z, et al. Copper and cobalt nanoparticles doped nitrogen-containing carbon frameworks derived from CuO-encapsulated ZIF-67 as high-efficiency catalyst for hydrogenation of 4-nitrophenol[J]. Applied Catalysis B: Environmental,2019,256:117792-117800. doi: 10.1016/j.apcatb.2019.117792 [41] BUDI C S, DEKA J R, HSU W C, et al. Bimetallic Co/Zn zeolitic imidazolate framework ZIF-67 supported Cu nanoparticles: An excellent catalyst for reduction of synthetic dyes and nitroarenes[J]. Journal of Hazardous Materials,2021,407:124392-124405. doi: 10.1016/j.jhazmat.2020.124392 [42] 魏磊, 刘洪燕, 王东升, 等. ZIF-67及其母液衍生Co3O4催化氨硼烷水解制氢[J]. 硅酸盐学报, 2020, 48(3):455-462.WEI Lei, LIU Hongyan, WANG Dongsheng, et al. ZIF-67 and its mother liquor derived Co3O4 as catalyst precursors for hydrolysis of ammonia borane to generate hydrogen[J]. Journal of the Chinese Ceramic Society,2020,48(3):455-462(in Chinese). [43] KIM D, KIM D, JEON Y, et al. Zeolitic imidazolate frameworks derived novel polyhedral shaped hollow Co-B-O@Co3O4 electrocatalyst for oxygen evolution reaction[J]. Electrochimica Acta,2019,299:213-221. doi: 10.1016/j.electacta.2019.01.005 [44] ZHAO J J, QUAN X, CHEN S, et al. Cobalt nanoparticles encapsulated in porous carbons derived from core-shell ZIF67@ZIF8 as efficient electrocatalysts for oxygen evolution reaction[J]. ACS Applied Materials & Interfaces,2017,9(34):28685-28694. [45] AMARANTE S F, FREIRE M A, MENDES D T S L, et al. Evaluation of basic sites of ZIFs metal organic frameworks in the knoevenagel condensation reaction[J]. Applied Catalysis A: General,2017,548:47-51. doi: 10.1016/j.apcata.2017.08.006 [46] 任勇, 袁涛, 刘德蓉, 等. Pd-Cu/Fe3O4@C催化1,4-丁炔二醇选择性加氢的研究[J]. 化学研究与应用, 2017, 29(11):1686-1692. doi: 10.3969/j.issn.1004-1656.2017.11.012REN Yong, YUAN Tao, LIU Derong, et al. Study on selective hydrogenation of 1,4-butynediol by Pd-Cu/Fe3O4@C catalyst[J]. Chemical Research and Application,2017,29(11):1686-1692(in Chinese). doi: 10.3969/j.issn.1004-1656.2017.11.012 [47] 林欣燕, 马静静, 周雪梅. 高效催化Suzuki偶联反应的催化剂–石墨烯负载纳米Pd-Cu合金纳米复合材料[J]. 合成化学研究, 2013, 1(2):5-9. doi: 10.12677/SSC.2013.12002LIN Xinyan, MA Jingjing, ZHOU Xuemei. A highly active catalyst for Suzuki cross-coupling reactions Pd-Cu bimetallic nanoparticles supported on graphene nanocomposites[J]. Studies in Synthetic Chemistry,2013,1(2):5-9(in Chinese). doi: 10.12677/SSC.2013.12002 [48] MUKHERJEE A, SU W N, PAN C J, et al. One pot synthesis of Pd@CuO core-shell nanoparticles for electro catalytic oxidation of ethylene glycol for alkaline direct fuel cell[J]. Journal of Electroanalytical Chemistry,2021,882:115006-115014. doi: 10.1016/j.jelechem.2021.115006 [49] ZHANG X, GUO Y C, ZHANG Z C, et al. High performance of carbon nanotubes confining gold nanoparticles for selective hydrogenation of 1,3-butadiene and cinnamaldehyde[J]. Journal of Catalysis,2012,292:213-226. doi: 10.1016/j.jcat.2012.05.017 [50] NAGY G, GÁL T, SRANKÓ D F, et al. Selective aerobic oxidation of benzyl alcohol on alumina supported Au-Ru and Au-Ir catalysts[J]. Molecular Catalysis,2020,492:110917-110930. doi: 10.1016/j.mcat.2020.110917 [51] PEREIRA M M, NORONHA F B, SCHMAL M. SMSI effect in the butadiene hydrogenation bimetallic catalysts[J]. Catalysis Today,1993,16(3-4):407-415. doi: 10.1016/0920-5861(93)80080-K [52] KANG M, SONG M W, KIM K L. SMSI effect on ceria supported Cu-Pd catalysts in the hydrogenation of 1,3-butadiene[J]. Reaction Kinetics & Catalysis Letters,2002,75(1):177-183. [53] COOPER A, BACHILLER-BAEZA B, ANDERSON J A, et al. Design of surface sites for the selective hydrogenation of 1,3-butadiene on Pd nanoparticles: Cu bimetallic formation and sulfur poisoning[J]. Catalysis Science & Technology,2014,4(5):1446-1455. [54] BACHILLER-BAEZA B, IGLESIAS-JUEZ A, AGOSTINI G, et al. Pd–Au bimetallic catalysts supported on ZnO for selective 1,3-butadiene hydrogenation[J]. Catalysis Science & Technology,2020,10:2503-2512. [55] LU F, XU Y, JIANG X, et al. Biosynthesized Pd/g-Al2O3 catalysts for low-temperature 1,3-butadiene hydrogenation: The effect of calcination atmosphere[J]. New Journal of Chemistry,2017,41(21):13036-13042. doi: 10.1039/C7NJ02557B -





 下载:
下载: