Supercritical CO2 fluid assisted synthesis of Si-Fe-Fe3O4-C composites and lithium storage performance
-
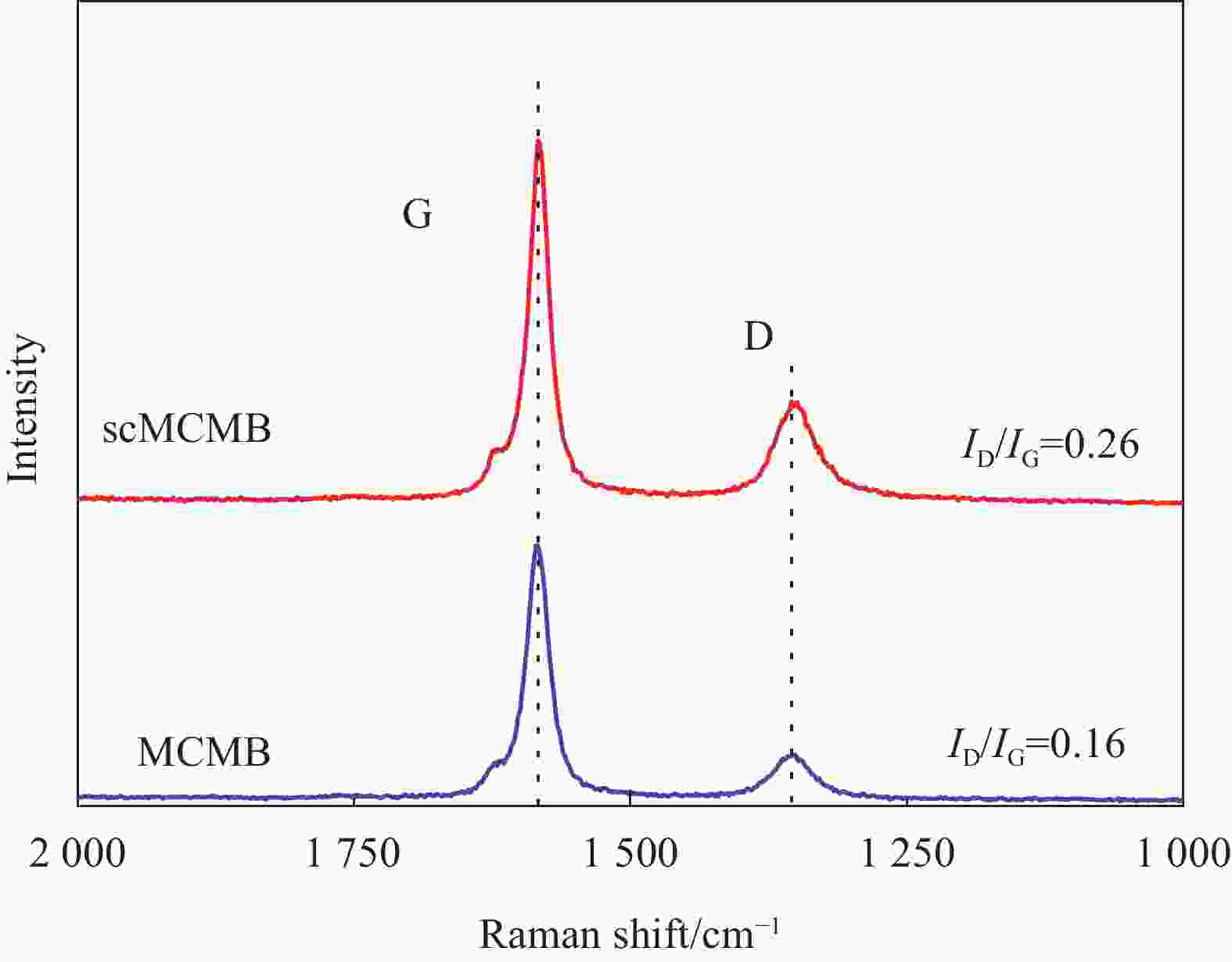
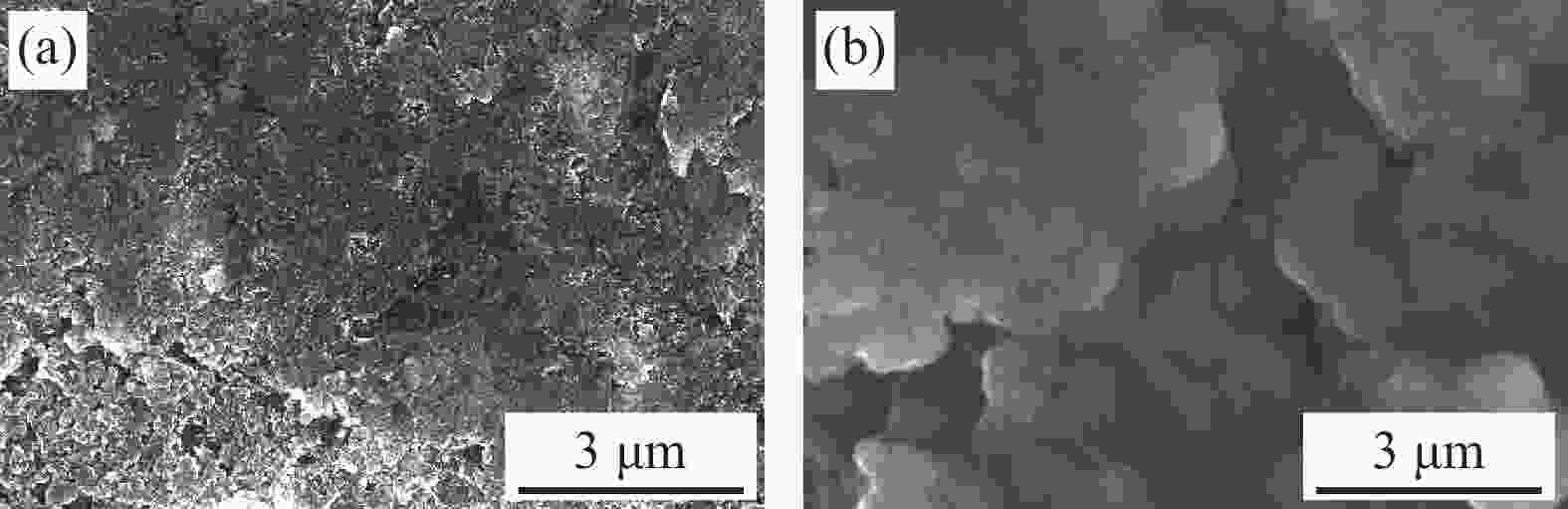
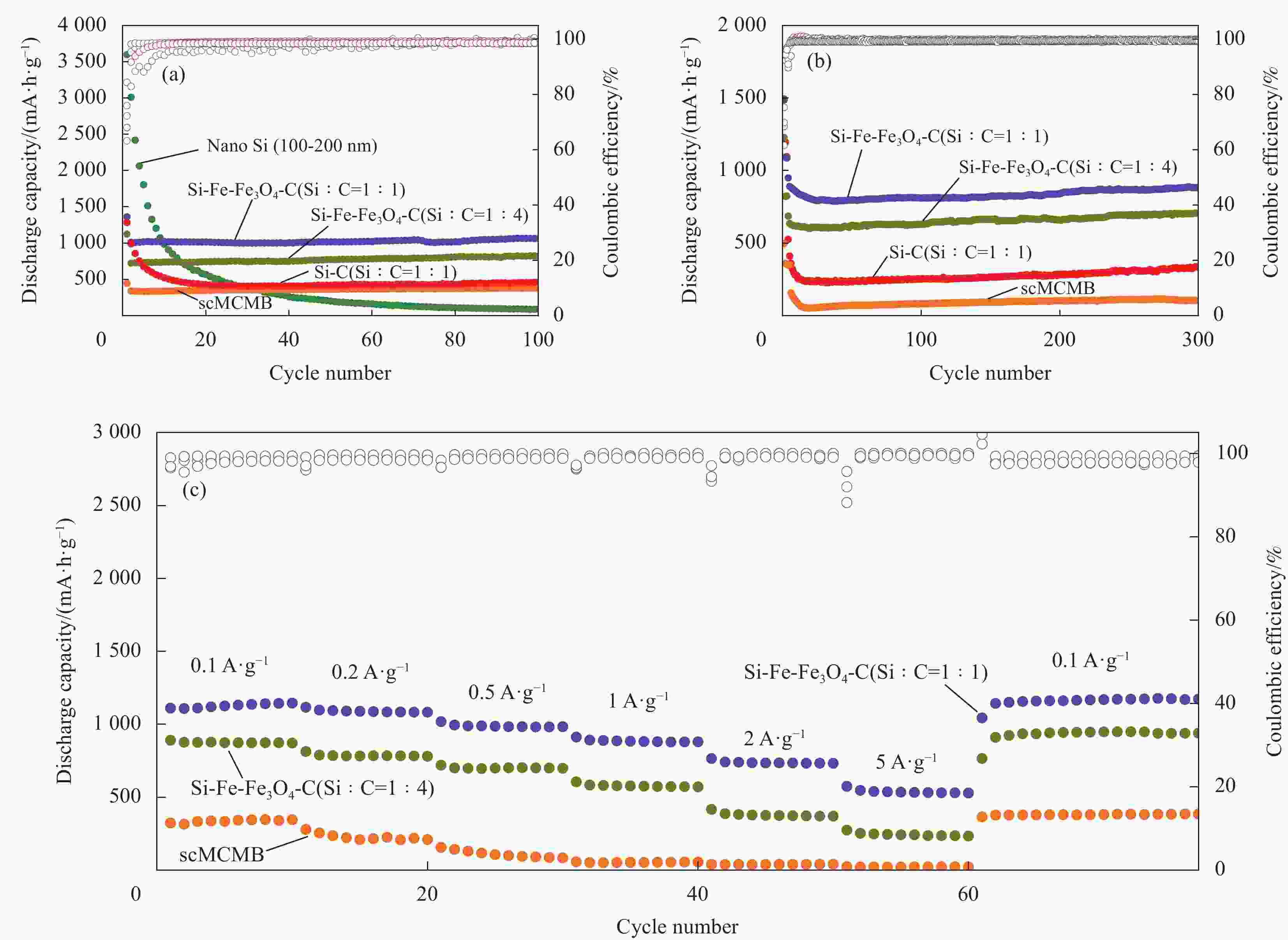
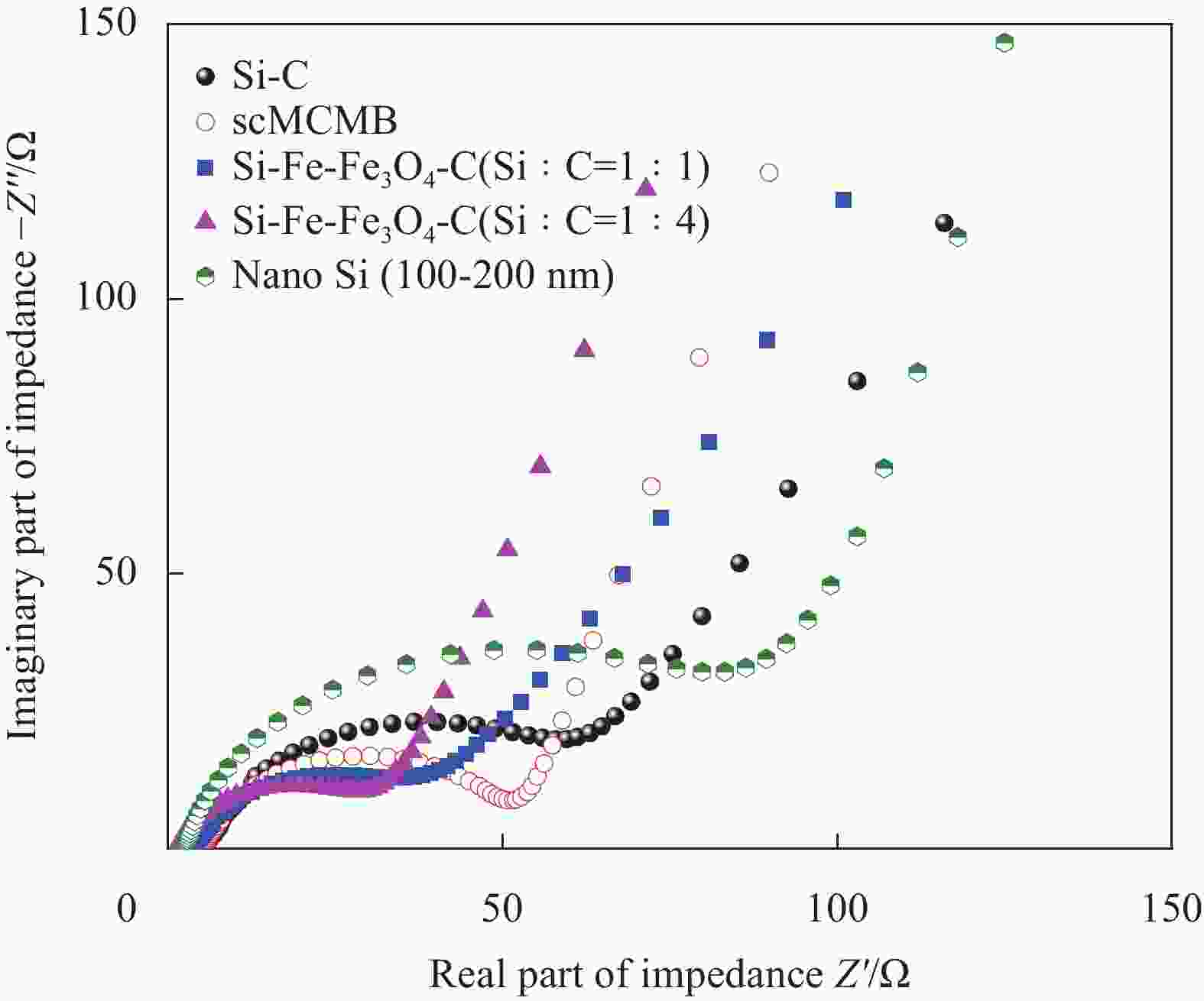
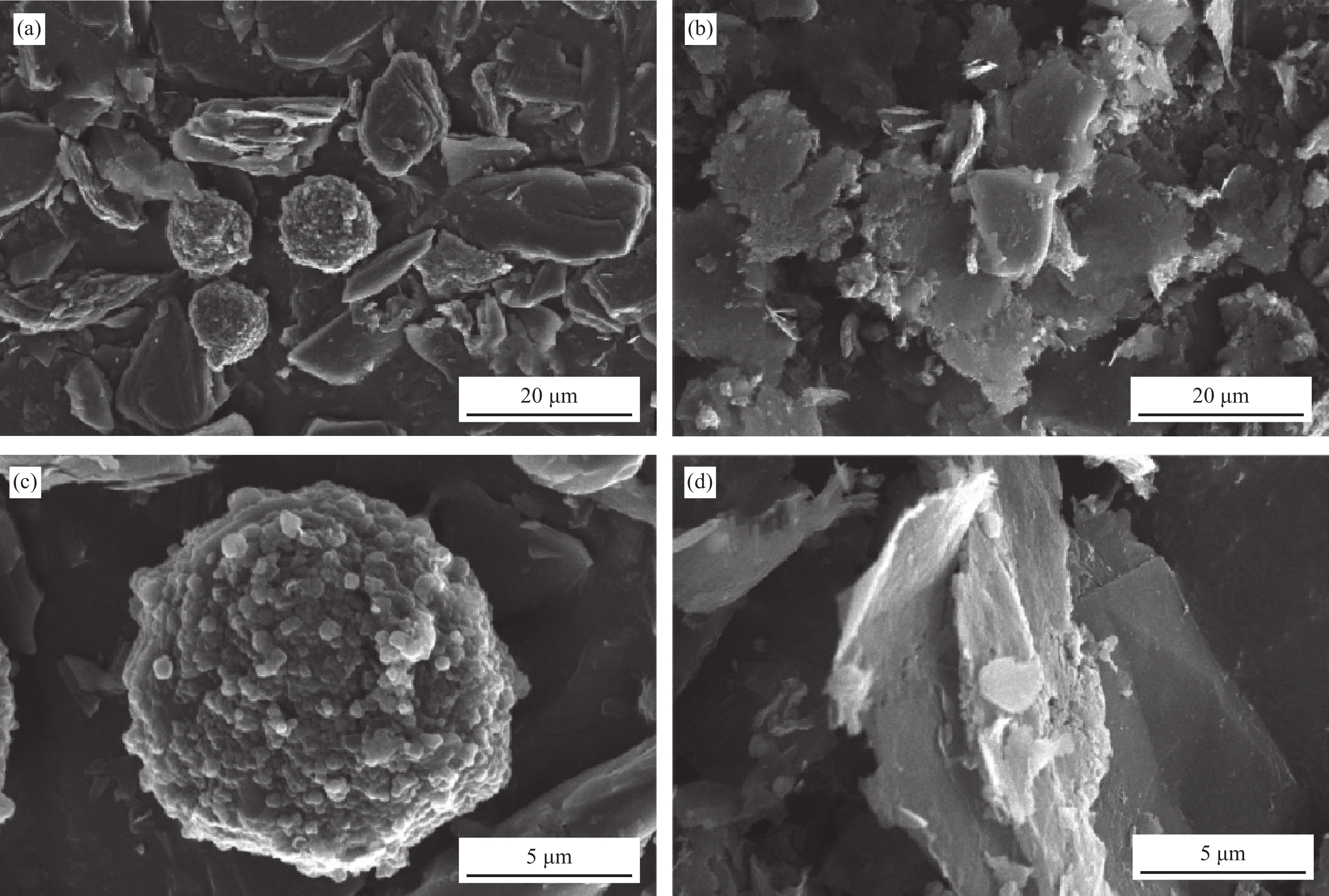
摘要: 硅碳负极是未来锂离子电池材料发展的重点方向之一,本文针对传统球磨法制备硅碳负极复合不均匀、界面融合差等问题,提出了一种超临界二氧化碳(scCO2)流体介质球磨合成Si-Fe-Fe3O4-C复合材料的新方法。研究发现,纳米硅和中间相碳微球(MCMB)在scCO2介质球磨混合过程中,CO2和Fe反应先得到均匀分散的Si-FeCO3-C前驱体,然后FeCO3原位高温固相分解得到Si-Fe-Fe3O4-C复合材料。同时,在scCO2流体渗透下,MCMB剥离成石墨片,并与纳米硅和Fe-Fe3O4实现较好的界面融合,Fe-Fe3O4的引入显著提升了硅碳负极的储锂容量、循环稳定性和倍率性能,Si-Fe-Fe3O4-C复合材料在0.2 A·g−1下100次循环后可逆容量保持在1065 mA·h·g−1。本方法利用超临界流体渗透性好、扩散能力强等特点,合成工艺简便,容易工业化实施,具有商业化开发潜力。
-
关键词:
- 硅碳负极 /
- 超临界流体 /
- CO2 /
- Si-Fe-Fe3O4-C复合材料 /
- 储锂性能
Abstract: Silicon-carbon anode is an important issue for the development of lithium-ion battery materials. Aiming at the problems of uneven combination and poor interfacial contact of silicon-carbon anode prepared by traditional ball milling, this paper proposes a new strategy to synthesize Si-Fe-Fe3O4-C composite by ball milling in supercritical carbon dioxide (scCO2) fluid medium. It is found that during the process of ball milling mixture of nano-silicon and mesophase carbon microspheres (MCMB) in the scCO2 medium, CO2 and Fe reacts firstly to form a uniformly dispersed Si-FeCO3-C precursor, and then in situ high temperature decomposition of FeCO3 solid phase results in final Si-Fe-Fe3O4-C product. Under the infiltration of scCO2 fluid, MCMB microspheres exfoliate into graphite flakes, and achieve ideal combination with nano-silicon and Fe-Fe3O4. The introduction of Fe-Fe3O4 in the composite has significantly improved the lithium storage capacity, cycle stability and rate performance of silicon-carbon anode, the synthesized Si-Fe-Fe3O4-C composite material maintains a reversible capacity of 1065 mA·h·g−1 after 100 cycles at 0.2 A·g−1. The method shows the merits of facile operation procedure, easy industrial production and potential commercial application basing on the supercritical fluid permeability and strong diffusion ability. -
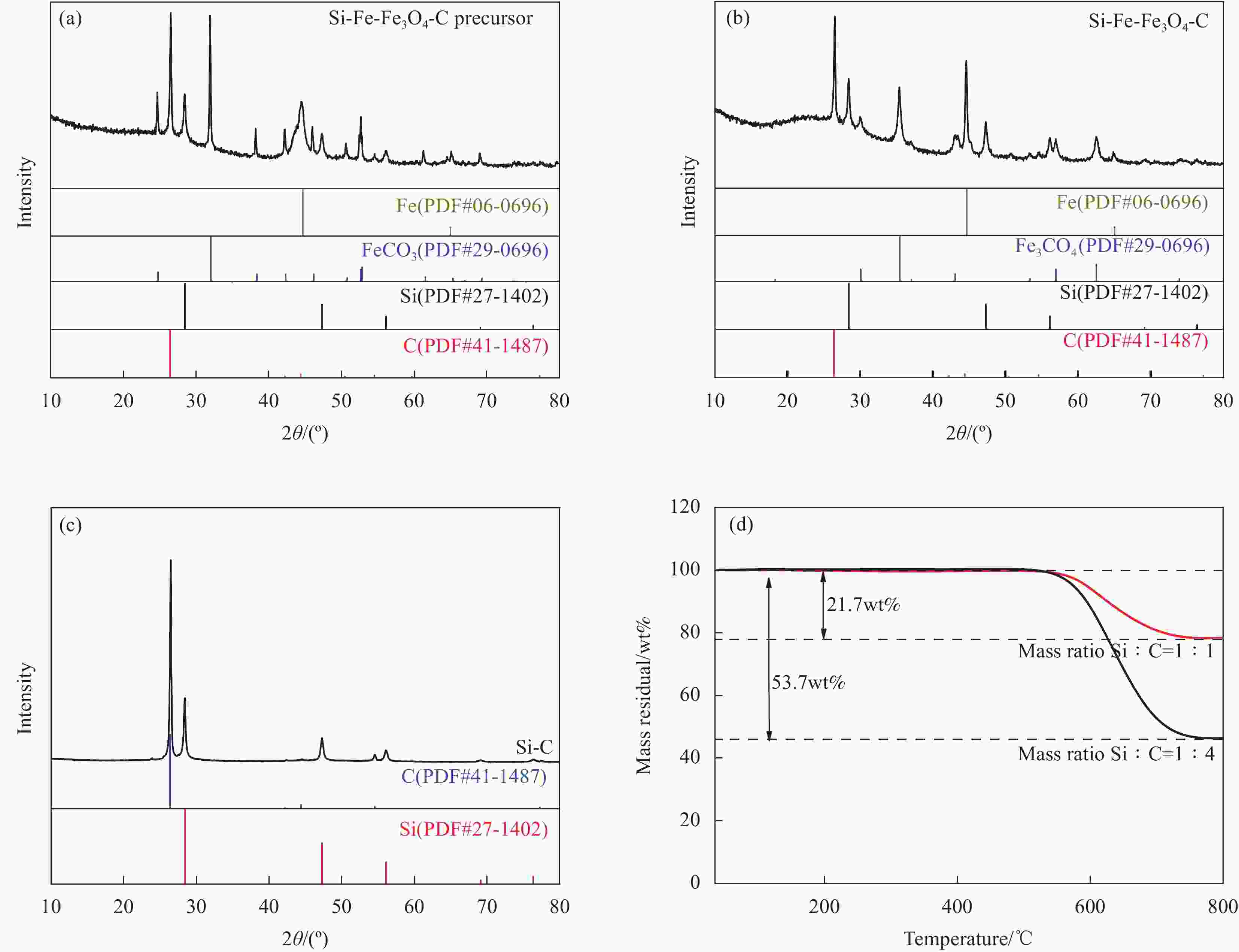
表 1 Si-Fe-Fe3O4-C复合材料制备过程中的质量变化及计算结果
Table 1. Mass changes and calculation results during the preparation of Si-Fe-Fe3O4-C composite
Sample Nano Si/g MCMB/g Si-FeCO3-C/g Si-Fe-Fe3O4-C/g Fe/g Fe4O3/g Mass residual/wt% Si-Fe-Fe3O4-C (Si∶C=1∶1) 0.250 0.250 0.995 0.966 0.285 0.119 21.7 Si-Fe-Fe3O4-C (Si∶C=1∶4) 0.250 1.000 1.730 1.660 0.320 0.091 53.7 表 2 Si-Fe-Fe3O4-C复合材料各物相组成
Table 2. Phase composition of Si-Fe-Fe3O4-C composite
Sample Si/wt% C/wt% Fe/wt% Fe3O4/wt% Si-Fe-Fe3O4-C
(Si∶C=1∶1)29.06 29.06 29.51 12.36 Si-Fe-Fe3O4-C
(Si∶C=1∶4)15.05 60.21 19.25 5.49 -
[1] OLABI A G, ONUMAEGBU C, WILBERFORCE T, et al. Critical review of energy storage systems[J]. Energy,2021,214:118987. doi: 10.1016/j.energy.2020.118987 [2] GE M Z, CAO C Y, BIESOLD G M, et al. Recent advances in silicon-based electrodes: From fundamental research toward practical applications[J]. Advanced Materials,2021,33(16):2004577. doi: 10.1002/adma.202004577 [3] GUO J P, DONG D Q, WANG J, et al. Silicon-based lithium ion battery systems: state-of-the-art from half and full cell viewpoint[J]. Advanced Functional Materials,2021,31(34):2102546. doi: 10.1002/adfm.202102546 [4] JIN Y, LI S, KUSHIMA A, et al. Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99.9%[J]. Energy & Environmental Science,2017,10(2):580-592. [5] SHI Q T, ZHOU J H, ULLAH S, et al. A review of recent developments in Si/C composite materials for Li-ion batteries[J]. Energy Storage Materials,2021,34:735-754. doi: 10.1016/j.ensm.2020.10.026 [6] ZAIDI S D A, WANG C, GYORGY B, et al. Iron and silicon oxide doped/PAN-based carbon nanofibers as free-standing anode material for Li-ion batteries[J]. Journal of Colloid and Interface Science,2020,569:164-176. doi: 10.1016/j.jcis.2020.02.059 [7] SUN L Y, YANG L, LI J, et al. Superior full-cell cycling and rate performance achieved by carbon coated hollow Fe3O4 nanoellipsoids for lithium ion battery[J]. Electrochimica Acta,2018,288:71-81. doi: 10.1016/j.electacta.2018.08.060 [8] YAN Y J, CHEN Y X, LI Y Y, et al. Synthesis of Si/Fe2O3-anchored rGO frameworks as high-performance anodes for Li-ion batteries[J]. International Journal of Molecular Sciences,2021,22(20):11041. doi: 10.3390/ijms222011041 [9] WANG H, DING Y, NONG J, et al. Bifunctional NaCl template for the synthesis of Si@graphitic carbon nanosheets as advanced anode materials for lithium ion batteries[J]. New Journal of Chemistry,2020,44(33):14278-14285. doi: 10.1039/D0NJ02547J [10] YANG Z X, DU Y, YANG Y J, et al. Large-scale production of highly stable silicon monoxide nanowires by radio-frequency thermal plasma as anodes for high-performance Li-ion batteries[J]. Journal of Power Sources,2021,497:229906. doi: 10.1016/j.jpowsour.2021.229906 [11] AN W, XIANG B, FU J, et al. Three-dimensional carbon-coating silicon nanoparticles welded on carbon nanotubes composites for high-stability lithium-ion battery anodes[J]. Applied Surface Science,2019,479:896-902. doi: 10.1016/j.apsusc.2019.02.145 [12] LIAO C, WU S. Pseudocapacitance behavior on Fe3O4-pillared SiOx microsphere wrapped by graphene as high performance anodes for lithium-ion batteries[J]. Chemical Engineering Journal,2019,355:805-814. doi: 10.1016/j.cej.2018.08.141 [13] GRINBOM G, MUALLEM M, ITZHAK A, et al. Synthesis of carbon nanotubes networks grown on silicon nanoparticles as Li-ion anodes[J]. Journal of Physical Chemistry C,2017,121(46):25632-25640. doi: 10.1021/acs.jpcc.7b05709 [14] ZHOU M J, GORDIN M L, CHEN S R, et al. Enhanced performance of SiO/Fe2O3 composite as an anode for rechargeable Li-ion batteries[J]. Electrochemistry Communications,2013,28:79-82. doi: 10.1016/j.elecom.2012.12.013 [15] SU L, ZHONG Y, ZHOU Z. Role of transition metal nanoparticles in the extra lithium storage capacity of transition metal oxides: A case study of hierarchical core-shell Fe3O4@C and Fe@C microspheres[J]. Journal of Materials Chemistry A,2013,1(47):15158-15166. doi: 10.1039/c3ta13233a [16] WANG Q, GUO C, HE J, et al. Fe2O3/C-modified Si nanoparticles as anode material for high-performance lithium-ion batteries[J]. Journal of Alloys and Compounds,2019,795:284-290. doi: 10.1016/j.jallcom.2019.05.038 [17] LIU C, XIA Q, LIAO C, et al. Pseudocapacitance contribution to three-dimensional micro-sized silicon@Fe3O4@few-layered graphene for high-rate and long-life lithium ion batteries[J]. Materials Today Communications,2019,18:66-73. doi: 10.1016/j.mtcomm.2018.11.004 [18] HERNANDHA R F H, RATH P C, UMESH B, et al. Supercritical CO2-assisted SiOx/carbon multi-layer coating on Si anode for lithium-ion batteries[J]. Advanced Functional Materials,2021,31(40):2104135. doi: 10.1002/adfm.202104135 [19] PAUL N, WANDT J, SEIDLMAYER S, et al. Aging behavior of lithium iron phosphate based 18650-type cells studied by in situ neutron diffraction[J]. Journal of Power Sources,2017,345:85-96. doi: 10.1016/j.jpowsour.2017.01.134 [20] FAN P, MU T, LOU S, et al. Amorphous carbon-encapsulated Si nanoparticles loading on MCMB with sandwich structure for lithium ion batteries[J]. Electrochimica Acta,2019,306:590-598. doi: 10.1016/j.electacta.2019.03.154 [21] XU Q, LI J Y, SUN J K, et al. Watermelon-inspired Si/C microspheres with hierarchical buffer structures for densely compacted lithium-ion battery anodes[J]. Advanced Energy Materials,2017,7(3):1601481. doi: 10.1002/aenm.201601481 [22] JIANG B, LIANG Y, YU X, et al. Facile synthesis of FeCO3/nitrogen-doped carbon dot composites for lithium-ion battery anodes[J]. Journal of Alloys and Compounds,2020,838:155508. doi: 10.1016/j.jallcom.2020.155508 [23] ZHAO X, XIA D, ZHENG K. Fe3O4/Fe/carbon composite and its application as anode material for lithium-ion batteries[J]. ACS Applied Materials & Interfaces,2012,4(3):1350-1356. [24] FURQUAN M, KHATRIBAIL A R, VIJAYALAKSHMI S, et al. Efficient conversion of sand to nano-silicon and its energetic Si-C composite anode design for high volumetric capacity lithium-ion battery[J]. Journal of Power Sources,2018,382:56-68. doi: 10.1016/j.jpowsour.2018.02.011 [25] LIN Y, CHEN Y, ZHAN Y, et al. Wet-chemical synthesized MCMB@Si@C microspheres for high-performance lithium-ion battery anodes[J]. Chemical Communications,2018,54(68):9466-9469. doi: 10.1039/C8CC04797A [26] XIE J, TONG L, SU L, et al. Core-shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance[J]. Journal of Power Sources,2017,342:529-536. doi: 10.1016/j.jpowsour.2016.12.094 -






 下载:
下载: