Preparation of magnetic hydrotalcite composite and its Eosin Y adsorption performance
-
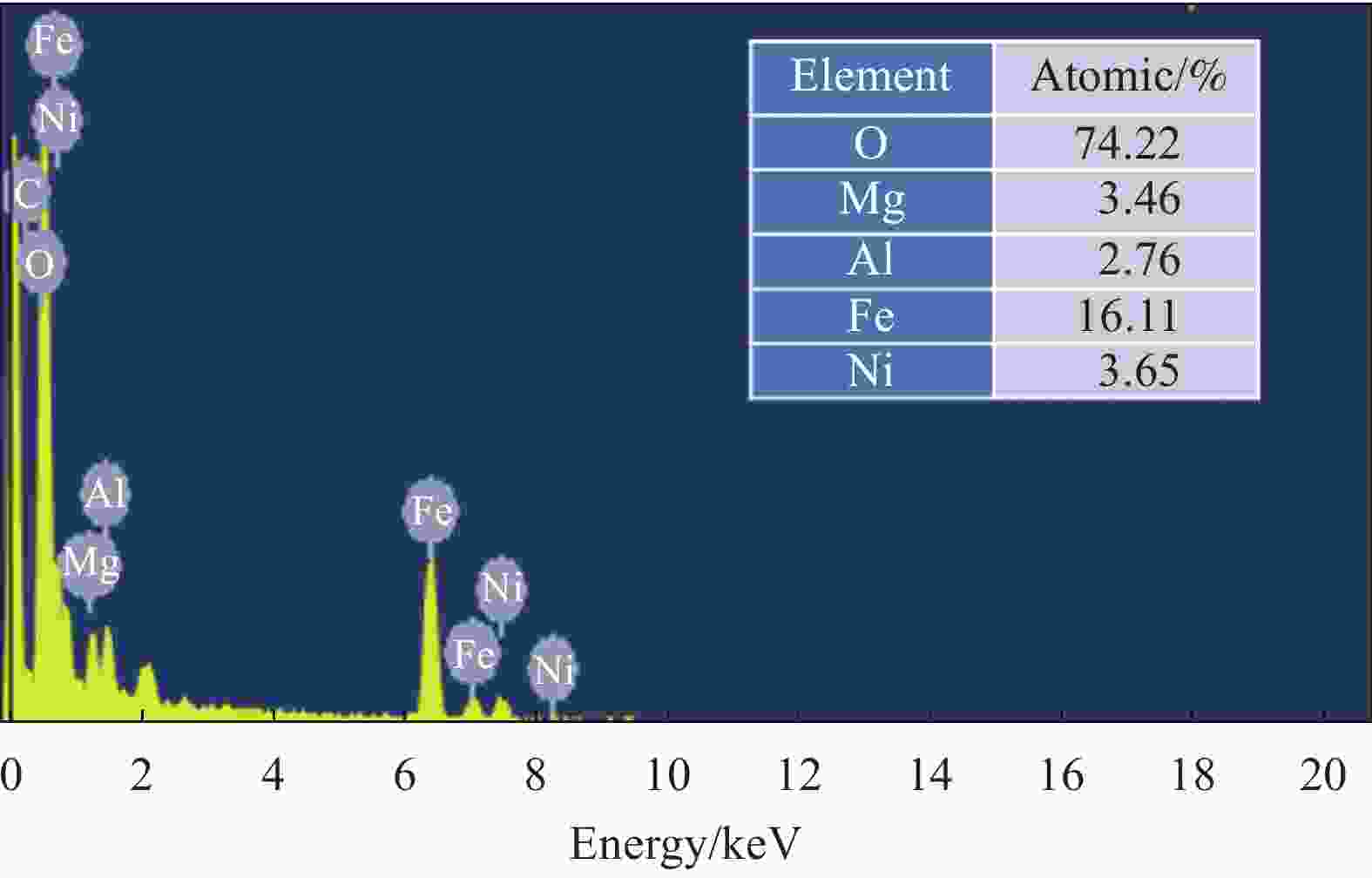
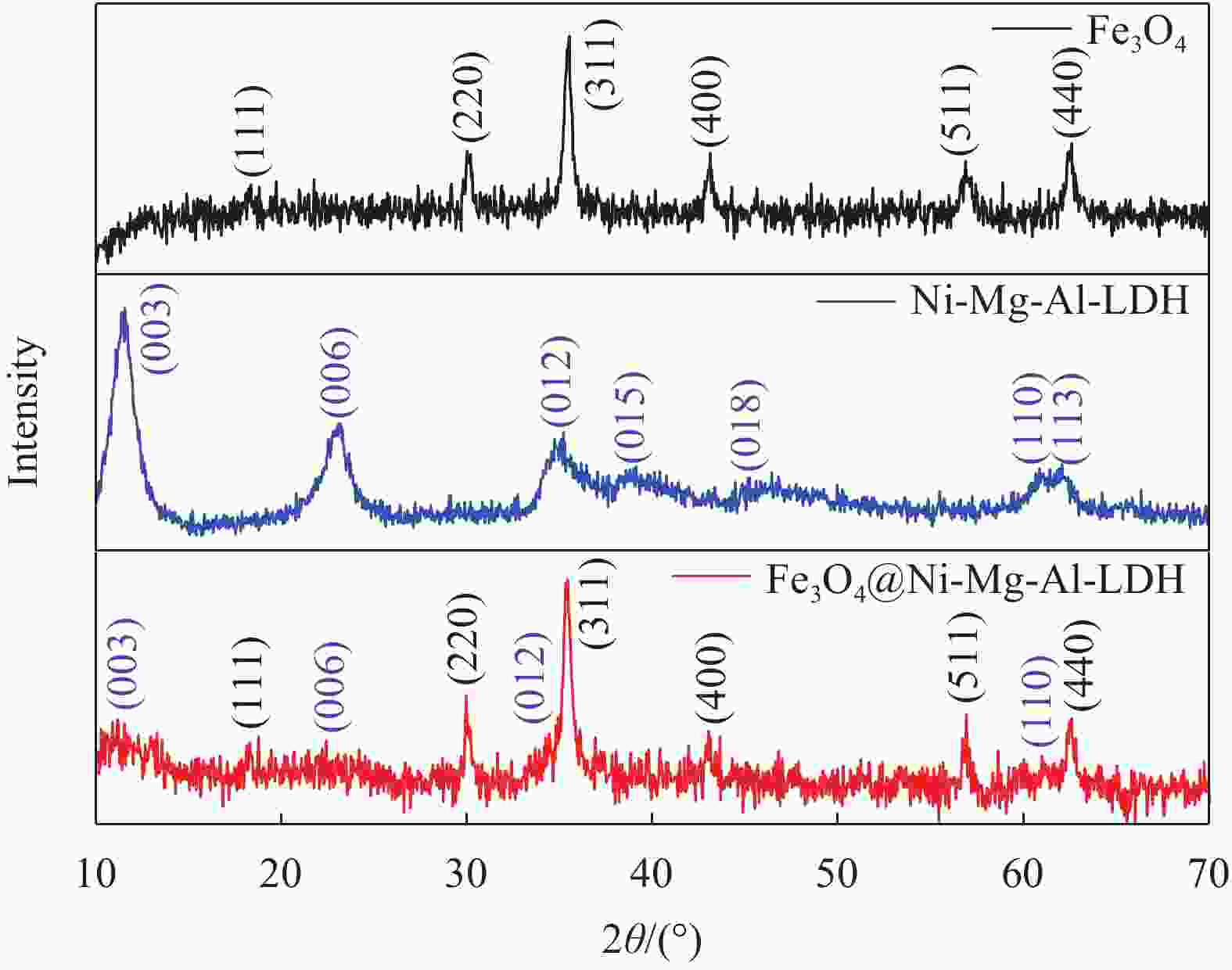
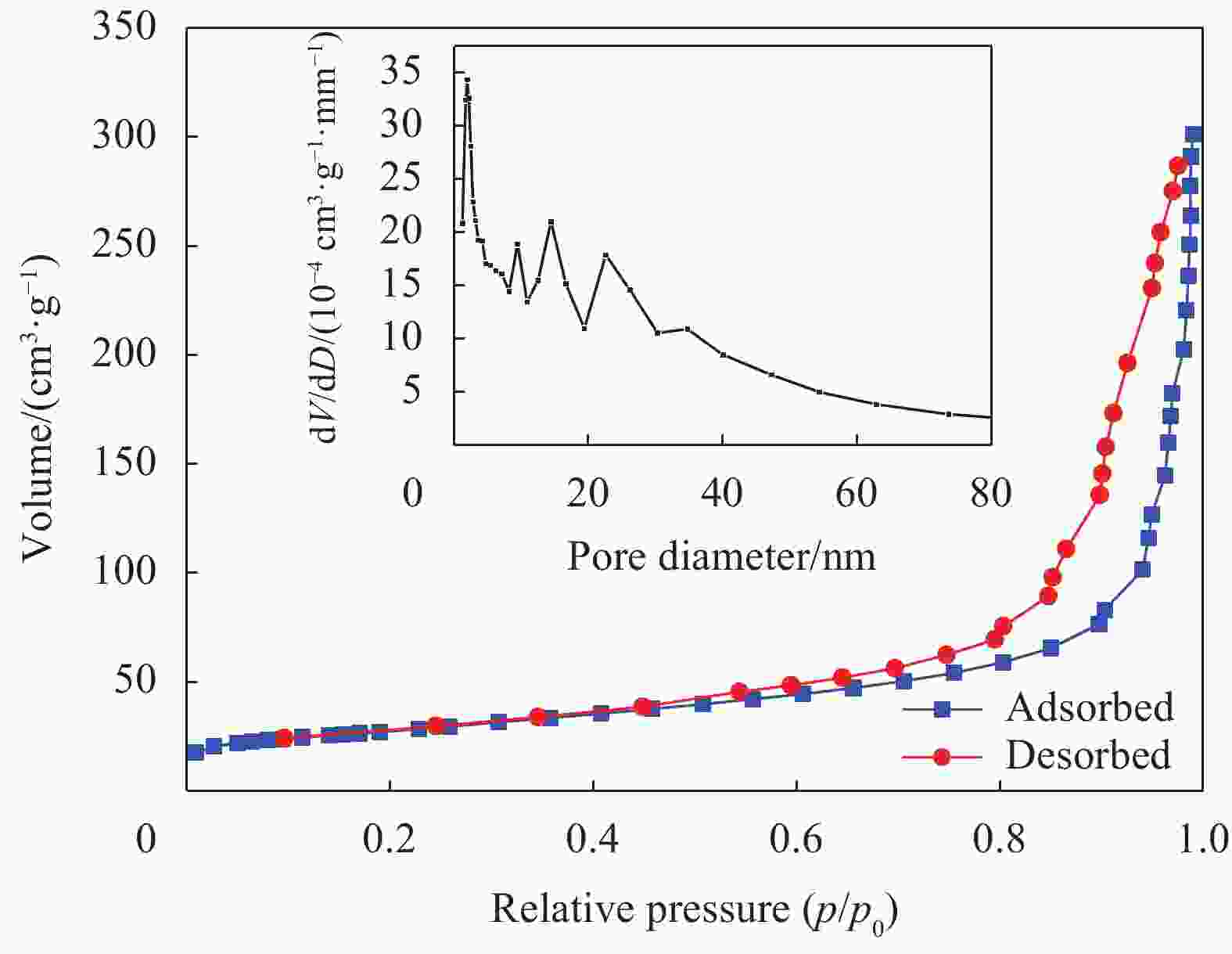
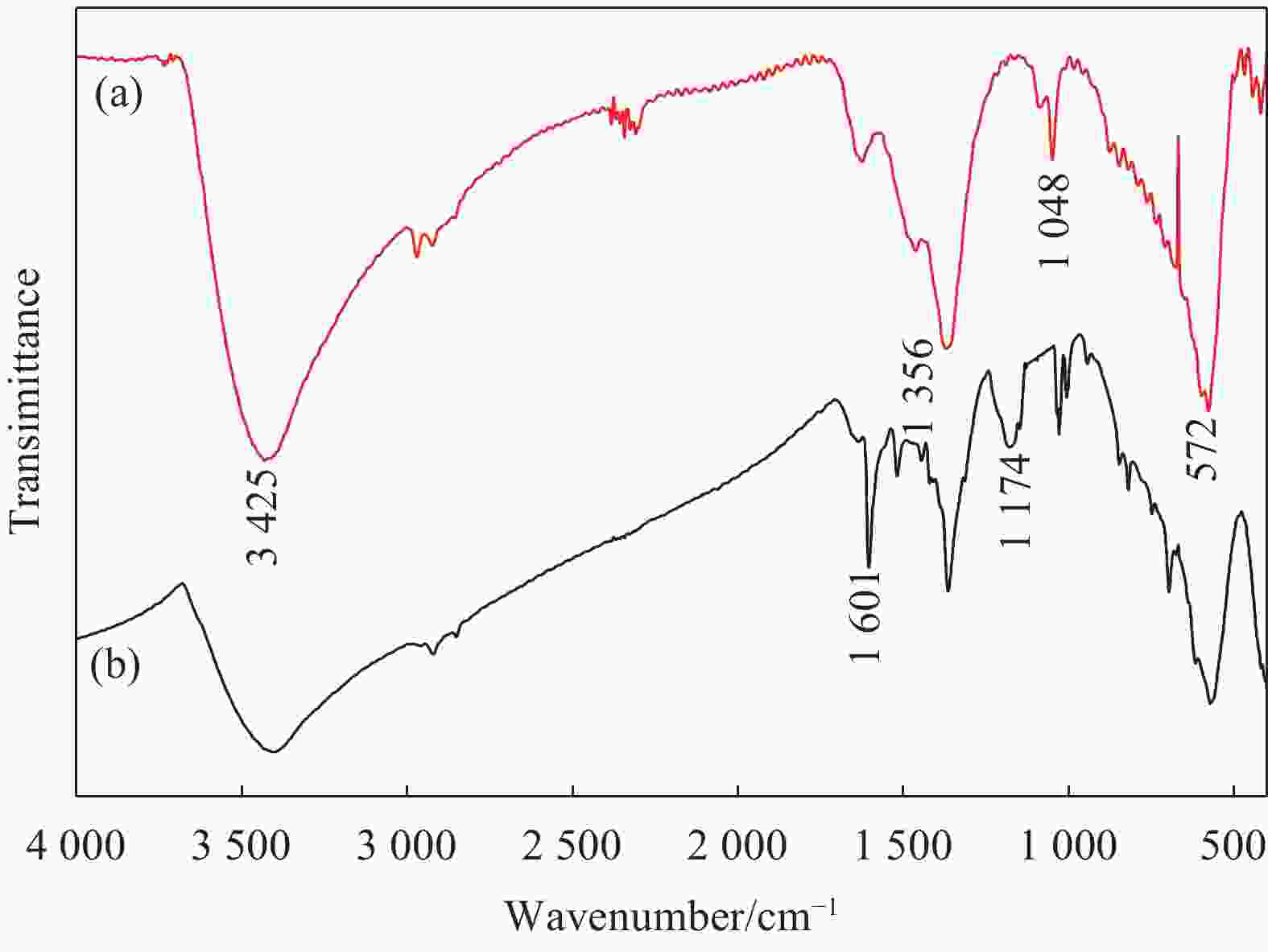
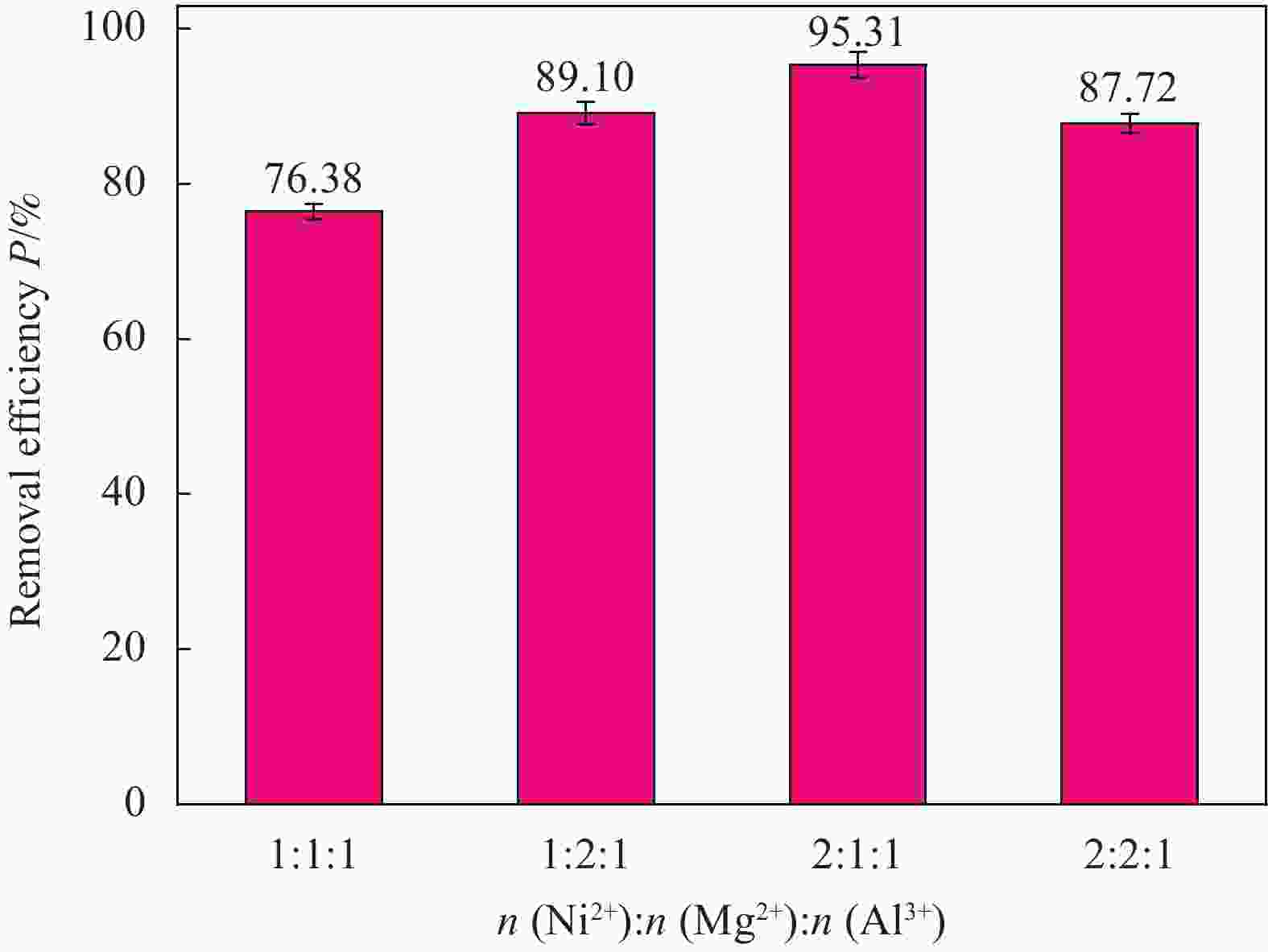
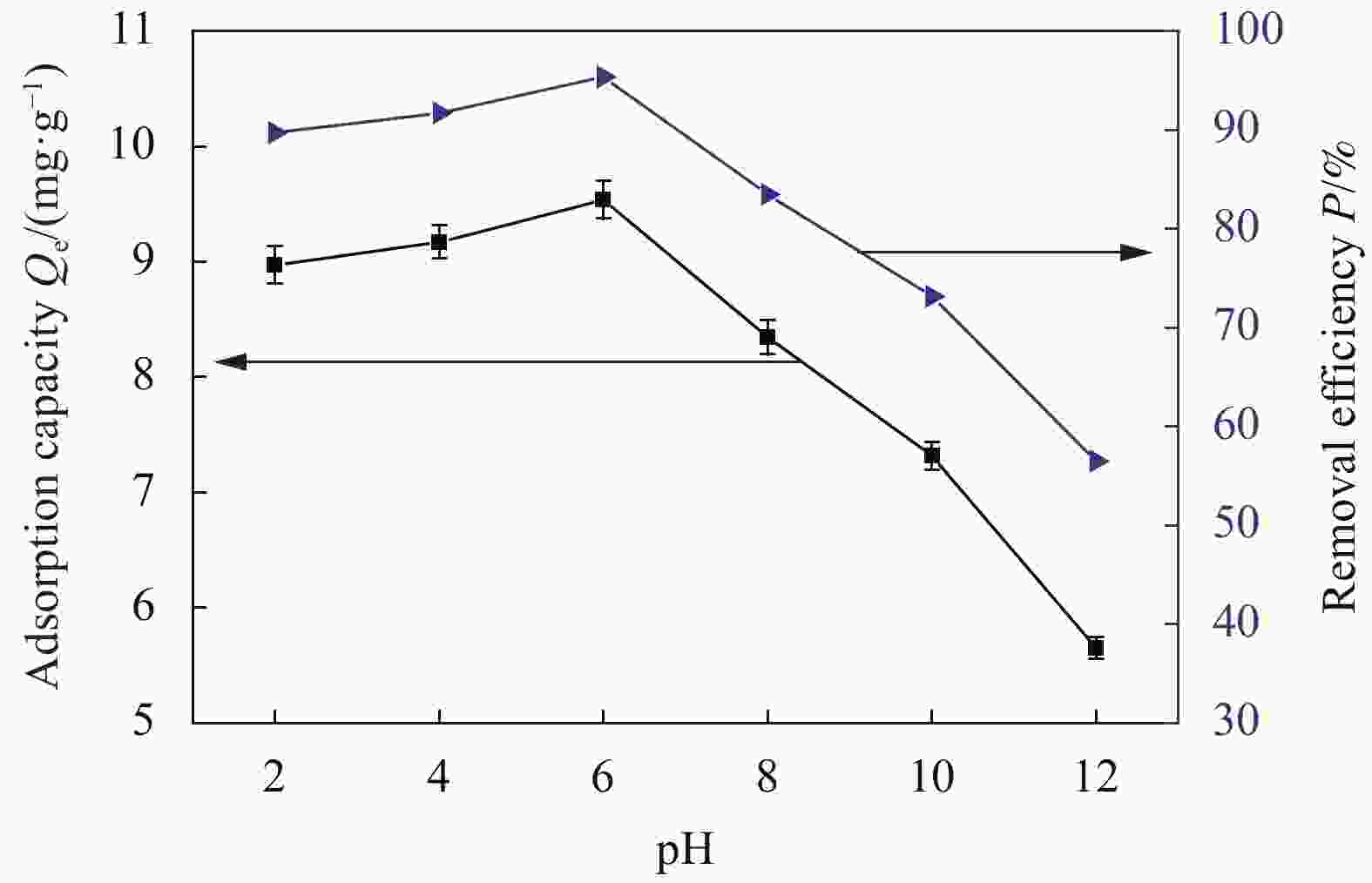
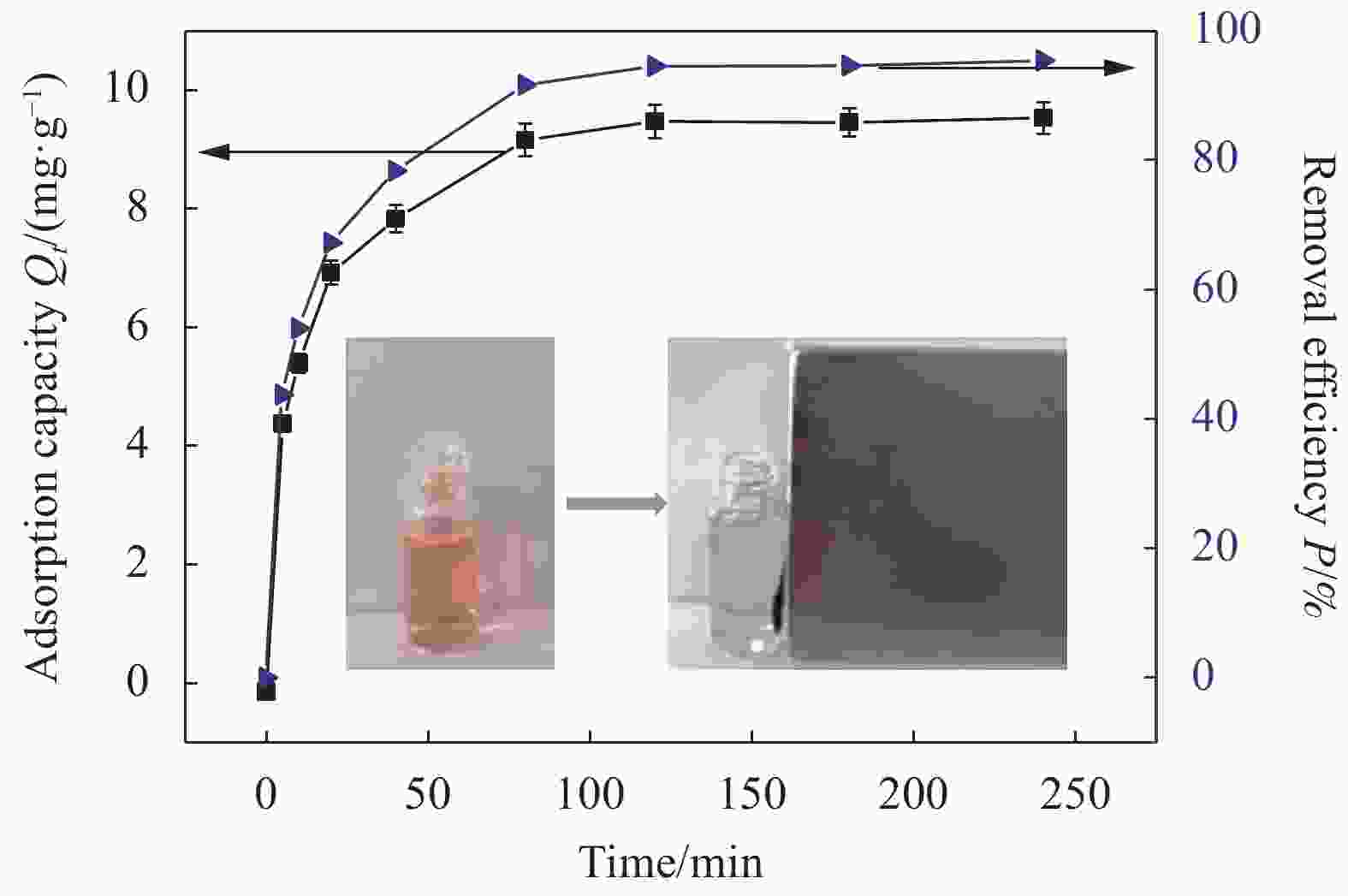
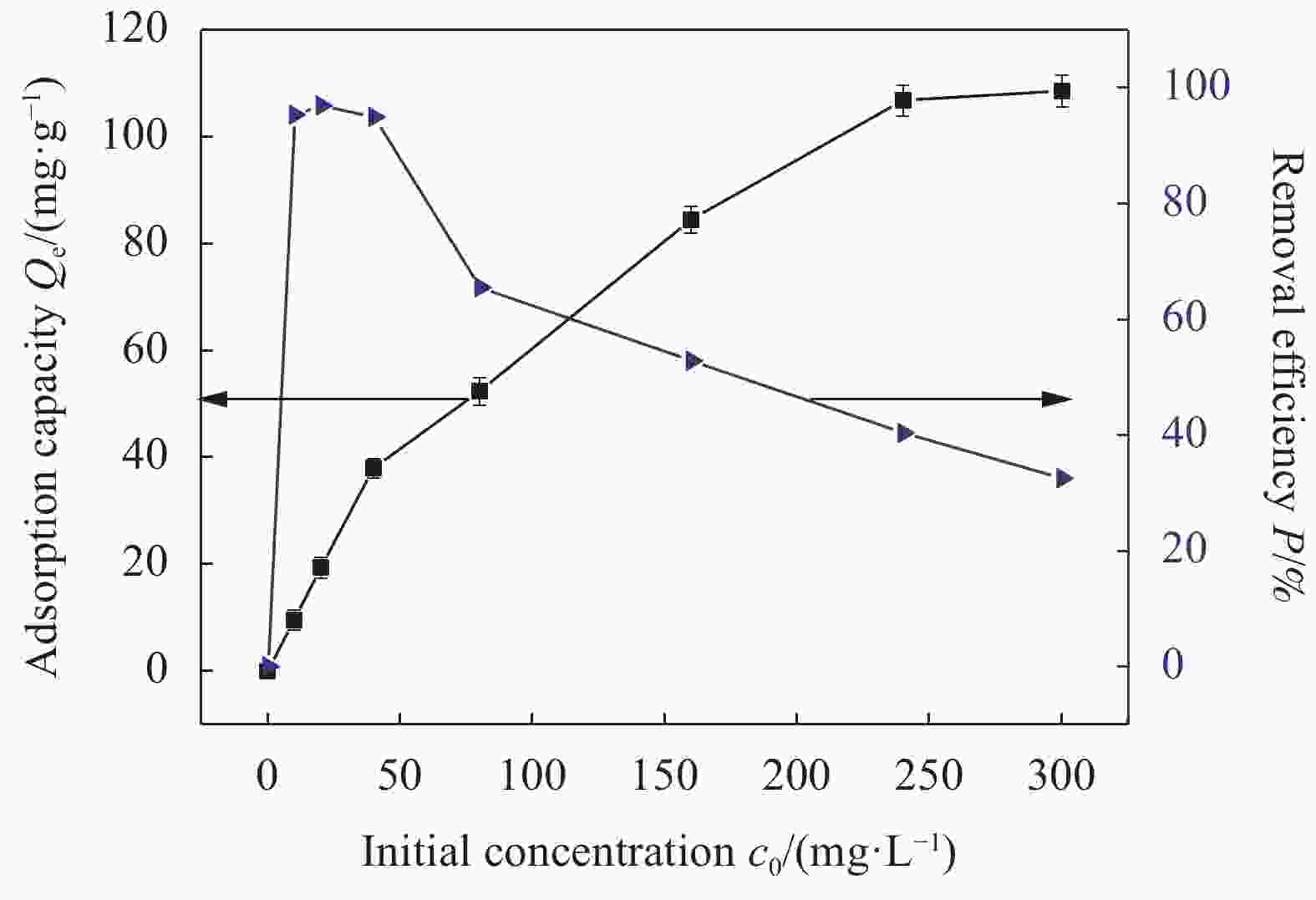
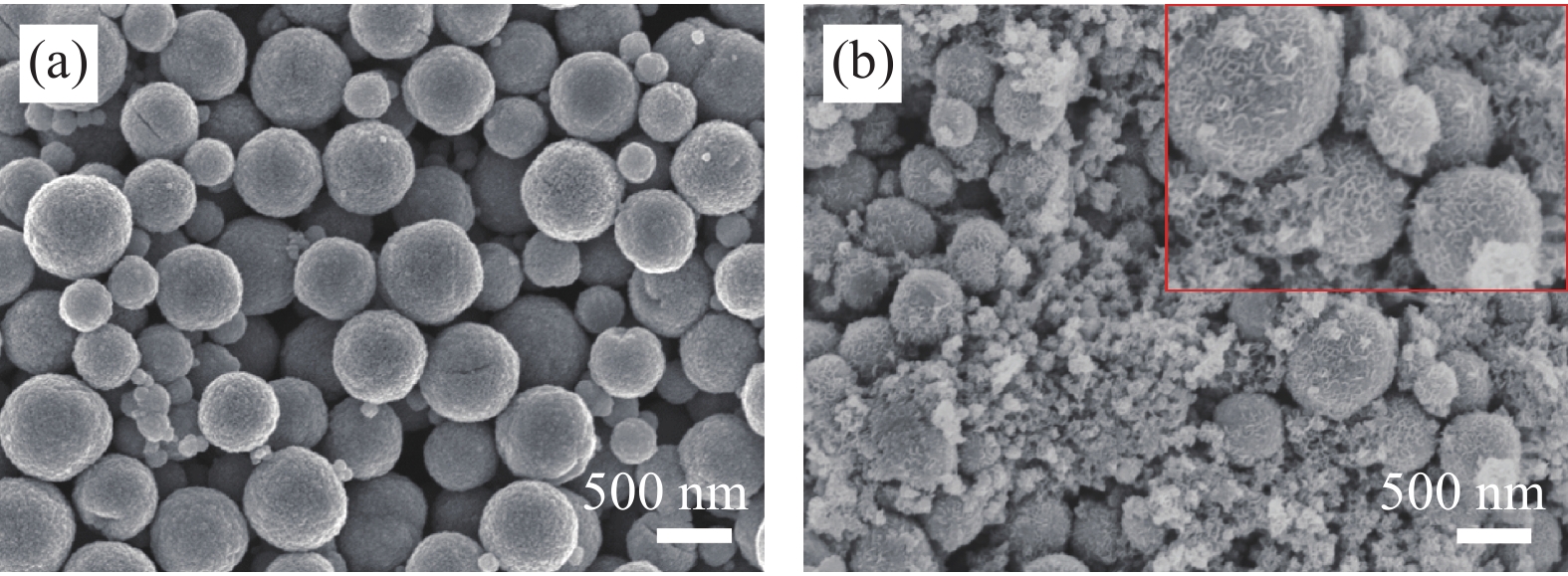
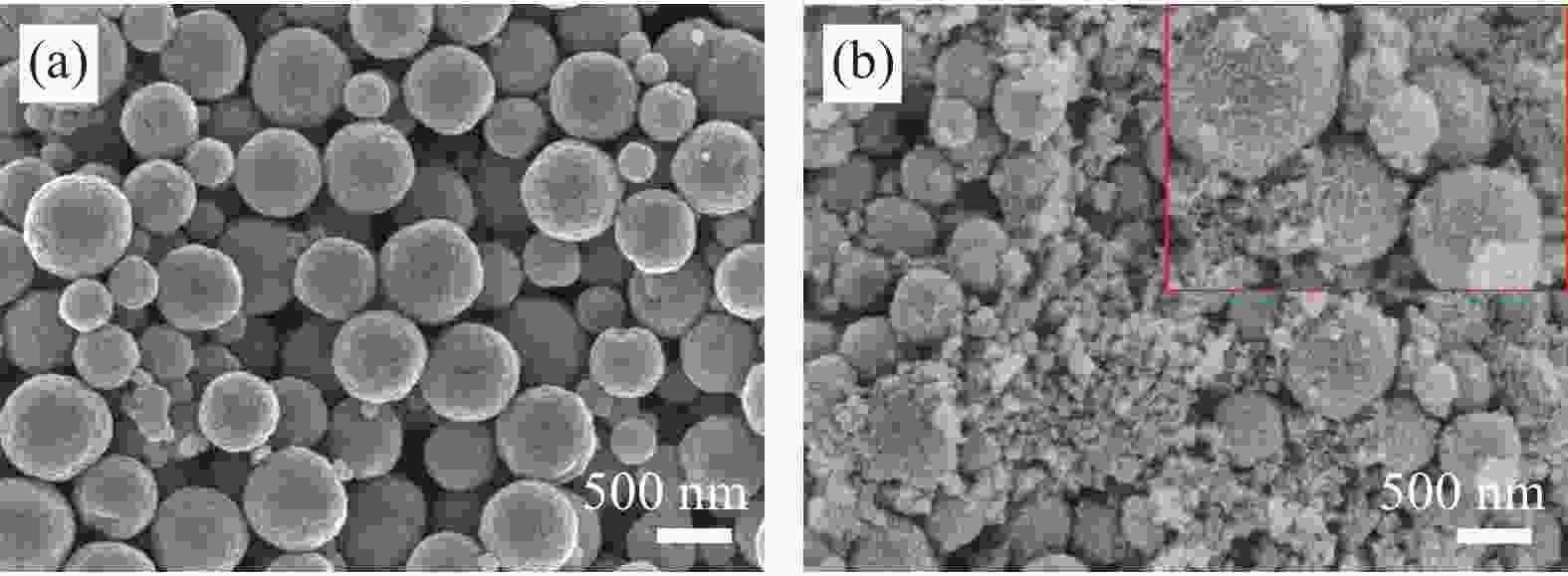
摘要: 为了解决水滑石型(LDH)吸附剂在污水处理中难回收的问题,采用双滴沉淀法将磁性Fe3O4颗粒与具有吸附性能的Ni-Mg-Al-LDH水滑石相结合,合成了Fe3O4@Ni-Mg-Al-LDH磁性水滑石复合吸附材料,利用SEM、XRD、FT-IR和氮气吸附脱附等表征对Fe3O4@Ni-Mg-Al-LDH材料的形貌和结构进行测试,并将其用于曙红Y染料废水处理。结果表明,Fe3O4@Ni-Mg-Al-LDH对曙红Y染料的吸附在20 min内较为迅速,120 min后吸附趋于平衡,且随着曙红Y初始浓度的升高,Fe3O4@Ni-Mg-Al-LDH对曙红Y染料的吸附量也逐渐增加,最大吸附量达到108.6 mg·g−1。同时,Fe3O4@Ni-Mg-Al-LDH对曙红Y的吸附过程符合Langmuir等温吸附模型和伪二级动力学方程,表明该吸附过程以单分子层化学吸附为主,且表面扩散和颗粒内扩散共同控制吸附速率。经五次循环后,吸附剂对曙红Y染料的去除率仍能保持80%以上,且吸附后易于磁分离,说明所制备的Fe3O4@Ni-Mg-Al-LDH磁性水滑石材料是一种良好的染料废水吸附剂。Abstract: In order to solve the problem of difficult recovery of hydrotalcite (LDH) adsorbent in sewage treatment, Fe3O4@Ni-Mg-Al-LDH magnetic hydrotalcite composite adsorption material was synthesized by combining magnetic Fe3O4 particles with Ni-Mg-Al-LDH hydrotalcite via double-drop precipitation method. The morphology and structure of the as-prepared Fe3O4@Ni-Mg-Al-LDH samples were characterized by SEM, XRD, FT-IR and N2 adsorption-desorption technologies. And it was used as adsorbent to simulate the wastewater treatment performance of Eosin Y dye. The results show that the adsorption of Eosin Y dye on Fe3O4@Ni-Mg-Al-LDH is very quickly within 20 min, while the adsorption tends to balance after 120 min. In addition, with the increase of the initial concentration of Eosin Y dye, the adsorption capacity of Fe3O4@Ni-Mg-Al-LDH sample for Eosin Y dye increases gradually and the maximum adsorption capacity is 108.6 mg·g−1. Meanwhile, the adsorption process of Eosin Y dye on Fe3O4@Ni-Mg-Al-LDH conforms to the Langmuir isothermal model and pseudo second-order kinetic equation, indicating that the adsorption process is dominated by the chemisorption of molecular layer, and the adsorption rate is controlled by surface diffusion and intra particle diffusion. After five cycles, the removal rate of Eosin Y dye still keeps above 80%, and the adsorbent is easy to be separated by magnetic field, implying that Fe3O4@Ni-Mg-Al-LDH magnetic hydrotalcite composite is a good adsorbent for dye wastewater.
-
Key words:
- adsorption /
- hydrotalcite /
- magnetism /
- removal rate /
- Eosin Y /
- wastewater
-
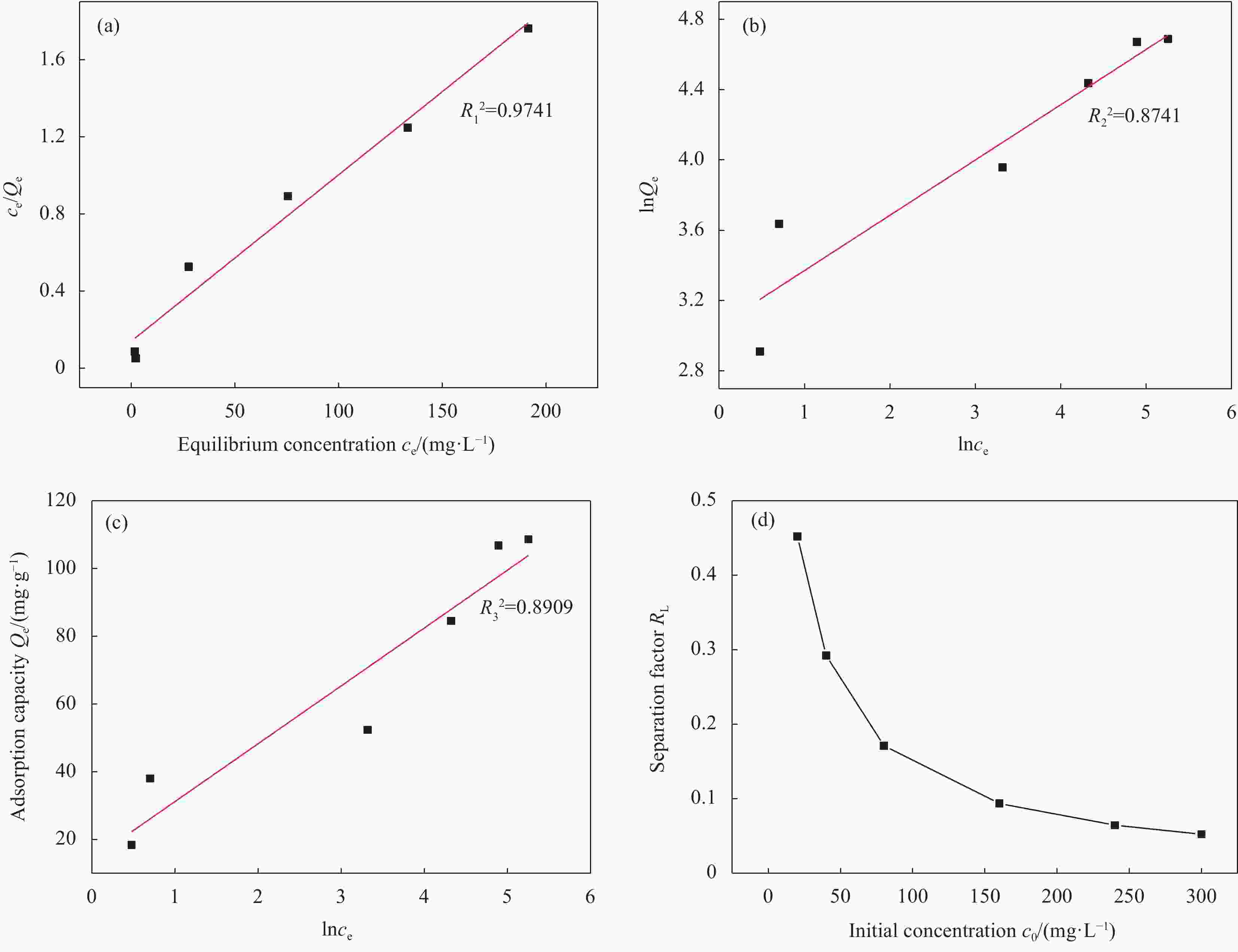
图 11 Fe3O4@Ni-Mg-Al-LDH吸附曙红Y的Langmuir (a)、Freundlich (b)、Temkin (c) 吸附等温线模型和Langmuir的RL参数变化曲线 (d)
Figure 11. Langmuir (a), Freundlich (b), Temkin (c) adsorption isotherm models and Langmuir parameter (RL) curve (d) of Eosin Y on Fe3O4@Ni-Mg-Al-LDH
Qe—Equilibrium adsorption capacity; ce—Equilibrium concentration; RL—Separation factor; c0—Initial concentration; R12, R22, R32—Correlation coefficient of Langmuir, Freundlich and Temkin models
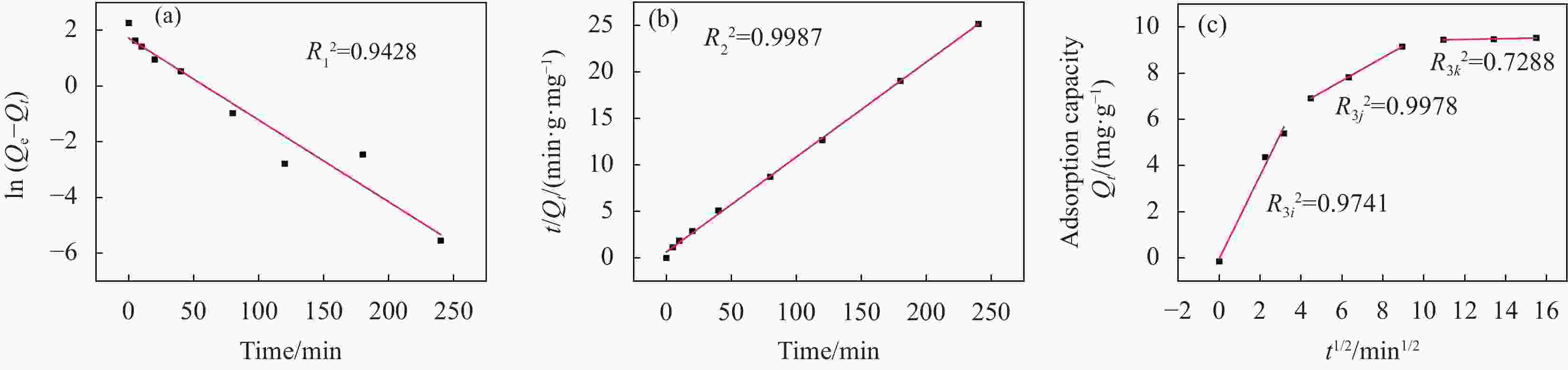
图 12 Fe3O4@Ni-Mg-Al-LDH吸附曙红Y的伪一级动力学 (a)、伪二级动力学 (b) 和颗粒内扩散 (c) 模型
Figure 12. Pseudo-first-order (a), pseudo-second-order (b) and intra-particle diffusion kinetics model (c) for the adsorption of Eosin Y on Fe3O4@Ni-Mg-Al-LDH
R3i2, R3j2, R3k2—Correlation coefficient of the first, second and third stage of intra-particle diffusion model
表 1 Fe3O4@Ni-Mg-Al-LDH对曙红Y的吸附等温线参数
Table 1. Adsorption isotherm parameters of Eosin Y on Fe3O4@Ni-Mg-Al-LDH
Isotherm models Parameters Fe3O4@Ni-Mg-Al-LDH Langmuir R12 0.9741 Qmax/(mg·g−1) 116.01 KL/(L·mg−1) 0.0606 Freundlich R22 0.8741 KF/(mg·g−1) 21.28 n 3.184 Temkin R32 0.8909 B/(J·mol−1) 17.07 KT/(L·mg−1) 2.291 Notes: R12, R22 and R32—Correlation coefficient of Langmuir, Freundlich and Temkin models; Qmax—Maximum adsorption capacity; KL, KF and KT—Adsorption equilibrium constant of Langmuir, Freundlich and Temkin models; n—Constant related to adsorption strength; B—Constant related to heat of adsorption. 表 2 Fe3O4@Ni-Mg-Al-LDH吸附曙红Y的动力学参数
Table 2. Kinetics parameters for the adsorption of Eosin Y on Fe3O4@Ni-Mg-Al-LDH
Kinetic models Parameters Fe3O4@Ni-Mg-Al-LDH Pseudo-first-order
dynamic modelR12 0.9428 K1/(min−1) 0.0293 Q1/(mg·g−1) 5.590 Pseudo-second-order
dynamic modelR22 0.9987 K2/(g·mg−1·min−1) 0.0169 Q2/(mg·g−1) 9.785 Intra-particle diffusion
modelR3i2 0.9741 K3i/(mg·g−1·min−0.5) 1.802 bi/(mg·g−1) −0.0321 R3j2 0.9978 K3j/(mg·g−1·min−0.5) 0.5015 bj/(mg·g−1) 4.671 R3k2 0.7288 K3k/(mg·g−1·min−0.5) 0.0168 bk/(mg·g−1) 9.266 Notes: R12, R22 —Correlation coefficient of pseudo-first-order and pseudo-second-order dynamic models; R3i2, R3j2 and R3k2—Correlation coefficient of the first, second and third stage of intra-particle diffusion model; K1, K2—Rate constant of pseudo-first-order and pseudo-second-order dynamic models; K3i, K3j and K3k—Rate constant of the first, second and third stage of intra-particle diffusion model; Q1, Q2—Adsorption capacity of pseudo-first-order and pseudo-second-order dynamic models; bi, bj and bk—Constant related to the thickness of the boundary layer of the first, second and third stage of intra-particle diffusion model. 表 3 Fe3O4@Ni-Mg-Al-LDH吸附剂使用后金属离子浸出量
Table 3. Metal ion leaching amount after the use of Fe3O4@Ni-Mg-Al-LDH adsorbent
Cycle
numberMetal ion leaching amount/(mg·L−1) Fe Ni Mg Al 1 0.21 0.11 0.06 0.07 2 0.17 0.09 0.04 0.03 3 0.12 0.05 0.02 0.02 4 0.09 0.02 0.01 0.02 5 0.07 0.01 − − -
[1] LIU Q. Pollution and treatment of dye waste-water[J]. IOP Conference Series: Earth and Environmental Science,2020,514(5):052001. [2] 席尚东, 高磊, 刘文宗, 等. 利用生活污水提升厌氧-生物电化学耦合系统处理染料废水的效能及关键功能微生物研究[J]. 环境科学学报, 2019, 39(2):15-25.XI Shangdong, GAO Lei, LIU Wenzong, et al. Domestic sewage enhancing azo dye wastewater treatment in anaerobic digestion-bioelectrochemical system and functional microbial community analysis[J]. Acta Scientiae Circumstantiae,2019,39(2):15-25(in Chinese). [3] AL-GUBURY H Y, SAAD S T, ALRAZZAK N A, et al. Photocatalytic removal of eosin dye from aqueous solution over titanium dioxide[J]. IOP Conference Series: Materials Science and Engineering, 2020, 871: 012031. [4] SIVAKUMAR D. Removal of color from textile industry wastewater using microorganism[J]. International Jour-nal of PharmTech Research,2015,8(5):836-842. [5] 刘颖琪, 翁文斌, 岑檠, 等. FeVO4/Cu3(BTC)2(H2O)3异质结制备及光催化性能[J]. 复合材料学报, 2020, 37(12):3128-3136.LIU Yingqi, WENG Wenbin, CEN Qin, et al. Preparation and photocatalytic properties of FeVO4/Cu3(BTC)2(H2O)3 heterojunction[J]. Acta Materiae Compositae Sinica,2020,37(12):3128-3136(in Chinese). [6] CHENG N, WANG B, WU P, et al. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review[J]. Environmental Pollution,2021,273:116448-116458. doi: 10.1016/j.envpol.2021.116448 [7] HUANG Z, LI Y, CHEN W, et al. Modified bentonite adsorption of organic pollutants of dye wastewater[J]. Materials Chemistry and Physics,2017,202:266-276. doi: 10.1016/j.matchemphys.2017.09.028 [8] 狄婧, 刘海霞, 姜永强, 等. 聚吡咯/壳聚糖复合膜的制备及其对Cu(Ⅱ)和Cr(Ⅵ)吸附机制[J]. 复合材料学报, 2021, 38(1):221-231.DI Jing, LIU Haixia, JIANG Yongqiang, et al. Preparation of polypyrrole/chitosan composite membrane and its adsorption mechanism for Cu(Ⅱ) and Cr(Ⅵ)[J]. Acta Materiae Compositae Sinica,2021,38(1):221-231(in Chinese). [9] WONG S, YAC’COB N A N, NGADI N, et al. From pollutant to solution of wastewater pollution: Synthesis of activated carbon from textile sludge for dye adsorption[J]. Chinese Journal of Chemical Engineering,2018,26(4):870-878. doi: 10.1016/j.cjche.2017.07.015 [10] NGULUBE T, GUMBO J R, MASINDI V, et al. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review[J]. Journal of Environmental Management,2017,191:35-57. [11] ZHENG L, DANG Z, YI X, et al. Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk[J]. Journal of Hazardous Materials,2010,176:650-656. doi: 10.1016/j.jhazmat.2009.11.081 [12] 徐然, 左华江, 唐春怡, 等. 壳聚糖类吸附材料的制备及应用研究进展[J]. 现代化工, 2020, 40(9):25-29.XU Ran, ZUO Huajiang, TANG Chunyi, et al. Advances in preparation and application of chitosan-based adsorption materials[J]. Modern Chemical Industry,2020,40(9):25-29(in Chinese). [13] LEI C, PI M, KUANG P, et al. Organic dye removal from aqueous solutions by hierarchical calcined Ni-Fe layered double hydroxide: Isotherm, kinetic and mechanism studies[J]. Journal of Colloid and Interface Science,2017,496:158-166. doi: 10.1016/j.jcis.2017.02.025 [14] BHARALI D, DEKA R C. Preferential adsorption of various anionic and cationic dyes from aqueous solution over ternary CuMgAl layered double hydroxide[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2017,525:64-76. [15] ZUBIR N A, YACOU C, MOTUZAS J, et al. Structural and functional investigation of graphene oxide–Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction[J]. Scientific Reports,2018,4:4594-4602. [16] 龚新怀, 李明春, 杨坤, 等. 纳米Fe3O4@茶渣/海藻酸钙磁性复合材料制备及其对亚甲基蓝的吸附性能与吸附机制[J]. 复合材料学报, 2021, 38(2):424-438.GONG Xinhuai, LI Mingchun, YANG Kun, et al. Preparation of nanosized Fe3O4@tea dregs/calcium alginate magnetic composite and its adsorption properties and adsorption mechanism for methylene blue[J]. Acta Materiae Compositae Sinica,2021,38(2):424-438(in Chinese). [17] 邢敏, 雷西萍, 韩丁, 等. Fe3O4/高岭土磁性复合材料对Cu2+的吸附性能[J]. 复合材料学报, 2019, 36(9):2204-2211.XING Min, LEI Xiping, HAN Ding, et al. Adsorption of Cu2+ by Fe3O4/kaolin magnetic composites[J]. Acta Materiae Compositae Sinica,2019,36(9):2204-2211(in Chinese). [18] MASOUDI M M, KASHANIAN F, AKBARI A, et al. Antibody-conjugated nontoxic arginine-doped Fe3O4 nanoparticles for magnetic circulating tumor cells separation[J]. International Journal of Medical and Health Sciences,2017,11(5):241-244. [19] PEI L, XUAN S, WU J, et al. Experiments and simulations on the magnetorheology of magnetic fluid based on Fe3O4 hollow chains[J]. Langmuir,2019,35(37):12158-12167. doi: 10.1021/acs.langmuir.9b01957 [20] WU Q, CHEN M, CHEN K, et al. Fe3O4-based core/shell nanocomposites for high-performance electrochemical supercapacitors[J]. Journal of Materials Science,2016,51(3):1572-1580. doi: 10.1007/s10853-015-9480-4 [21] ENIOLA J O, KUMAR R, MOHAMED O A, et al. Synthesis and characterization of CuFe2O4/NiMgAl-LDH composite for the efficient removal of oxytetracycline antibiotic[J]. Journal of Saudi Chemical Society,2019,24(1):139-150. [22] BAO H, YANG J, HUANG Y, et al. Synthesis of well-dispersed layered double hydroxide core@ordered mesoporous silica shell nanostructure (LDH@mSiO2) and its application in drug delivery[J]. Nanoscale,2011,3(10):4069-4073. doi: 10.1039/c1nr10718f [23] LEI C, ZHU X, ZHU B, et al. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions[J]. Journal of Hazardous Materials,2017,321:801-811. doi: 10.1016/j.jhazmat.2016.09.070 [24] GUO X Y, MAO F, WANG W, et al. Sulfhydryl-modified Fe3O4@SiO2 core/shell nanocomposite: Synthesis and toxicity assessment in vitro[J]. ACS Applied Materials Interfaces,2015,7(27):14983-14991. doi: 10.1021/acsami.5b03873 [25] 魏刚, 顾峥烨, 龚水水, 等. 红外光谱法氧化石墨烯表面氧化度的测定[J]. 光谱学与光谱分析, 2020, 40(6):68-73.WEI Gang, GU Zhengye, GONG Shuishui, et al. Determination of the oxidiz ability on the surface of the graphene oxide layer by infrared spectroscopy[J]. Spectroscopy and Spectral Analysis,2020,40(6):68-73(in Chinese). [26] AI L, ZHANG C, MENG L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg-Al layered double hydroxide[J]. Journal of Chemical and Engineering Data,2011,56(11):4217-4225. doi: 10.1021/je200743u [27] YANG K, YAN L G, YANG Y M, et al. Adsorptive removal of phosphate by Mg-Al and Zn-Al layered double hydroxides: Kinetics, isotherms and mechanisms[J]. Separation and Purification Technology,2014,124:36-42. doi: 10.1016/j.seppur.2013.12.042 [28] YAN L G, YANG K, SHAN R R, et al. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core-shell Fe3O4@LDHs composites with easy magnetic separation assistance[J]. Journal of Colloid and Interface Science,2015,448:508-516. doi: 10.1016/j.jcis.2015.02.048 [29] 王洪杰, 兰依博, 李晓东. KMnO4改性稻壳、稻杆水热炭吸附染料的研究[J]. 应用化工, 2019, 6:1344-1350. doi: 10.3969/j.issn.1671-3206.2019.06.022WANG Hongjie, LAN Yibo, LI Xiaodong. Hydrothermal synthesis of KMnO4 modified rice husk andrice straw and its adsorption properties[J]. Applied Chemical Industry,2019,6:1344-1350(in Chinese). doi: 10.3969/j.issn.1671-3206.2019.06.022 [30] SARUCHI, KUMAR V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pbions from aqueous solutions by a hybrid ion-exchanger[J]. Arabian Journal of Chemistry,2019,3(12):316-329. [31] ZHANG J, LIU M, YANG T, et al. Synthesis and characterization of a novel magnetic biochar from sewage sludge and its effectiveness in the removal of methyl orange from aqueous solution[J]. Water Science & Technology ,2017,75(7):1539-1547. [32] SANTHOSH C, DANESHVAR E, TRIPATHI K M, et al. Synthesis and characterization of magnetic biochar adsorbents for the removal of Cr(VI) and acid orange 7 dye from aqueous solution[J]. Environmental Science and Pollution Research,2020,27(2):1-14. -





 下载:
下载: