Light-driven photothermal synergistic catalytic CO2 reduction over CoOx/WO3-x
-
摘要: 基于半导体光催化还原的人工光合成技术可在室温常压下将CO2转化为碳基燃料,被认为是同时缓解能源短缺和环境危机的理想策略,但因已有光催化剂对太阳光利用不足、光生电荷复合快,致使CO2光还原能量转换效率仍较低。采用水热法并结合表面浸渍过程首次制备出无定型CoOx/WO3-x复合光催化剂,通过XRD、TEM、XPS、EPR和紫外-可见-近红外吸收光谱等测试技术对催化剂的晶相组成、微观形貌、光吸收特性与氧空位缺陷进行系统表征。CO2光还原实验结果表明可见-近红外光照射3 h后,WO3-x为催化剂仅可检测到3.2 μmol·g−1的CH4,复合CoOx可显著提升WO3-x的CO2光催化还原性能,相同条件下最优催化剂2.5wt%CoOx/WO3-x的CO与CH4产生量分别可达78.2和19.7 μmol·g−1。引入氧空位可在WO3-x的能带结构中形成一新的中间能级,增强近红外光吸收并使催化剂表面产生局部温升;复合CoOx可在调控WO3-x导带电势的同时,增强光生电荷的分离与迁移,光热效应和CoOx助催化剂的协同作用是CO2光催化转化性能增强的主要原因。此外,复合光催化剂CoOx/WO3-x具有优异的长期催化与结构稳定性。Abstract: The conversion of CO2 into carbon-based fuels through artificial photosynthesis technology based on semiconductor photocatalytic reduction has been identified as an ideal strategy to alleviate energy shortage and environmental crisis. However, due to insufficient utilization of solar energy and rapid recombination of photogenerated charges for the reported photocatalysts, the energy conversion efficiency of CO2 photoreduction is still low. Amorphous CoOx/WO3-x composite photocatalysts were synthesized by a hydrothermal method combining with surface impregnation process for the first time. Crystal phase composition, microstructure, optical absorption properties and oxygen vacancy defects of the prepared catalysts were systematically characterized by XRD, TEM, XPS, EPR and UV-Vis-NIR DRS. The results of CO2 photoreduction experiments show that only 3.2 μmol·g−1 CH4 can be detected when using WO3-x as a catalyst after Vis-NIR light irradiation for 3 h, whereas introducing CoOx can significantly boost the CO2 photocatalytic reduction performance of WO3-x. Under the same experimental conditions, the yield of CO and CH4 on 2.5wt%CoOx/WO3-x catalyst can reach 78.2 and 19.7 μmol·g−1 respectively. Introducing oxygen vacancies can form a new intermediate energy level in the band structure of WO3-x, which enhances NIR absorption and causes local temperature rise of the catalysts surface. Incorporating CoOx contributes to enhance the separation and migration of photogenerated charges, and meanwhile can regulate the conduction-band potential of WO3-x. The synergistic effect of photothermal effect and CoOx cocatalyst is the primary reason for the promoted performance of CO2 photocatalytic conversion. Additionally, the composite photocatalysts CoOx/WO3-x shows excellent long-term catalytic and structural stability.
-
Key words:
- photocatalysis /
- WO3-x /
- CoOx /
- CO2 photoreduction /
- photothermal synergy
-
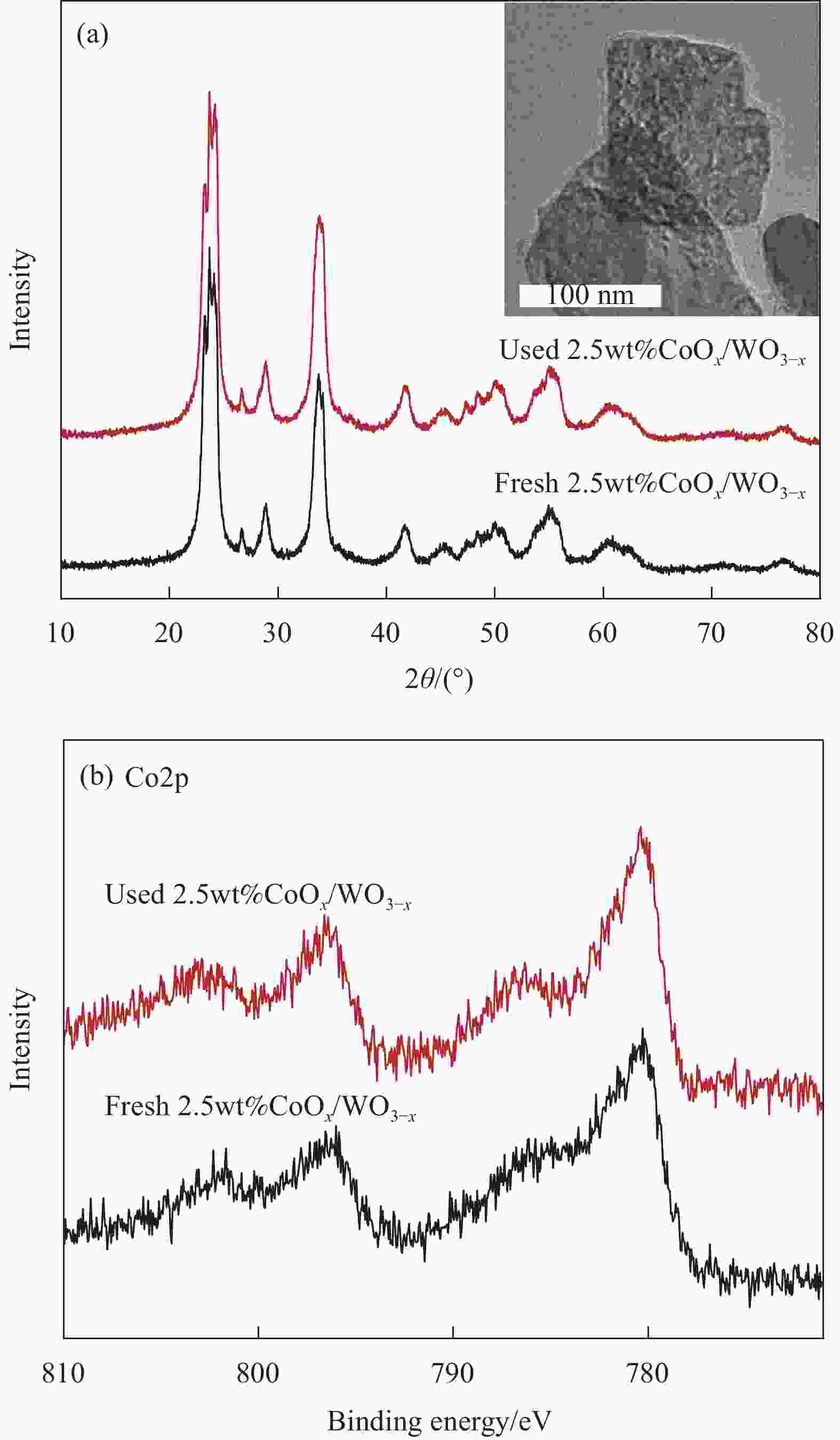
图 5 单一WO3-x与2.5wt%CoOx/WO3-x的XPS全谱 (a)、W4f高分辨XPS图谱 (b) 和O1s高分辨XPS图谱 (c)、2.5wt%CoOx/WO3-x的Co2p高分辨XPS图谱 (d)
Figure 5. XPS survey spectra of bare WO3-x and 2.5wt%CoOx/WO3-x (a), high-resolution W4f XPS spectra (b) and O1s XPS spectra of WO3-x and 2.5wt%CoOx/WO3-x (c), high-resolution Co2p XPS spectrum of 2.5wt%CoOx/WO3-x (d)
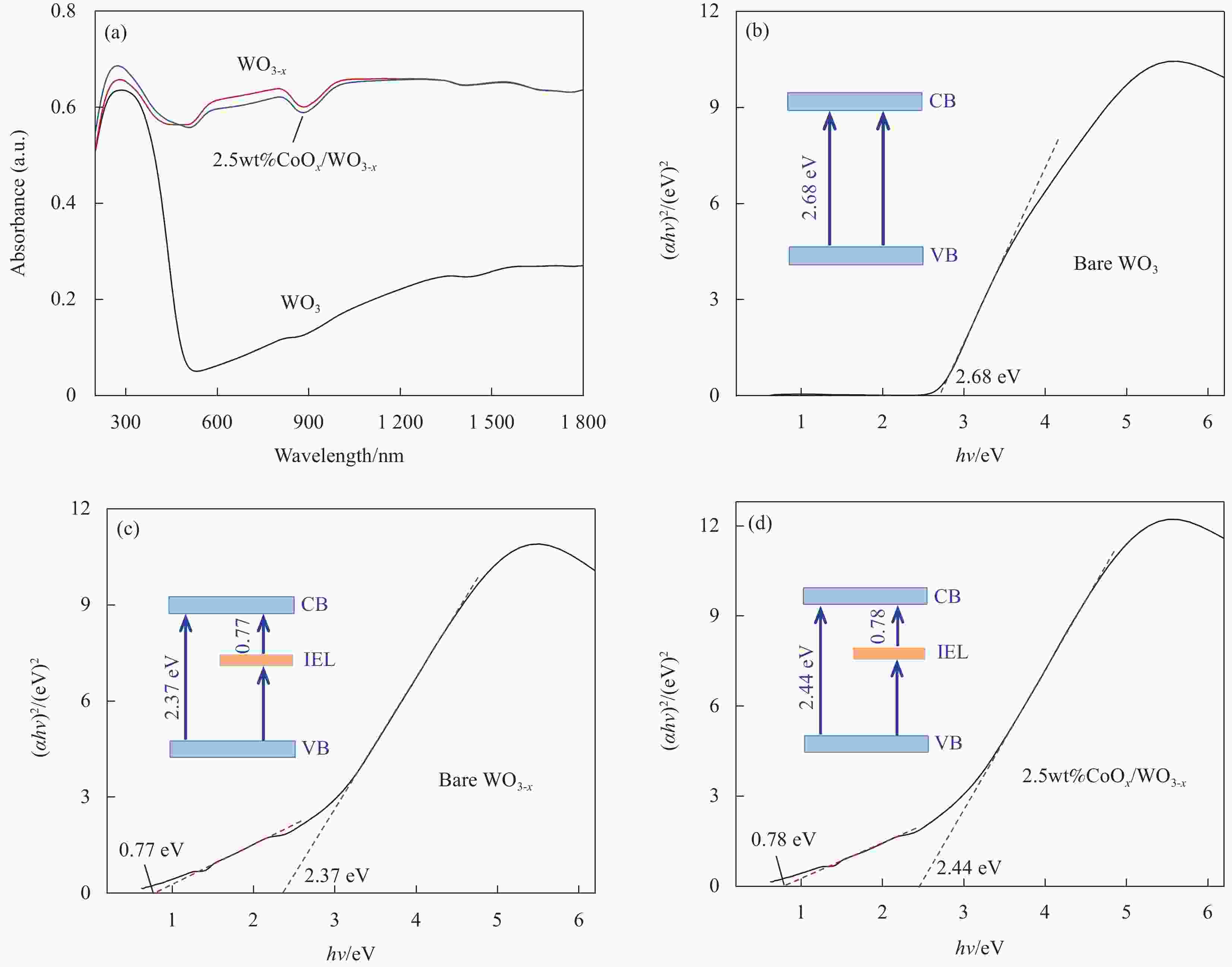
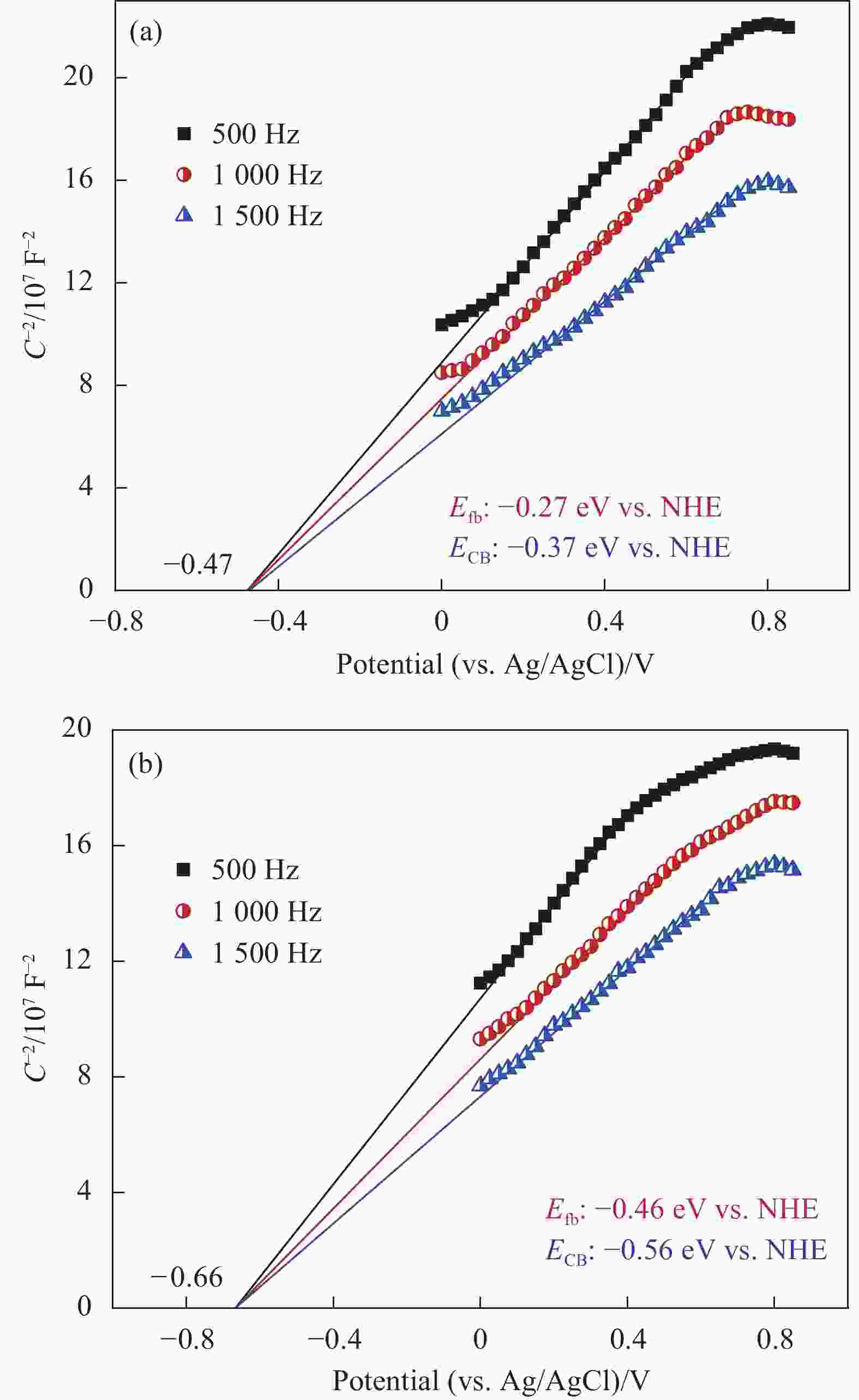
图 7 WO3、WO3-x与2.5wt%CoOx/WO3-x的紫外-可见-近红外吸收光谱 (a) 及相应的(αhv)2对hv曲线 ((b)~(d))
Figure 7. UV-Vis-NIR absorption spectra (a) and corresponding (αhv)2 vs. hv curves ((b)-(d)) of WO3, WO3-x and 2.5wt%CoOx/WO3-x
CB—Conduction band; VB—Valence band; IEL—Intermediate energy level; h—Planck constant; v—Frequency; α—Absorptivity index
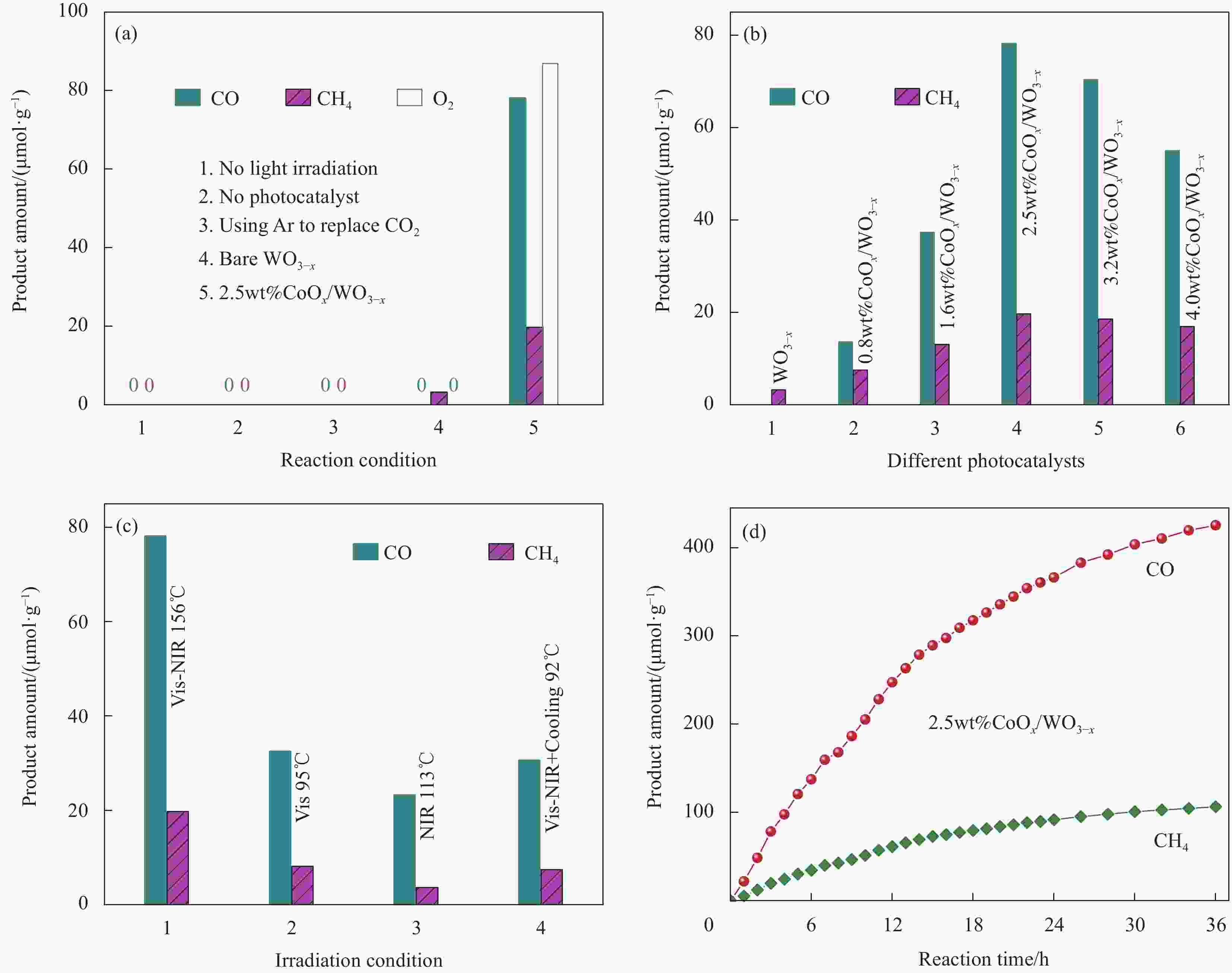
图 8 (a) 不同反应条件下C1产物与O2生成量;可见-近红外光照射(b)和不同光源照射条件下(c)CoOx/WO3-x催化剂的C1产物生成量;(d) 可见-近红外光照射下2.5wt%CoOx/WO3-x的长期催化稳定性
Figure 8. (a) Generation amount of C1 products and O2 under different reaction conditions; Generation amount of C1 products on different CoOx/WO3-x under Vis-NIR light (b) and under different light source irradiation (c); (d) Long-time catalytic stability of 2.5wt%CoOx/WO3-x under Vis-NIR light
NIR—Near infrared ray
表 1 CoOx/WO3-x复合催化剂中Co元素的实测含量
Table 1. Actual content of Co element in CoOx/WO3-x composite catalysts
Sample Addition content
of Co/wt%Measured content
of Co/wt%0.8wt%CoOx/WO3-x 0.8 0.79 1.6wt%CoOx/WO3-x 1.6 1.57 2.5wt%CoOx/WO3-x 2.5 2.48 3.2wt%CoOx/WO3-x 3.2 3.17 4.0wt%CoOx/WO3-x 4.0 3.95 表 2 CO2光催化还原产物生成速率的比较
Table 2. Comparison of products generation rate for CO2 photocatalytic reduction
Photocatalyst CO yield/(μmol·g−1·h−1) CH4 yield/(μmol·g−1·h−1) Reaction condition Reference MoO3-x 10.3 2.08 300 W Xe lamp UV-Vis-IR [20] Bi2S3/UiO-66 25.6 – 300 W Xe lamp UV-Vis-IR [17] C-doped WO3-x 23.2 1.01 300 W Xe lamp an AM1.5 G filter [36] Bi4TaO8Cl/W18O49 23.42 – 180 mW/cm2 solar light, 393 K [42] WO3 nanosheets – 1.19 300 W Xe lamp Visible light [43] TiO2-x(A/B)-CoOx 16.46 10.02 150 W UV lamp 393 K [44] WO3/LaTiO2N 2.21 0.36 300 W Xe lamp Visible light [45] WO3-x/g-C3N4 8.3 – 500 W Xe lamp [46] 2.5wt%CoOx/WO3-x 26.1 6.57 300 W Xe lamp Vis-NIR This work -
[1] SHARIFZADEH M, WANG L, SHAH N. Integrated biorefineries: CO2 utilization for maximum biomass conversion[J]. Renewable and Sustainable Energy Reviews,2015,47:151-161. doi: 10.1016/j.rser.2015.03.001 [2] JIA J, QIAN C X, DONG Y C, et al. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering-perfecting imperfection[J]. Chemical Society Reviews,2017,46(15):4631-4644. doi: 10.1039/C7CS00026J [3] SHEN H, GU Z X, ZHENG G F. Pushing the activity of CO2 electroreduction by system engineering[J]. Science Bulletin,2019,64(24):1805-1816. doi: 10.1016/j.scib.2019.08.027 [4] INOUE T, FUJISHIMA A, KONISHI S, et al. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders[J]. Nature,1979,277(5698):637-638. doi: 10.1038/277637a0 [5] LI X, YU J G, JARONIEC M, et al. Cocatalysts for selective photoreduction of CO2 into solar fuels[J]. Chemical Reviews,2019,119(6):3962-4179. doi: 10.1021/acs.chemrev.8b00400 [6] JI Y F, LUO Y. Theoretical study on the mechanism of photoreduction of CO2 to CH4 on the anatase TiO2 (101) surface[J]. ACS Catalysis,2016,6(3):2018-2025. doi: 10.1021/acscatal.5b02694 [7] ZHAO L H, YE F, WANG D M, et al. Lattice engineering on metal cocatalysts for enhanced photocatalytic reduction of CO2 into CH4[J]. ChemSusChem,2018,11(19):3524-3533. doi: 10.1002/cssc.201801294 [8] LIU L J, ZHAO H L, ANDINO J M, et al. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry[J]. ACS Catalysis,2012,2(8):1817-1828. doi: 10.1021/cs300273q [9] LI P, HU H F, LUO G, et al. Crystal facet-dependent CO2 photoreduction over porous ZnO nanocatalysts[J]. ACS Applied Materials & Interfaces,2020,12(50):56039-56048. [10] WANG S H, ZHAN J W, CHEN K, et al. Potassium-doped g-C3N4 achieving efficient visible-light-driven CO2 reduction[J]. ACS Sustainable Chemistry & Engineering,2020,8(22):8214-8222. [11] ZHANG Z J, LI L, JIANG Y, et al. Step-scheme photocatalyst of CsPbBr3 quantum dots/BiOBr nanosheets for efficient CO2 photoreduction[J]. Inorganic Chemistry,2022,61(7):3351-3360. doi: 10.1021/acs.inorgchem.2c00012 [12] CHO K M, KIM K H, PARK K, et al. Amine-functionalized graphene/CdS composite for photocatalytic reduction of CO2[J]. ACS Catalysis,2017,7(10):7064-7069. doi: 10.1021/acscatal.7b01908 [13] CAI X Y, ZHU M S, ELBANNA O A, et al. Au nanorod photosensitized La2Ti2O7 nanosteps: Successive surface heterojunctions boosting visible to near-infrared photocatalytic H2 evolution[J]. ACS Catalysis,2018,8:122-131. doi: 10.1021/acscatal.7b02972 [14] LIU S, CHAI J, SUN S H, et al. Site-selective photosynthesis of Ag–AgCl@Au nanomushrooms for NIR-II light-driven O2- and O2·– evolving synergistic photothermal therapy against deep hypoxic tumors[J]. ACS Applied Materials & Interfaces,2021,13(39):46451-46463. [15] 李成欣, 高助威, 刘钟馨, 等. 氧化石墨烯负载无纺布复合膜的制备及光热转换性能[J]. 复合材料学报, 2021, 38(12):4255-4264. doi: 10.13801/j.cnki.fhclxb.20210304.001LI Chengxin, GAO Zhuwei, LIU Zhongxin, et al. Preparation of graphene oxide supported non-woven fabric composite membrane and its photothermal conversion performance[J]. Acta Materiae Compositae Sinica,2021,38(12):4255-4264(in Chinese). doi: 10.13801/j.cnki.fhclxb.20210304.001 [16] MOON H K, LEE S H, CHOI H C. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes[J]. ACS Nano,2009,3(11):3707-3713. doi: 10.1021/nn900904h [17] CHEN X, LI Q, LI J J, et al. Modulating charge separation via in situ hydrothermal assembly of low content Bi2S3 into UiO-66 for efficient photothermocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental,2020,270:118915. doi: 10.1016/j.apcatb.2020.118915 [18] LI R Y, ZHANG L B, SHI L, et al. MXene Ti3C2: An effective 2D light-to-heat conversion material[J]. ACS Nano,2017,11(4):3752-3759. doi: 10.1021/acsnano.6b08415 [19] KUWAHARA Y, YOSHIMURA Y, HAEMATSU K, et al. Mild deoxygenation of sulfoxides over plasmonic molybdenum oxide hybrid with dramatic activity enhancement under visible light[J]. Journal of the American Chemical Society,2018,140(29):9203-9210. doi: 10.1021/jacs.8b04711 [20] LI J, YE Y H, YE L Q, et al. Sunlight induced photo-thermal synergistic catalytic CO2 conversion via localized surface plasmon resonance of MoO3-x[J]. Journal of Materials Chemistry A,2019,7(6):2821-2830. doi: 10.1039/C8TA10922B [21] JI Y F, LUO Y. New mechanism for photocatalytic reduction of CO2 on the anatase TiO2(101) surface: The essential role of oxygen vacancy[J]. Journal of the American Che-mical Society,2016,138(49):15896-15902. doi: 10.1021/jacs.6b05695 [22] WANG M, SHEN M, JIN X X, et al. Oxygen vacancy generation and stabilization in CeO2–x by Cu introduction with improved CO2 photocatalytic reduction activity[J]. ACS Catalysis,2019,9(5):4573-4581. doi: 10.1021/acscatal.8b03975 [23] LI X, WEN J Q, LOW J, et al. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel[J]. Science China Materials,2014,57(1):70-100. doi: 10.1007/s40843-014-0003-1 [24] WANG S Y, TERAMURA K, HISATOMI T, et al. Highly selective photocatalytic conversion of carbon dioxide by water over Al-SrTiO3 photocatalyst modified with silver–metal dual cocatalysts[J]. ACS Sustainable Chemistry& Engineering,2021,9(28):9327-9335. [25] WANG Y O, GODIN R, DURRANT J R, et al. Efficient hole trapping in carbon dot/oxygen-modified carbon nitride heterojunction photocatalysts for enhanced methanol production from CO2 under neutral conditions[J]. Angewandte Chemie-International Edition,2021,60(38):20811-20816. doi: 10.1002/anie.202105570 [26] WANG Y O, CHEN E Q, TANG J W. Insight on reaction pathways of photocatalytic CO2 conversion[J]. ACS Catalysis,2022,12(12):7300-7316. doi: 10.1021/acscatal.2c01012 [27] YIN G, NISHIKAWA M, NOSAKA Y, et al. Photocatalytic carbon dioxide reduction by copper oxide nanocluster-grafted niobate nanosheets[J]. ACS Nano,2015,9(2):2111-2119. doi: 10.1021/nn507429e [28] YAMAKATA A, KAWAGUCHI M, NISHIMURA N, et al. Behavior and energy states of photogenerated charge carriers on Pt- or CoOx-loaded LaTiO2N photocatalysts: Time-resolved visible to mid-infrared absorption study[J]. Journal of Physical Chemistry C,2014,118(41):23897-23906. doi: 10.1021/jp508233z [29] ZHANG H Y, GUO C F, REN J B, et al. Beyond CoOx: A versatile amorphous cobalt species as an efficient cocatalyst for visible-light-driven photocatalytic water oxidation[J]. Chemical Communications,2019,55(93):14050-14053. doi: 10.1039/C9CC07835E [30] ZHANG J K, YU Z B, GAO Z, et al. Porous TiO2 nanotubes with spatially separated platinum and CoOx cocatalysts produced by atomic layer deposition for photocatalytic hydrogen production[J]. Angewandte Chemie-International Edition,2017,56(3):816-820. doi: 10.1002/anie.201611137 [31] BILLO T, FU F, RAGHUNATH P, et al. Ni-nanocluster modified black TiO2 with dual active sites for selective photocatalytic CO2 reduction[J]. Small,2018,14(2):1702928. doi: 10.1002/smll.201702928 [32] YANG J, CHEN P Y, DAI J, et al. Solar-energy-driven conversion of oxygen-bearing low-concentration coal mine methane into methanol on full-spectrum-responsive WO3-x catalysts[J]. Energy Conversion and Management,2021,247:114767. doi: 10.1016/j.enconman.2021.114767 [33] 刘颖琪, 翁文斌, 岑檠, 等. FeVO4/Cu3(BTC)2(H2O)3异质结制备及光催化性能[J]. 复合材料学报, 2020, 37(12):3128-3136.LIU Yingqi, WENG Wenbin, CEN Qin, et al. Preparation and photocatalytic properties of FeVO4/Cu3(BTC)2(H2O)3 heterojunction[J]. Acta Materiae Compositae Sinica,2020,37(12):3128-3136(in Chinese). [34] HUANG J W, ZHANG Y, DING Y. Rationally designed/constructed CoOx/WO3 anode for efficient photoelectrochemical water oxidation[J]. ACS Catalysis,2017,7(3):1841-1845. doi: 10.1021/acscatal.7b00022 [35] ZHANG N, LI X Y, YE H C, et al. Oxide defect engineering enables to couple solar energy into oxygen activation[J]. Journal of the American Chemical Society,2016,138(28):8928-8935. doi: 10.1021/jacs.6b04629 [36] LEI B, CUI W, CHEN P, et al. C−doping induced oxygen-vacancy in WO3 nanosheets for CO2 activation and photoreduction[J]. ACS Catalysis,2022,12(15):9670-9678. doi: 10.1021/acscatal.2c02390 [37] MONIRUDDIN M, OPPONG E, STEWART D, et al. Designing CdS-based ternary heterostructures consisting of co-metal and CoOx cocatalysts for photocatalytic H2 evolution under visible light[J]. Inorganic Chemistry,2019,58(18):12325-12333. doi: 10.1021/acs.inorgchem.9b01854 [38] 杨玉蓉, 王佳慧, 马远驰, 等. 双缺陷调控的Z型BiVO4−x/g-C3N4−x异质结的制备及其光催化全解水[J]. 复合材料学报, 2022, 39(10):4642-4651.YANG Yurong, WANG Jiahui, MA Yuanchi, et al. Preparation of Z-scheme BiVO4−x/g-C3N4−x heterojunction mediated by double defects and photocatalytic overall water splitting[J]. Acta Materiae Compositae Sinica,2022,39(10):4642-4651(in Chinese). [39] YANG J, HU S Y, FANG Y R, et al. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation[J]. ACS Catalysis,2019,9(11):9751-9763. doi: 10.1021/acscatal.9b02408 [40] ZHENG Y F, FU K X, YU Z H, et al. Oxygen vacancies in a catalyst for VOCs oxidation: Synthesis, characterization, and catalytic effects[J]. Journal of Materials Chemistry A,2022,10(27):14171-14186. doi: 10.1039/D2TA03180A [41] LI A, CAO Q, ZHOU G Y, et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface[J]. Angewandte Chemie International Edition,2019,58(41):14549-14555. doi: 10.1002/anie.201908058 [42] YAN J Y, WANG C H, MA H, et al. Photothermal synergic enhancement of direct Z-scheme behavior of Bi4TaO8Cl/W18O49 heterostructure for CO2 reduction[J]. Applied Catalysis B: Environmental,2020,268:118401. doi: 10.1016/j.apcatb.2019.118401 [43] CHEN X Y, ZHOU Y, LIU Q, et al. Ultrathin, single-crystal WO3 nanosheets by two-dimensional oriented attachment toward enhanced photocatalystic reduction of CO2 into hydrocarbon fuels under visible light[J]. ACS Applied Materials & Interfaces,2012,4(7):3372-3377. [44] LI Y Y, WANG C H, SONG M, et al. TiO2-x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with H2O steam[J]. Applied Catalysis B: Environmental,2019,243:760-770. doi: 10.1016/j.apcatb.2018.11.022 [45] LIN N S, LIN Y A, QIAN X J, et al. Construction of a 2D/2D WO3/LaTiO2N direct Z-scheme photocatalyst for enhanced CO2 reduction performance under visible light[J]. ACS Sustainable Chemistry & Engineering,2021,9(40):13686-13694. [46] HUANG S L, LONG Y J, RUAN S C, et al. Enhanced photocatalytic CO2 reduction in defect-engineered Z-scheme WO3-x/g-C3N4 heterostructures[J]. ACS Omega,2019,4(13):15593-15599. doi: 10.1021/acsomega.9b01969 [47] YANG J, WANG X H, ZHAO X L, et al. Synthesis of uniform Bi2WO6-reduced graphene oxide nanocomposites with significantly enhanced photocatalytic reduction activity[J]. The Journal of Physical Chemistry C,2015,119(6):3068-3078. doi: 10.1021/jp510041x -






 下载:
下载: