Preparation of graphene oxide load Ag3PO4@polyaniline composite and its photocatalytic performance
-
摘要: 为了解决Ag3PO4严重的光腐蚀问题,采用化学吸附法制备了核壳结构的聚苯胺(PANI)包覆磷酸银(Ag3PO4@PANI),并用氧化石墨烯(GO)作为Ag3PO4@PANI复合光催化剂的载体,通过PANI和GO的协同作用提升了载流子的分离效率。当GO与Ag3PO4@PANI质量比为4%时,催化剂在24 min内降解苯酚的去除率可达98.1%,18 min内对环丙沙星(CIP)的去除率可达90.3%,15 min内对四环素(TC)的去除率可达98.6%,在5 min内对各类染料的去除率为100%。经过6次重复反应,Ag3PO4@PANI/GO仍保持较好的稳定性。自由基捕获实验证实•h+和•O2−是光催化降解的主要活性物种。实验结果表明,PANI与Ag3PO4之间形成了核壳结构,GO的引入提升了电子的传输速率,PANI和GO对Ag3PO4的协同作用促进了光生电子-空穴的分离,进而提升了Ag3PO4的稳定性和光催化活性。Abstract: To solve severe photocorrosion of Ag3PO4, which was used to prepare a core-shell Ag3PO4@polyaniline (PANI) composite photocatalyst by chemisorption, and graphene oxide (GO) was used as the carrier of Ag3PO4@PANI composite photocatalyst, reaching the superior carrier separation efficiency via synergetic effect of GO and PANI. The photocatalyst with mass ratio of GO to Ag3PO4@PANI of 4% shows visible light activity for the degradation of phenol, ciprofloxacin (CIP), tetracycline (TC) and dyes of 98.1%, 90.3%, 98.6% and 100% in 24 min, 18 min, 15 min and 5 min, respectively. Even after six repeat reactions, Ag3PO4@PANI/GO still maintain a high degradation rate. The trapping experiments confirm that •h+ and •O2 are the main active species in the photocatalytic degradation. The experimental results show that a core-shell structure is formed between PANI and Ag3PO4, the introduction of GO increases the electron transport rate, and the synergistic effect of PANI and GO on Ag3PO4 promotes the separation of photogenerated electrons and holes, thereby improving the stability of Ag3PO4 and photocatalytic activity.
-
Key words:
- Ag3PO4 /
- graphene oxide /
- core-shell /
- photocatalyst /
- degradation /
- phenol /
- antibiotic /
- dye
-
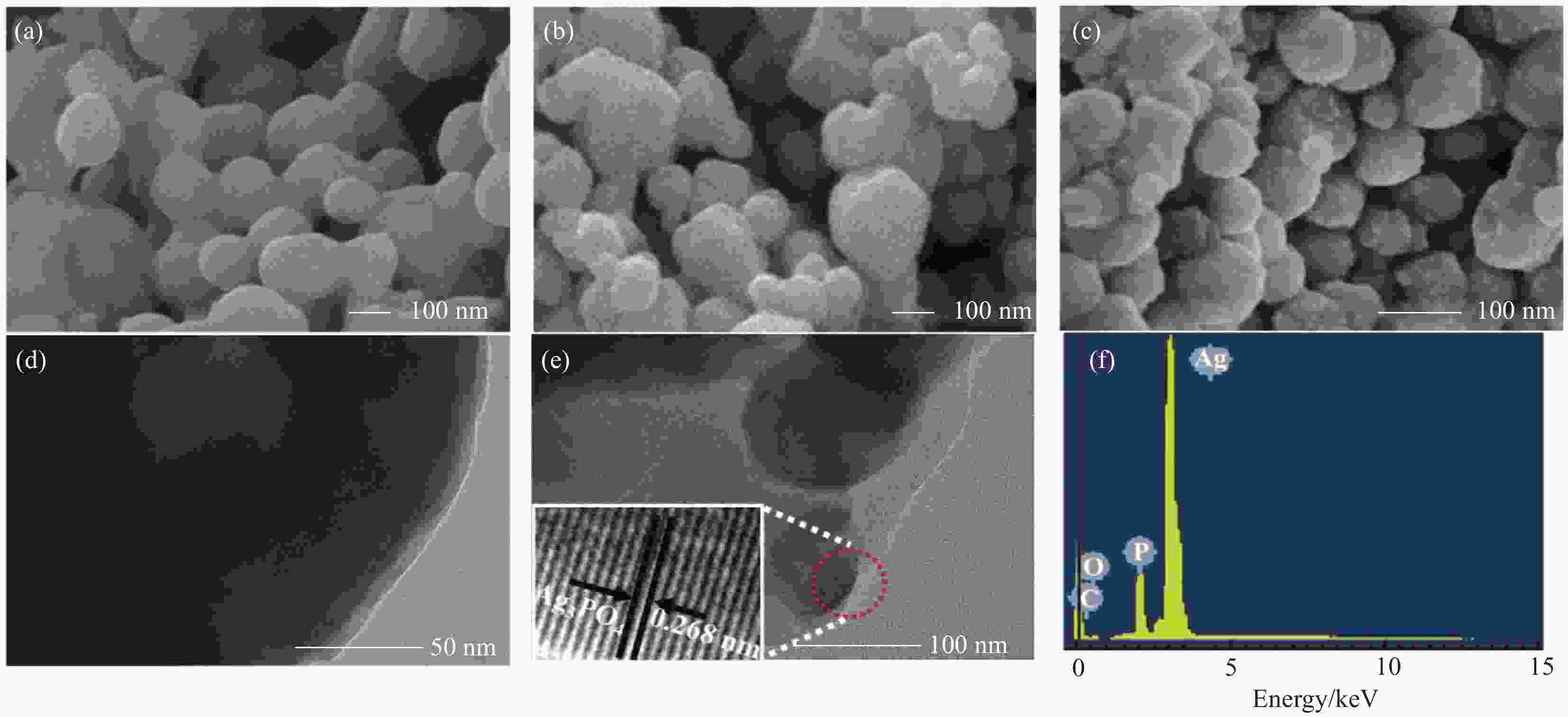
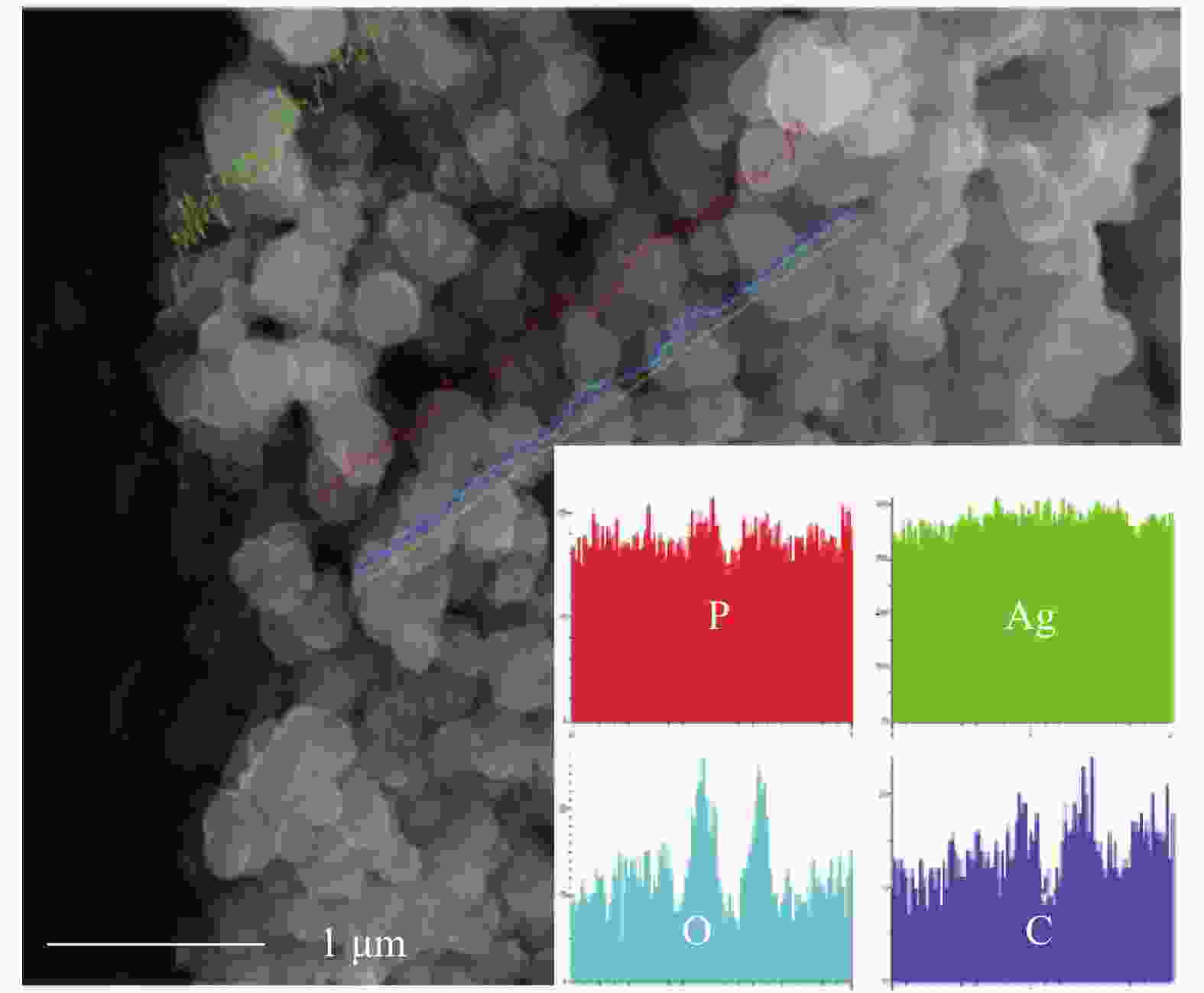
图 4 纯Ag3PO4 (a)、Ag3PO4@PANI (b) 和Ag3PO4@PANI/GO (c) 的SEM图像;Ag3PO4@PANI (d) 和Ag3PO4@PANI/4%GO (e) 的TEM图像;Ag3PO4@PANI/4%GO的EDS能谱图 (f)
Figure 4. SEM images of Ag3PO4 (a), Ag3PO4@PANI (b) and Ag3PO4@PANI/GO (c); TEM images of Ag3PO4@PANI (d) and Ag3PO4@PANI/4%GO (e); EDS spectrum of Ag3PO4@PANI/4%GO (f)
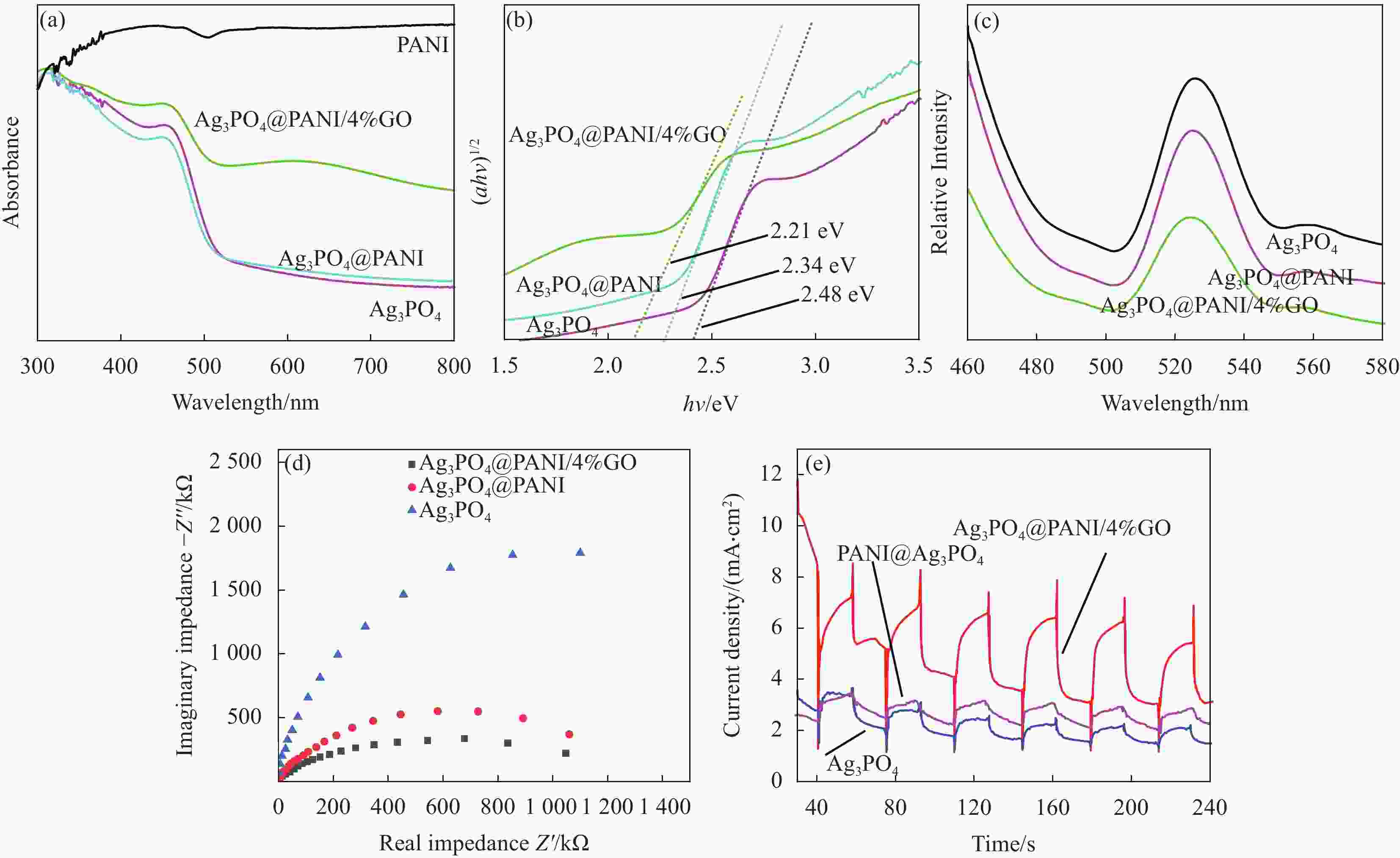
图 7 PANI、Ag3PO4、Ag3PO4@PANI和Ag3PO4@PANI/4%GO的UV-Vis (a)、Kubelka-Munk图 (b)、PL图 (c)、EIS图 (d) 和光电流响应图 (e)
Figure 7. UV-Vis diffuse reflectance spectra (a), plot of (αhν)1/2 vs. hν (b), Photoluminescence spectrum (c), EIS of Nyquist plots (d) and photocurrent responses (e) of PANI, Ag3PO4, Ag3PO4@PANI and Ag3PO4@PANI/4%GO
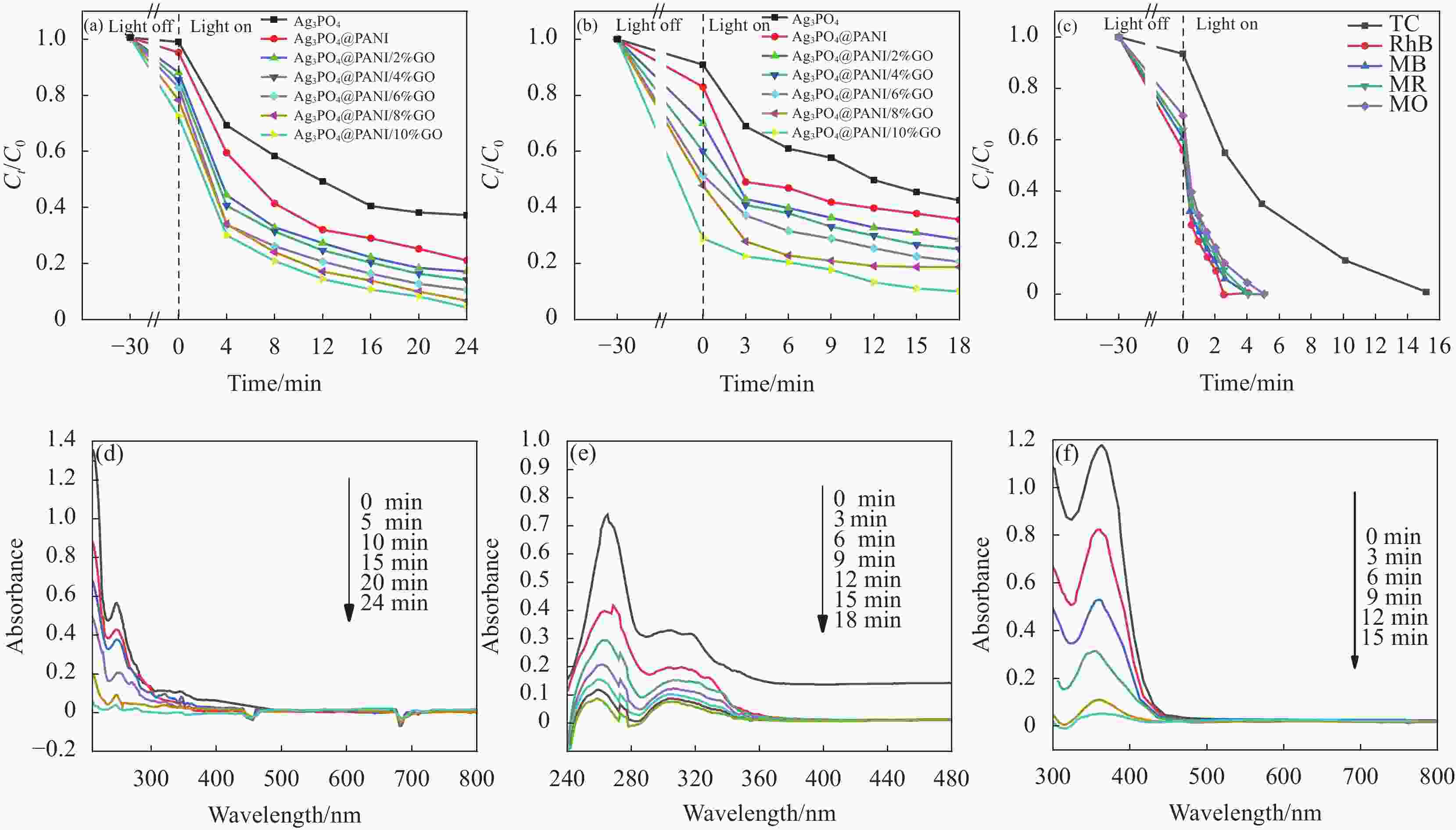
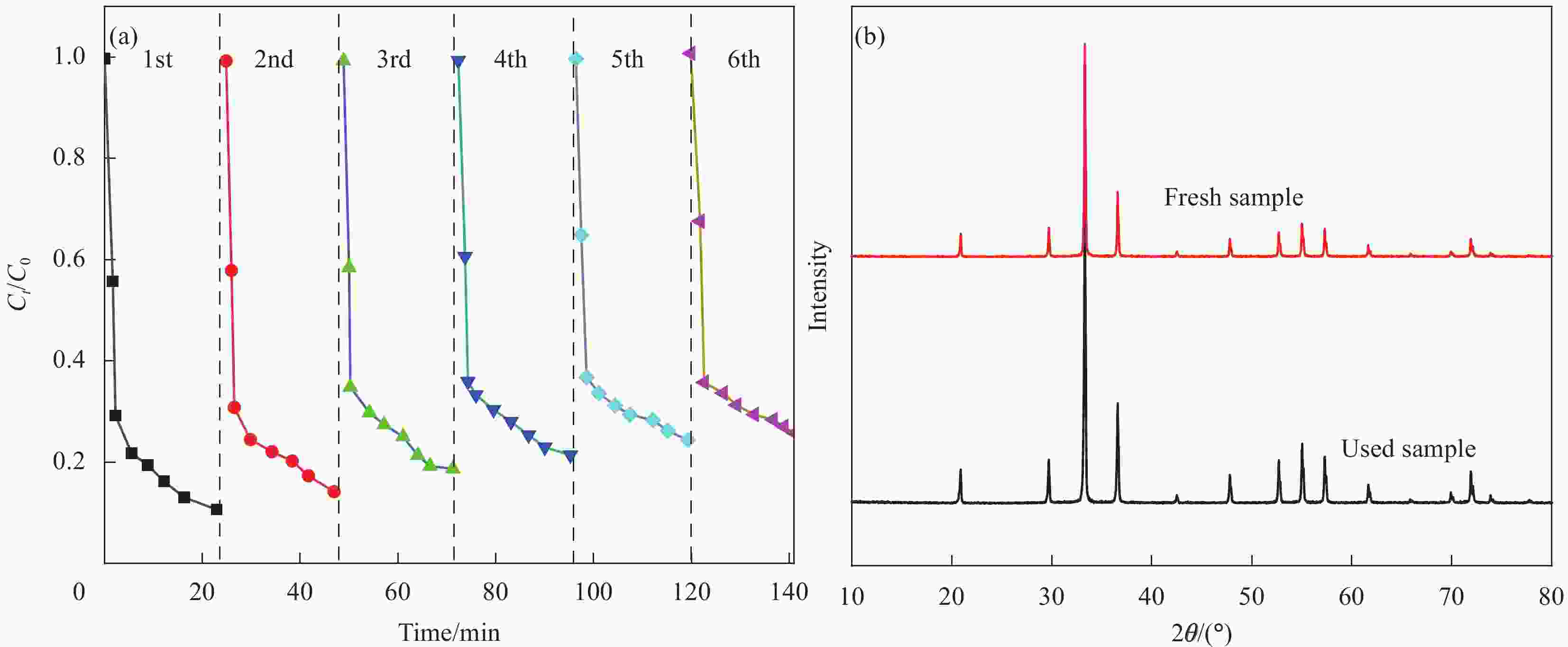
图 8 PANI、Ag3PO4、Ag3PO4@PANI和Ag3PO4@PANI/GO可见光下降解苯酚曲线 (a)、降解环丙沙星(CIP)曲线 (b);Ag3PO4@PANI/GO降解四环素(TC)、罗丹明B(RhB)、亚甲基蓝(MB)、亚甲基红(MR)和亚甲基橙 (MO)的曲线 (c);Ag3PO4@PANI/4%GO降解苯酚 (d)、CIP (e) 和TC (f) 的紫外-可见吸收光谱曲线
Figure 8. Under visible light curves of degradation of phenol (a), curves of degradation of ciprofloxacin (CIP) (b) of PANI, Ag3PO4, Ag3PO4@PANI and Ag3PO4@PANI/GO; Curves of degradation of tetracycline (TC), rhodamine B (RhB), methylene blue (MB), methylene red (MR) and methylene orange (MO) by Ag3PO4@PANI/GO (c); Degradation of phenol (d), CIP (e) and TC (f) by Ag3PO4@PANI/4%GO ultraviolet-visible absorption spectrum curves
Ct—Concentration after time t of degradation; C0—Initial concentration
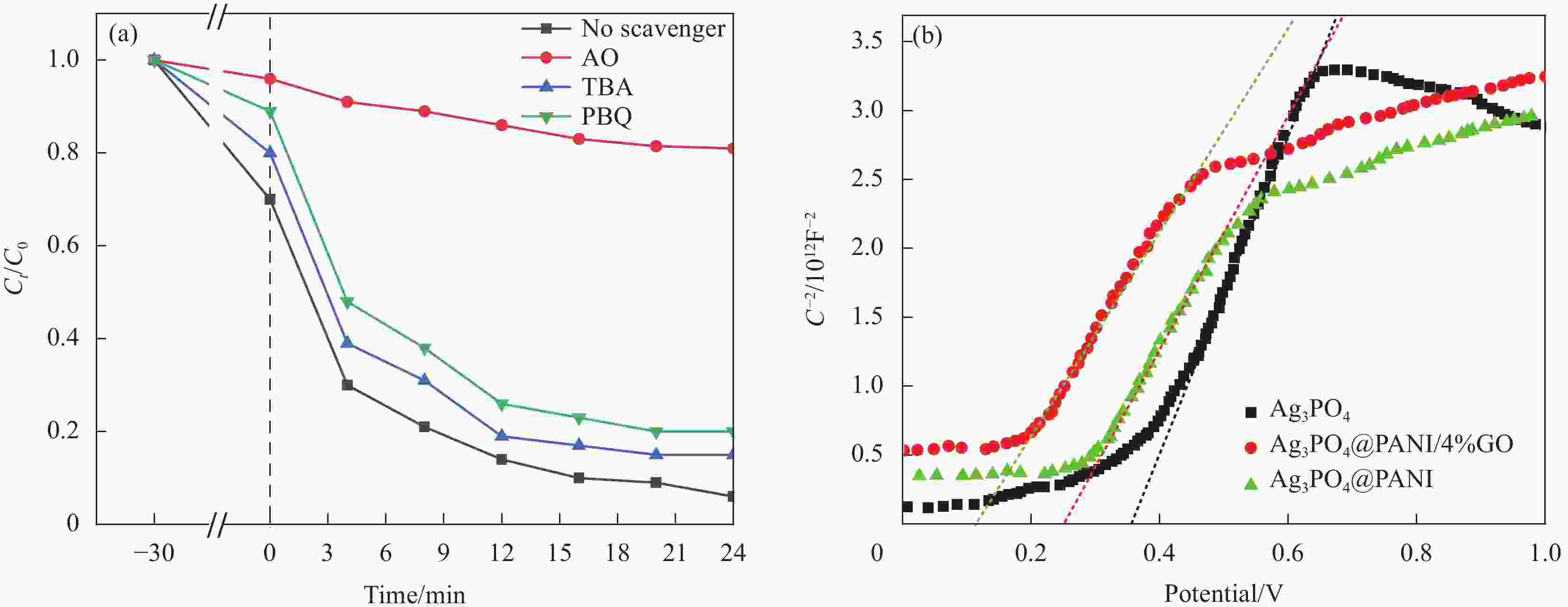
图 10 (a) Ag3PO4@PANI/4%GO的活性物种捕获实验;(b) Ag3PO4@PANI/4%GO、Ag3PO4和Ag3PO4@PANI的Mott–Schottky曲线
Figure 10. (a) Trapping experiments for active species of Ag3PO4@PANI/4%GO; (b) Mott–Schottky curves obtained for Ag3PO4@PANI/4%GO, Ag3PO4 and Ag3PO4@PANI
AO—Ammonium oxalate; TBA—Tertiary butyl alcohol; PBQ—Benzoquinone; C—Interface capacitance
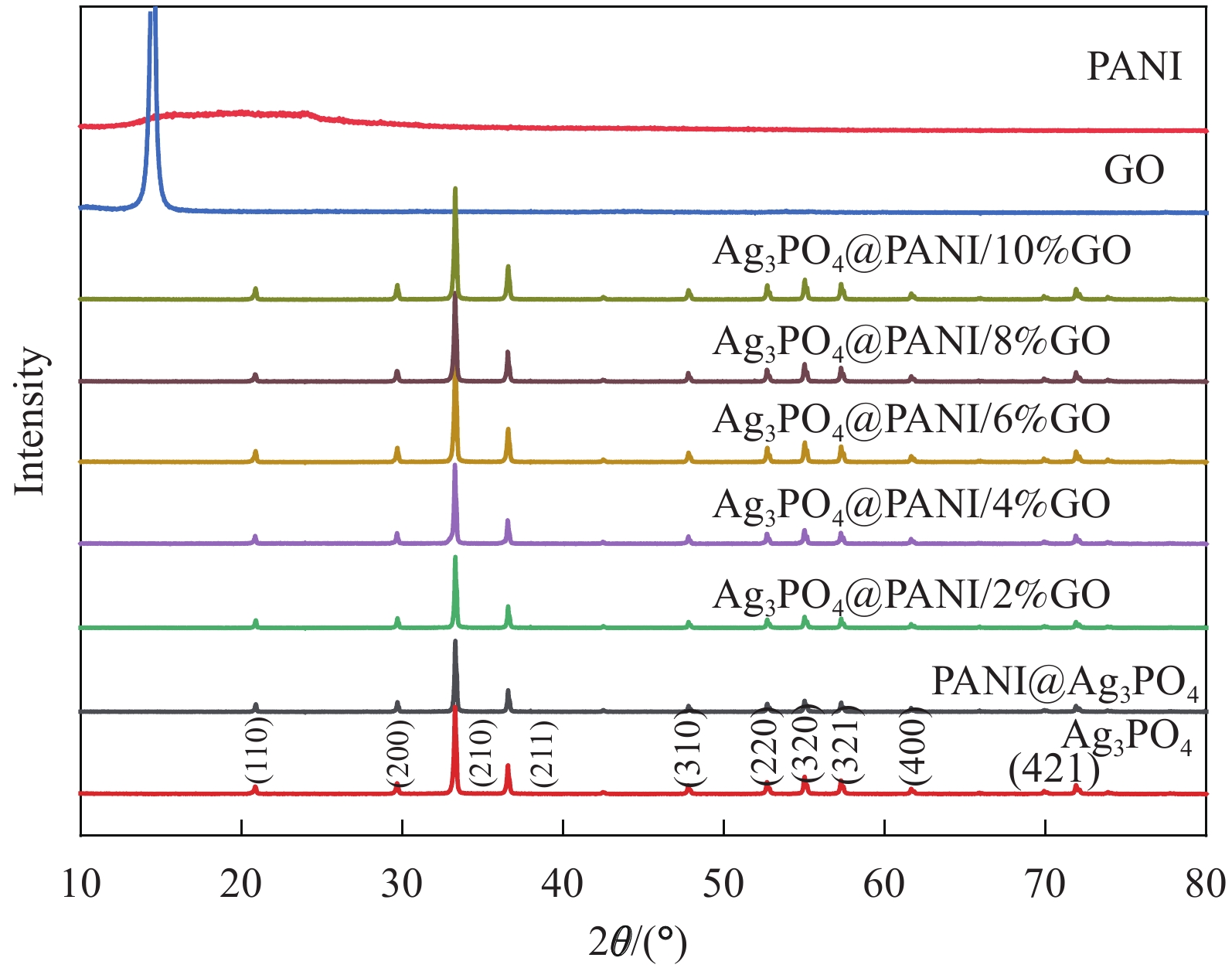
表 1 Ag3PO4@聚苯胺(PANI)/氧化石墨烯(GO)复合材料的命名
Table 1. Naming of Ag3PO4@polyaniline (PANI)/graphene oxide (GO) composites
Sample Mass ratio
of GO/%Mass ratio of
Ag3PO4@PANI/%Ag3PO4@PANI/2%GO 2 100 Ag3PO4@PANI/4%GO 4 100 Ag3PO4@PANI/6%GO 6 100 Ag3PO4@PANI/8%GO 8 100 Ag3PO4@PANI/10%GO 10 100 -
[1] KERGARAVAT S V, HERNÁNDEZ S R, GAGNETEN A M. Second-, third-and fourth-generation quinolones: Ecotoxicity effects on daphnia and ceriodaphnia species[J]. Chemosphere,2021,262:127823. doi: 10.1016/j.chemosphere.2020.127823 [2] DORIVAL-GARCÍA N, ZAFRA-GÓMEZ A, NAVALÓN A, et al. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions[J]. Jour-nal of Environmental Management,2013,120:75-83. [3] WANG H, LI J, HUO P, et al. Preparation of Ag2O/Ag2CO3/MWNTs composite photocatalysts for enhancement of ciprofloxacin degradation[J]. Applied Surface Science,2016,366:1-8. doi: 10.1016/j.apsusc.2015.12.229 [4] LI N, ZHANG J, TIAN Y, et al. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight[J]. Chemical Engineering Journal,2017,308:377-385. doi: 10.1016/j.cej.2016.09.093 [5] BI Y, OUYANG S, UMEZAWA N, et al. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties[J]. Journal of the American Chemical Society,2011,133(17):6490-6492. doi: 10.1021/ja2002132 [6] HE Y, ZHANG L, TENG B, et al. New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel[J]. Environmental Science & Technology,2015,49(1):649-656. [7] YANG X, CUI H, LI Y, et al. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance[J]. ACS Catalysis,2013,3(3):363-369. doi: 10.1021/cs3008126 [8] WU A, TIAN C, CHANG W, et al. Morphology-controlled synthesis of Ag3PO4 nano/microcrystals and their antibacterial properties[J]. Materials Research Bulletin,2013,48(9):3043-3048. doi: 10.1016/j.materresbull.2013.04.054 [9] WU S, LIN Y, YANG C, et al. Enhanced activation of peroxymonosulfte by LaFeO3 perovskite supported on Al2O3 for degradation of organic pollutants[J]. Chemosphere,2019,237:124478. doi: 10.1016/j.chemosphere.2019.124478 [10] XU Y S, ZHANG W D. Morphology-controlled synthesis of Ag3PO4 microcrystals for high performance photocatalysis[J]. CrystEngComm,2013,15(27):5407-5411. doi: 10.1039/c3ce40172c [11] YU H, DONG Q, JIAO Z, et al. Ion exchange synthesis of PAN/Ag3PO4 core-shell nanofibers with enhanced photocatalytic properties[J]. Journal of Materials Chemistry A,2014,2(6):1668-1671. doi: 10.1039/C3TA14447J [12] KHASEVANI S G, MOHAGHEGH N, GHOLAMI M R. Kinetic study of navy blue photocatalytic degradation over Ag3PO4/BiPO4@MIL-88B (Fe)@gC3N4 core@shell nanocomposite under visible light irradiation[J]. New Journal of Chemistry,2017,41(18):10390-10396. doi: 10.1039/C7NJ01968H [13] DENG J, LIU L, NIU T, et al. Synthesis and characterization of highly efficient and stable Pr6O11/Ag3PO4/Pt ternary hybrid structure[J]. Applied Surface Science,2017,403:531-539. doi: 10.1016/j.apsusc.2017.01.257 [14] KIANI M, BAGHERZADEH M, KAVEH R, et al. Novel Pt- Ag3PO4/CdS/chitosan nanocomposite with enhanced photocatalytic and biological activities[J]. Nanomaterials,2020,10(11):2320. doi: 10.3390/nano10112320 [15] PEI S, CHENG H M. The reduction of graphene oxide[J]. Carbon,2012,50(9):3210-3228. doi: 10.1016/j.carbon.2011.11.010 [16] NACIRI Y, HSINI A, AJMAL Z, et al. Recent progress on the enhancement of photocatalytic properties of BiPO4 using π–conjugated materials[J]. Advances in Colloid and Interface Science,2020:102160. [17] CHEN S, HUANG D, ZENG G, et al. In-situ synthesis of facet-dependent BiVO4/Ag3PO4/PANI photocatalyst with enhanced visible-light-induced photocatalytic degradation performance: Synergism of interfacial coupling and hole-transfer[J]. Chemical Engineering Journal,2020,382:122840. doi: 10.1016/j.cej.2019.122840 [18] TAO R, YANG S, SHAO C, et al. Reusable and flexible g-C3N4/Ag3PO4/polyacrylonitrile heterojunction nanofibers for photocatalytic dye degradation and oxygen evolution[J]. ACS Applied Nano Materials,2019,2(5):3081-3090. doi: 10.1021/acsanm.9b00428 [19] LIU L, HU P, LI Y, et al. P3HT-coated Ag3PO4 core-shell structure for enhanced photocatalysis under visible light irradiation[J]. Applied Surface Science,2019,466:928-936. doi: 10.1016/j.apsusc.2018.10.112 [20] SUN X, LIU Z, GUO J, et al. Novel stable enhanced visible light photocatalytic system based on a Ag3PO4@polypyrrole core-shell Z-scheme with in-situ generated metallic Ag ohmic contacts[J]. Journal of Physics and Chemistry of Solids,2020,146:109572. doi: 10.1016/j.jpcs.2020.109572 [21] LIU L, DING L, LIU Y, et al. A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation[J]. Applied Catalysis B: Environmental,2017,201:92-104. doi: 10.1016/j.apcatb.2016.08.005 [22] WANG Y, WU M, LEI W, et al. Preparation of 3D grid structure rGH/α-Ag3VO4/GOQDs and its catalytic performance under visible light[J]. Journal of Alloys and Compounds, 2022, 895: 162410-162415. [23] ISHIKAWA A, TAKATA T, KONDO J N, et al. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ≤650 nm)[J]. Journal of the American Chemical Society,2002,124(45):13547-13553. doi: 10.1021/ja0269643 -






 下载:
下载: