Preparation and properties of sodium nitrate/semi-coke ash shape-stable phase change composite
-
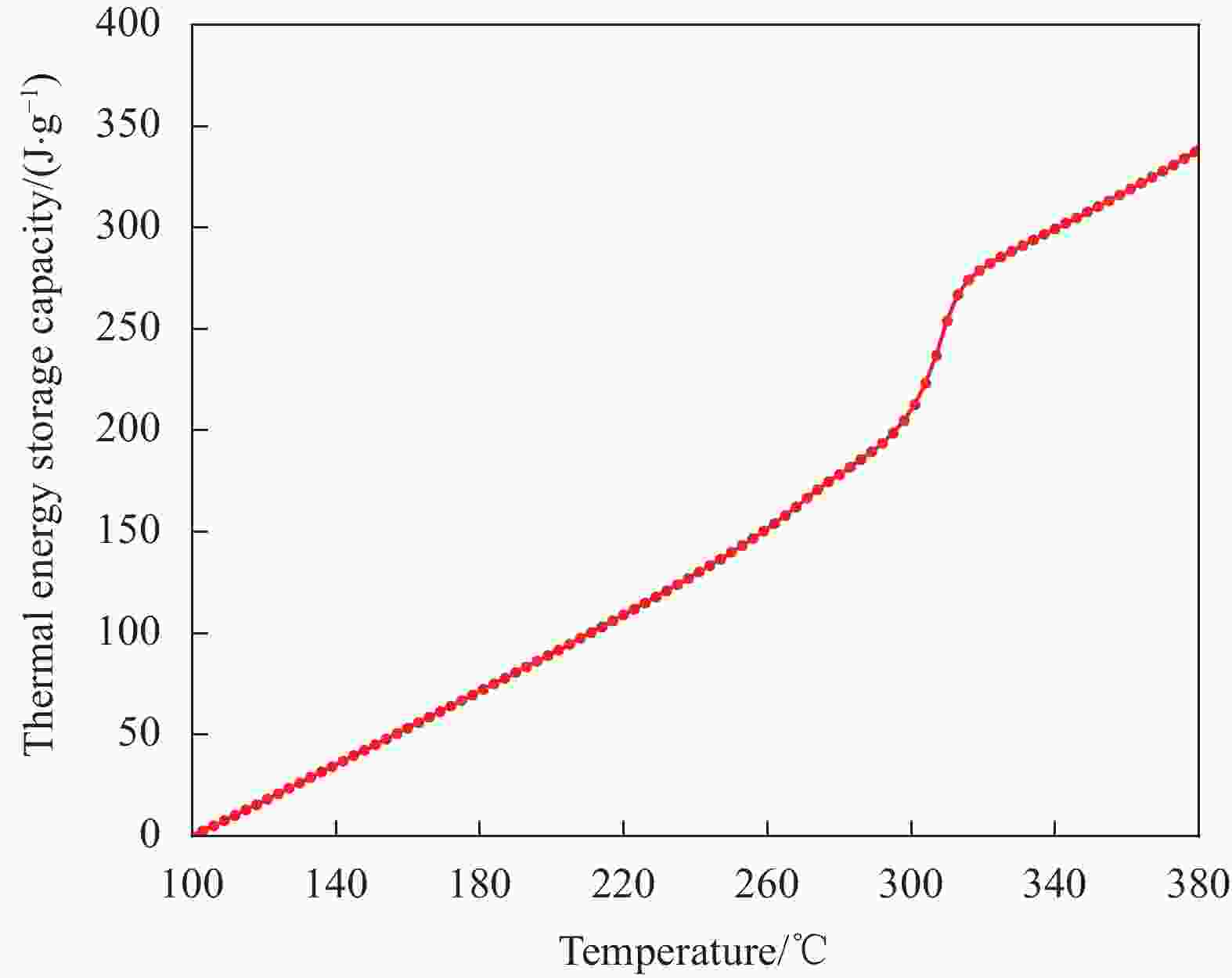
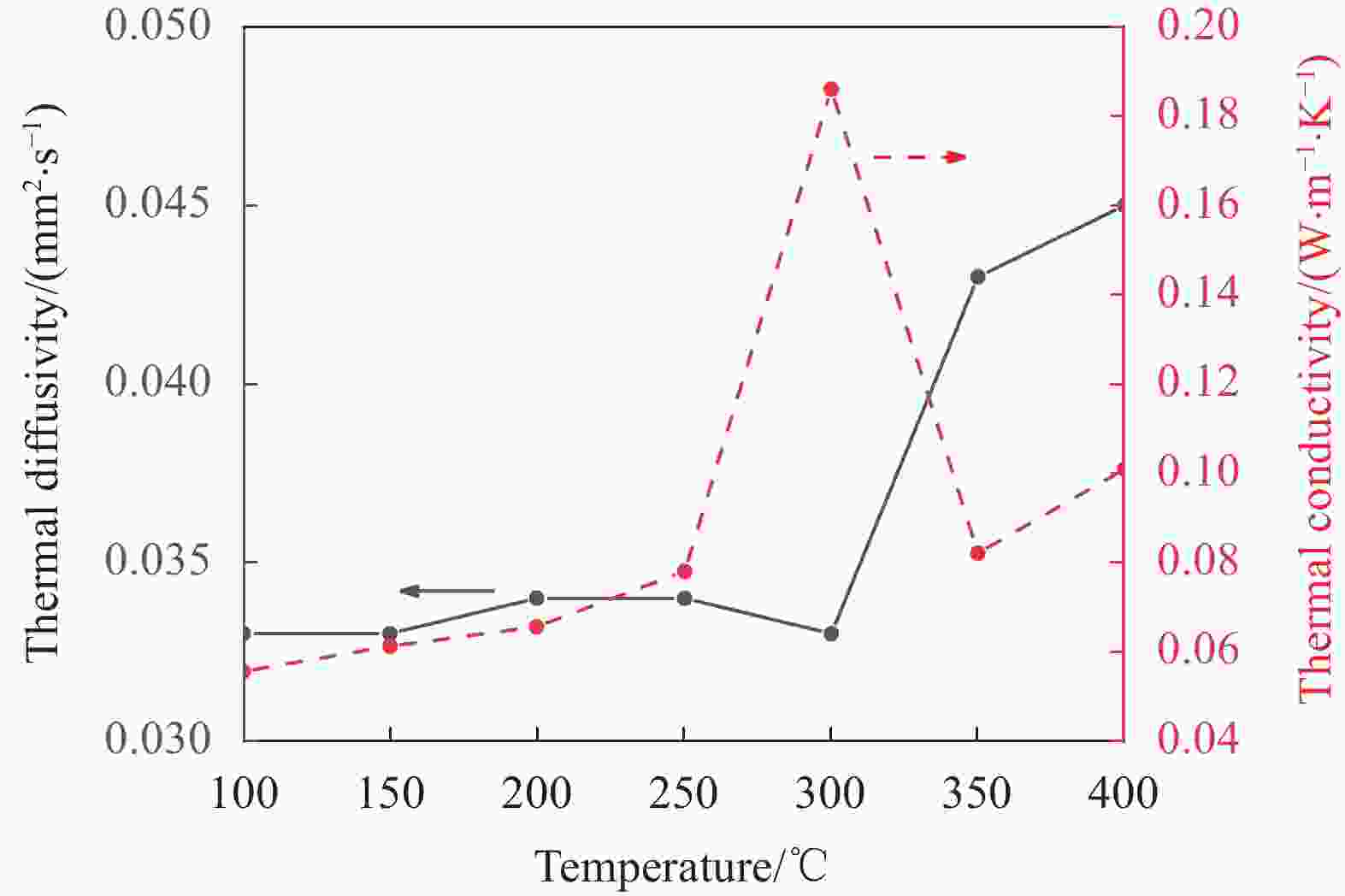
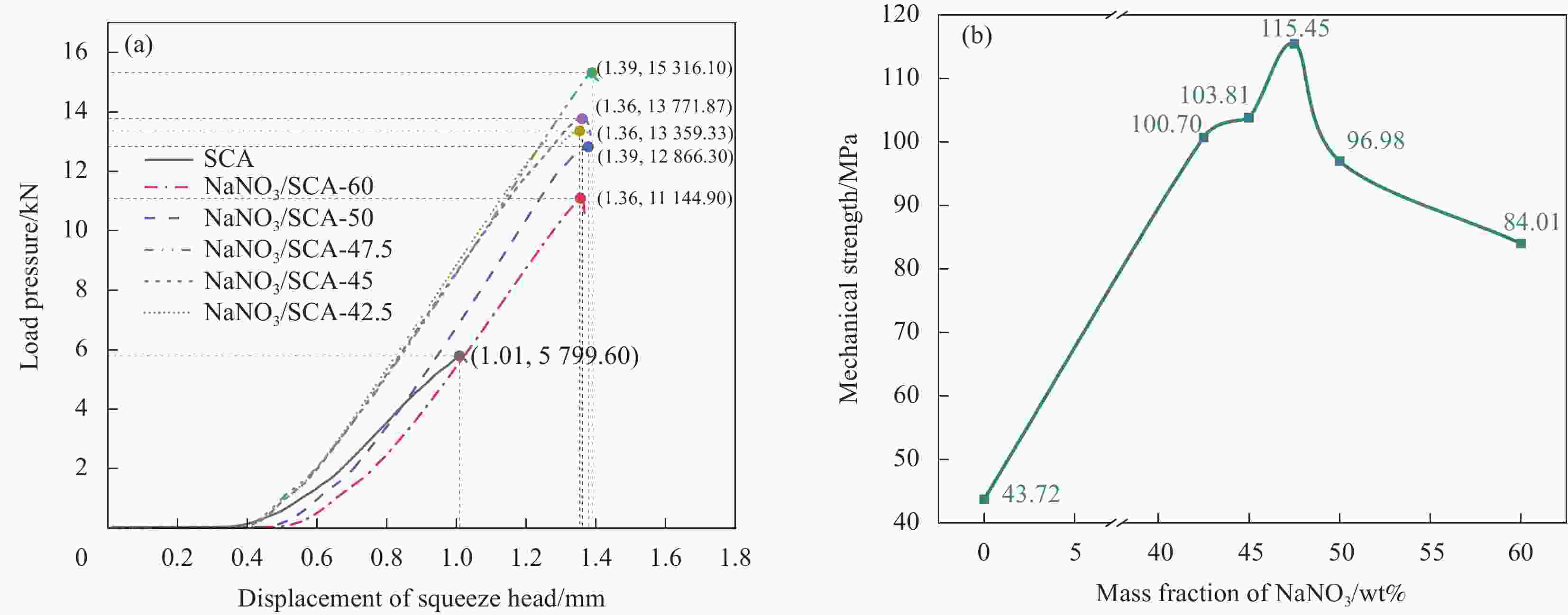
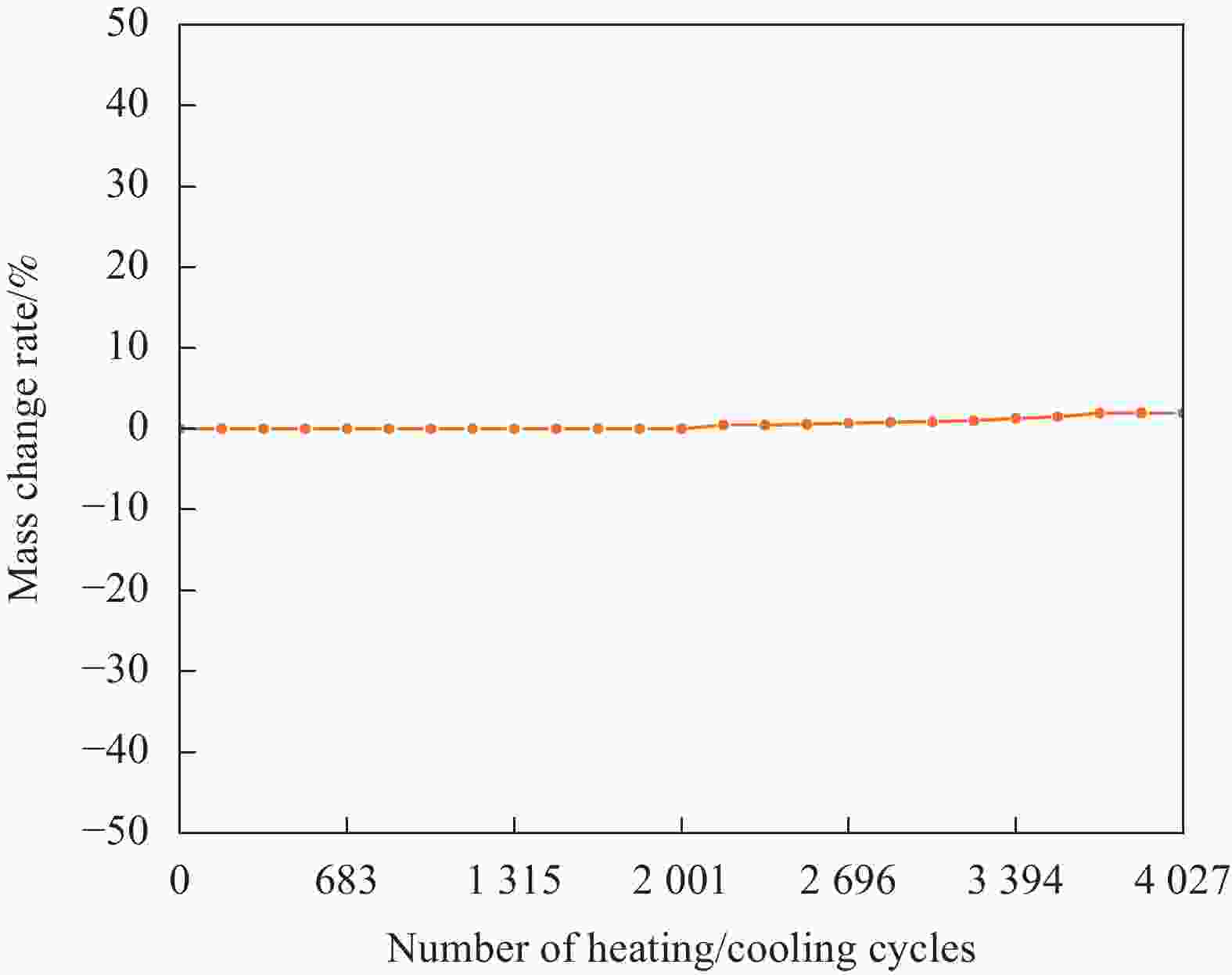
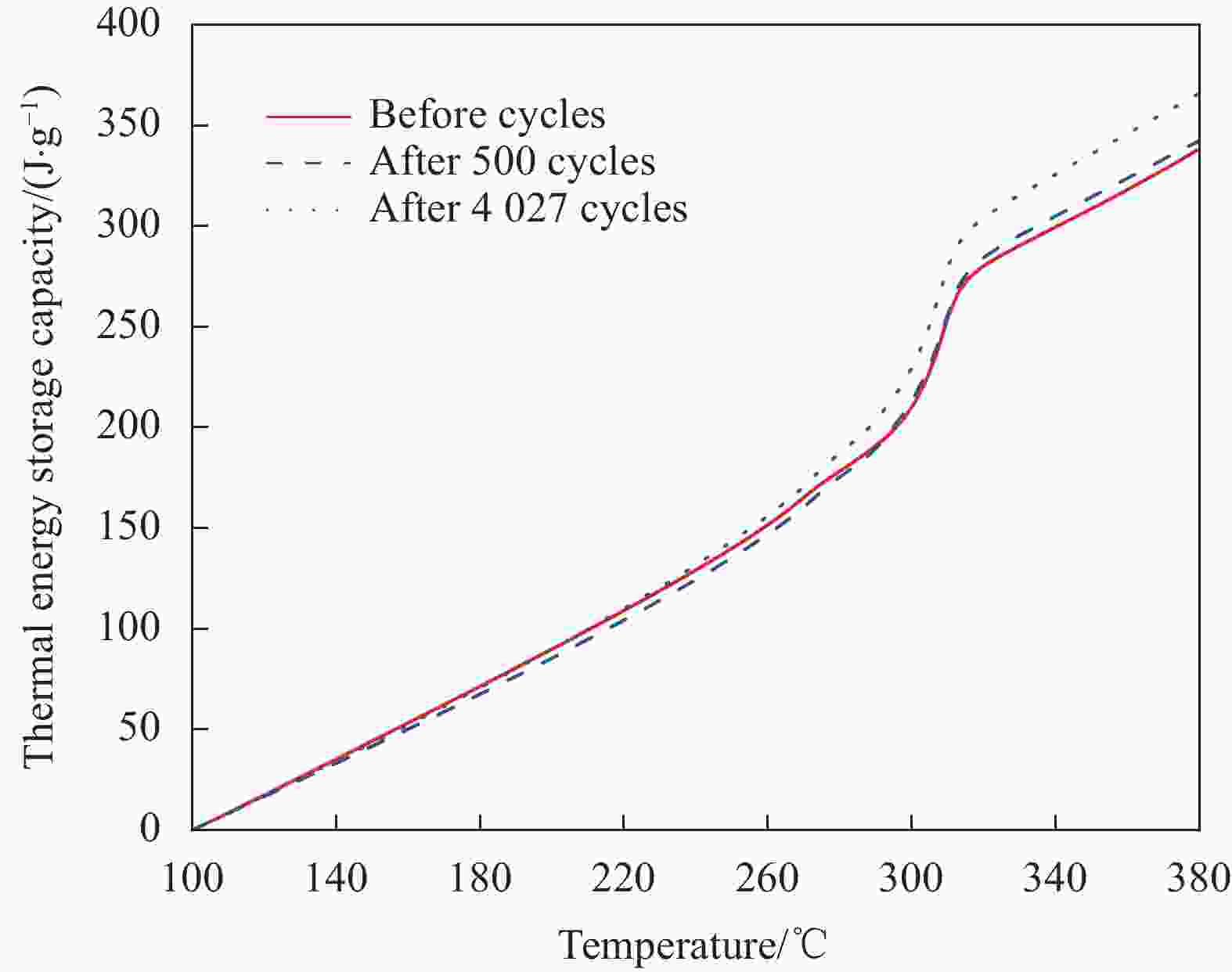
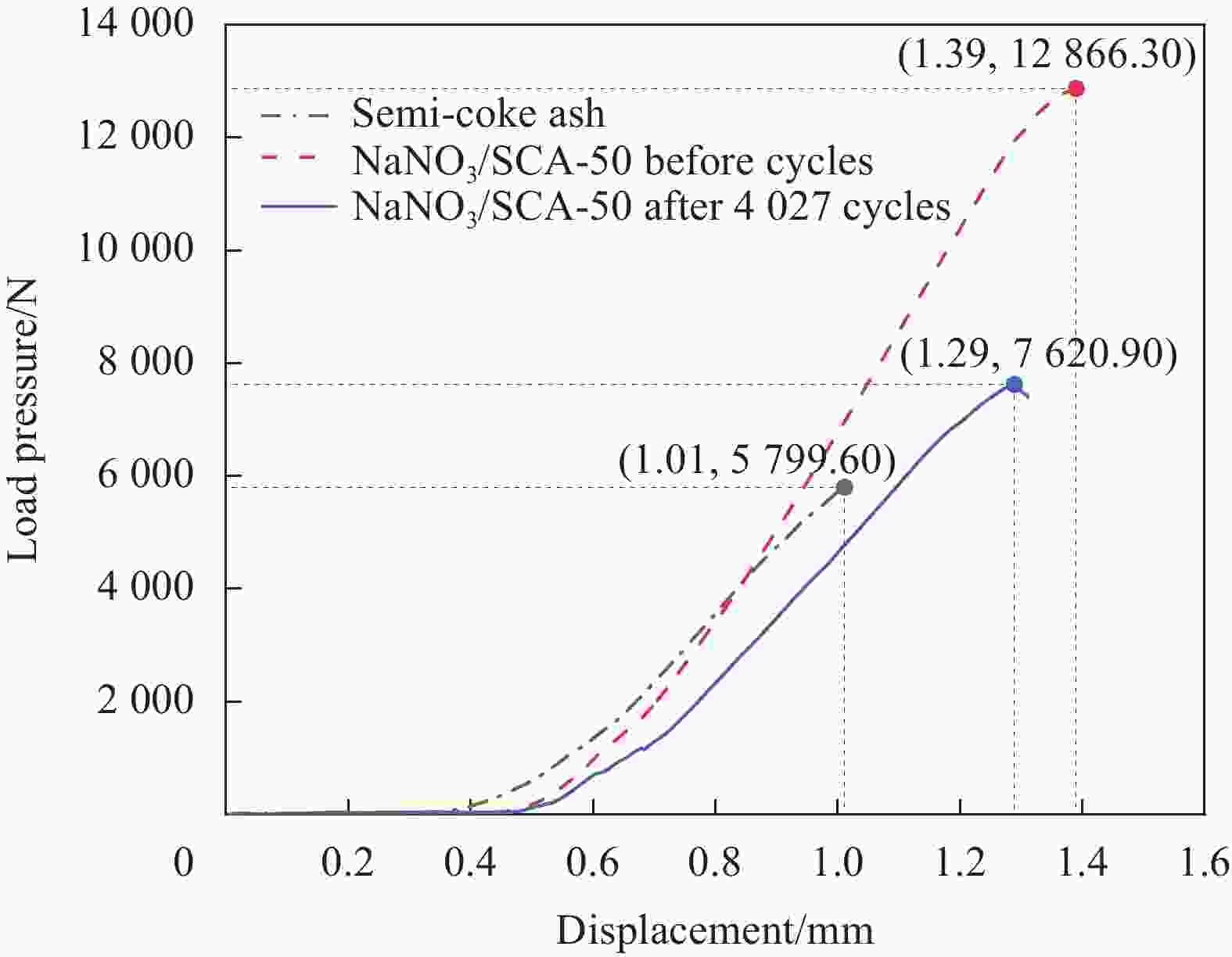
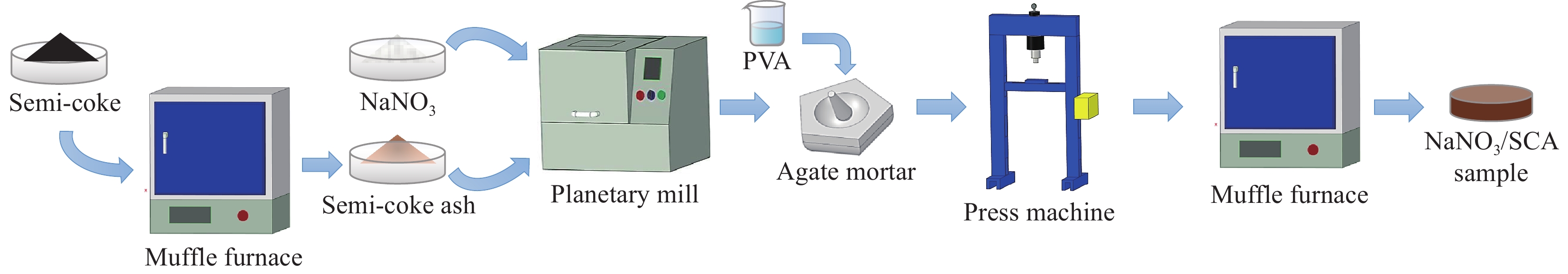
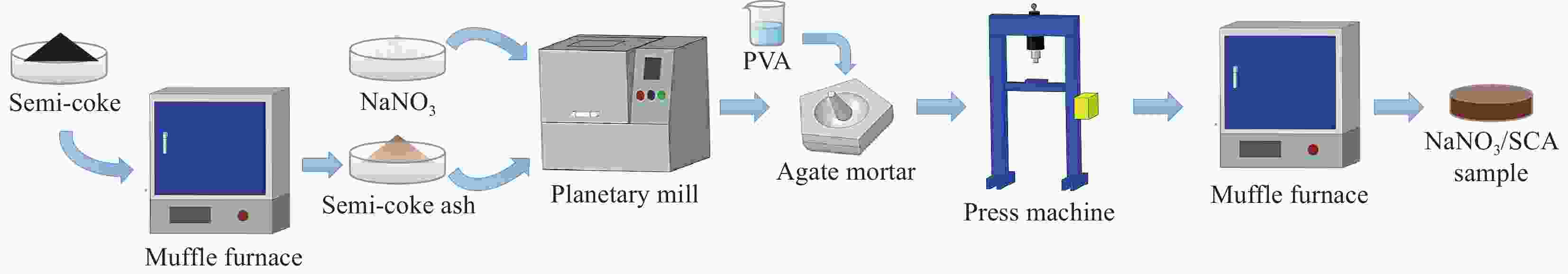
摘要: 工业固废兰炭灰(SCA)大量堆积会造成局部生态环境破坏,为促进工业固废兰炭灰的规模化消纳及复合相变储热材料(SSPCCs)的低成本制备,创新提出兰炭灰作为骨架材料制备复合相变储热材料。为研究兰炭灰骨架复合相变储热材料的可行性,制备了8种不同比例的NaNO3/兰炭灰复合相变储热材料,采用差示扫描量热法、激光导热分析法、微观形貌分析法、定速加压法和X射线衍射法对NaNO3/兰炭灰复合相变储热材料的关键性能进行表征。结果表明,未发生明显泄露和形变的样品NaNO3/SCA-50具有最佳兰炭灰与NaNO3质量比(5∶5),其抗压强度达到96.98 MPa,100~380℃范围内储热密度达到338.24 J/g;经4027次加热/冷却循环后,样品NaNO3/SCA-50保持良好的热稳定性和化学相容性。兰炭灰作为骨架材料制备复合相变储热材料具有较好的可行性,为工业固废兰炭灰的资源化利用提供了新的途径。Abstract: The massive accumulation of industrial solid waste semi-coke ash (SCA) will cause damage to local ecological environment. To promote the large-scale consumption of industrial solid waste semi-coke ash and fabricate low-cost shape-stable phase change composites (SSPCCs), semi-coke ash was innovatively proposed as a skeleton material to fabricate the SSPCCs. To evaluate the feasibility of the semi-coke ash as the skeleton material of the SSPCCs, eight NaNO3/semi-coke ash SSPCC samples with different mass ratio of the semi-coke ash to NaNO3 were fabricated. The key performance of the NaNO3/semi-coke ash SSPCCs were investigated by the differential scanning calorimetry, laser flash analysis, scanning electron microscopy, constant speed pressurization method and X-ray diffraction. Results show that the sample NaNO3/SCA-50 without visual leakage and deformation holds the optimal mass ratio 5:5 of the semi-coke ash to NaNO3 with the thermal energy storage capacity of 338.24 J/g ranging from 100 to 380℃ and the mechanical strength of 96.98 MPa. After 4027 heating/cooling cycles, the sample NaNO3/SCA-50 keeps good thermal stability and chemical compatibility, which indicates that the semi-coke ash is feasible for the SSPCCs fabrication, and provides a new way for recycling the resource of the solid waste semi-coke ash.
-
表 1 兰炭灰(SCA)的组成成分
Table 1. Chemical compositions of semi-coke ash (SCA)
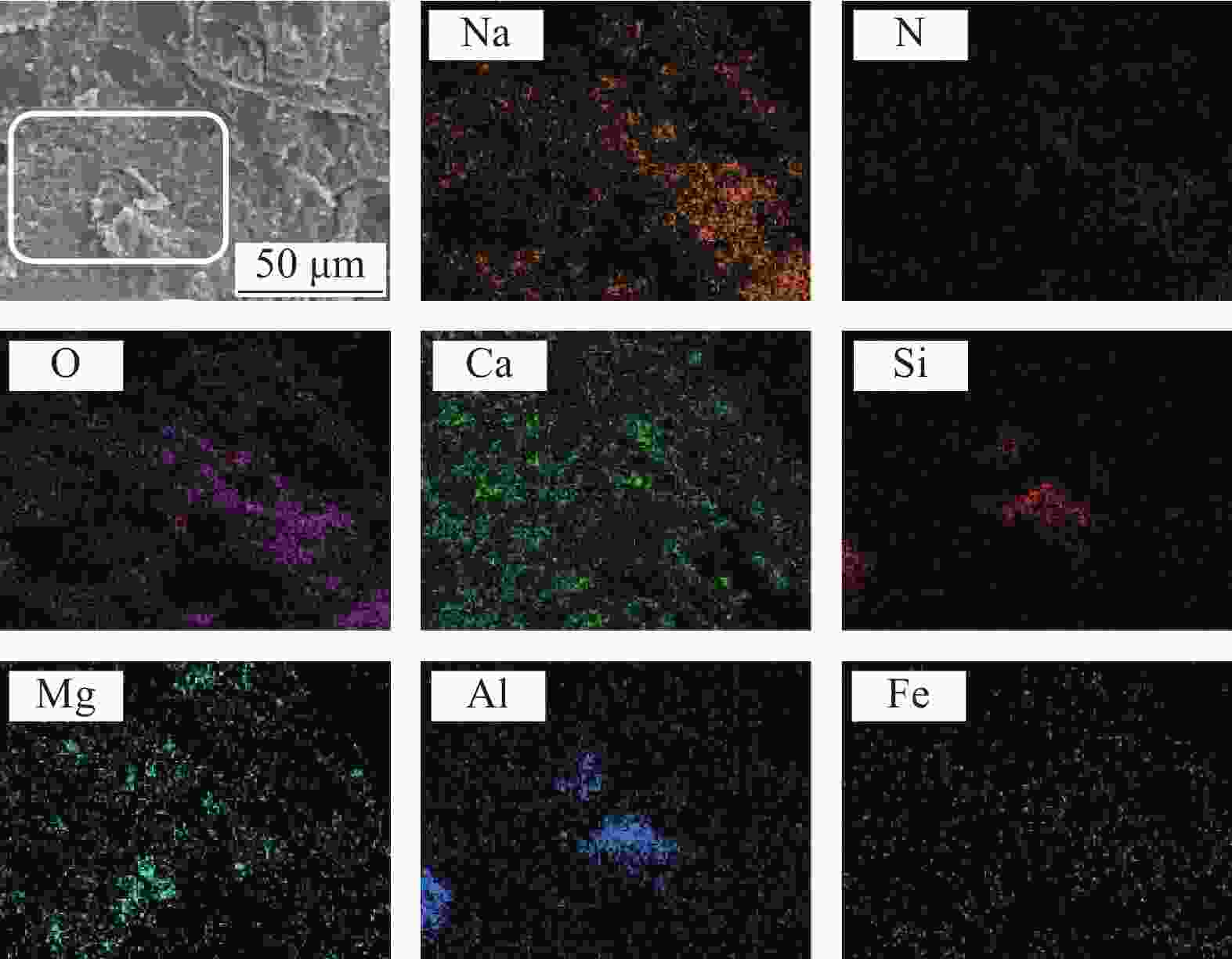
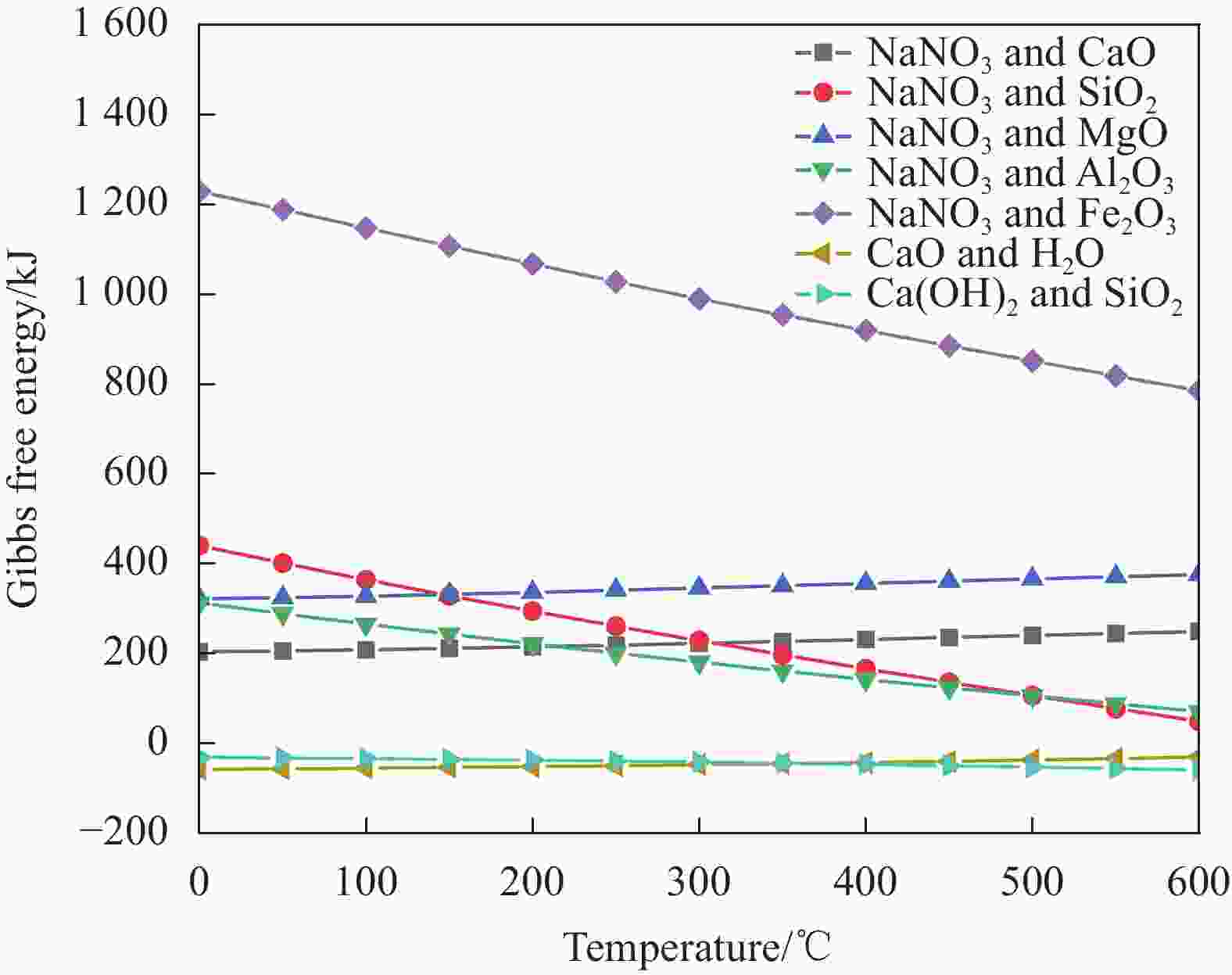
Composition Content/wt% CaO 39.49 SiO2 18.22 Fe2O3 16.71 Al2O3 10.61 MgO 2.05 Others 12.92 表 2 不同配比NaNO3/SCA样品的详细情况
Table 2. Details of the NaNO3/SCA samples with different proportions
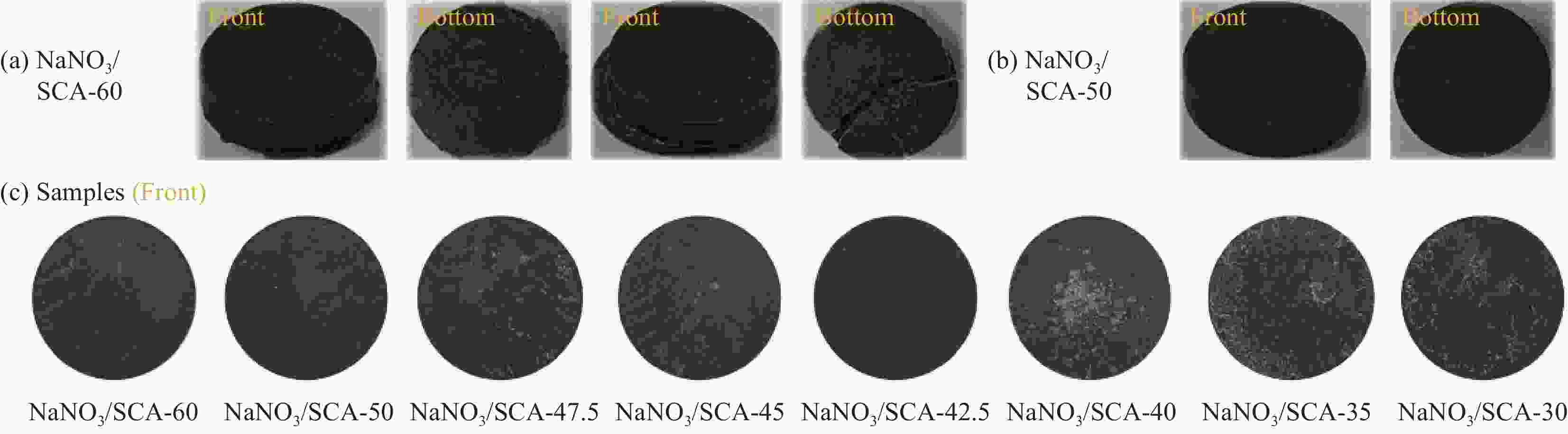
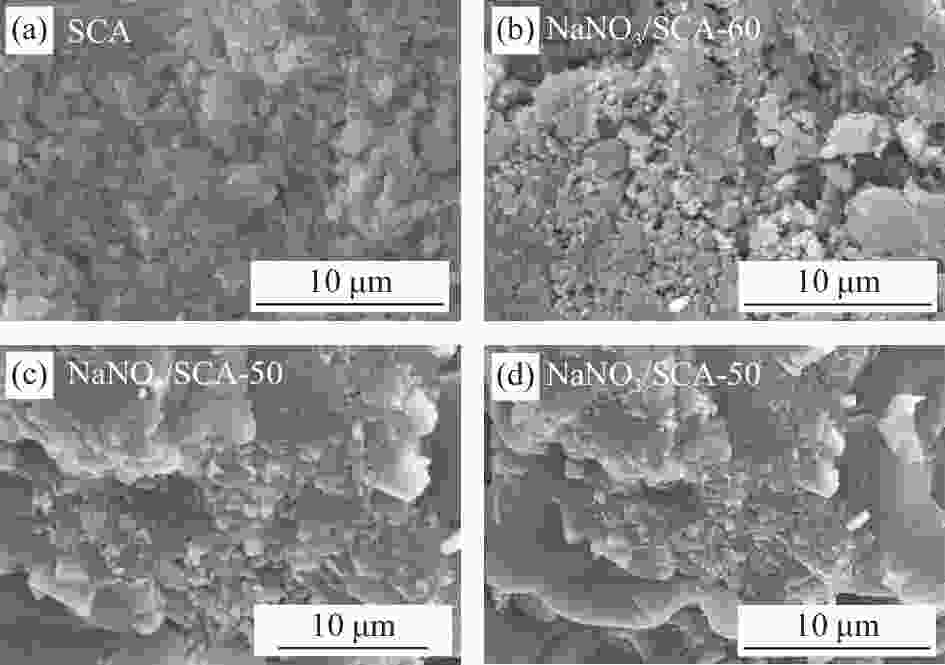
Sample NaNO3/wt% SCA/wt% Degree of PCM leakage NaNO3/SCA-60 60.0 40.0 Slight NaNO3/SCA-50 50.0 50.0 No NaNO3/SCA-47.5 47.5 52.5 No NaNO3/SCA-45 45.0 55.0 No NaNO3/SCA-42.5 42.5 57.5 No NaNO3/SCA-40 40.0 60.0 No NaNO3/SCA-35 35.0 65.0 No NaNO3/SCA-30 30.0 70.0 No Note: PCM—Phase change material. 表 3 4027次加热/冷却循环过程中NaNO3/SCA-50的形貌记录(直径13 mm)
Table 3. Morphologies recording of NaNO3/SCA-50 during 4027 heating/cooling cycles (Diameter of 13 mm)
Sample morphology 



Number of cycles 0 508 2001 4027 表 4 NaNO3/SCA-50加热/冷却循环前后的相变性能
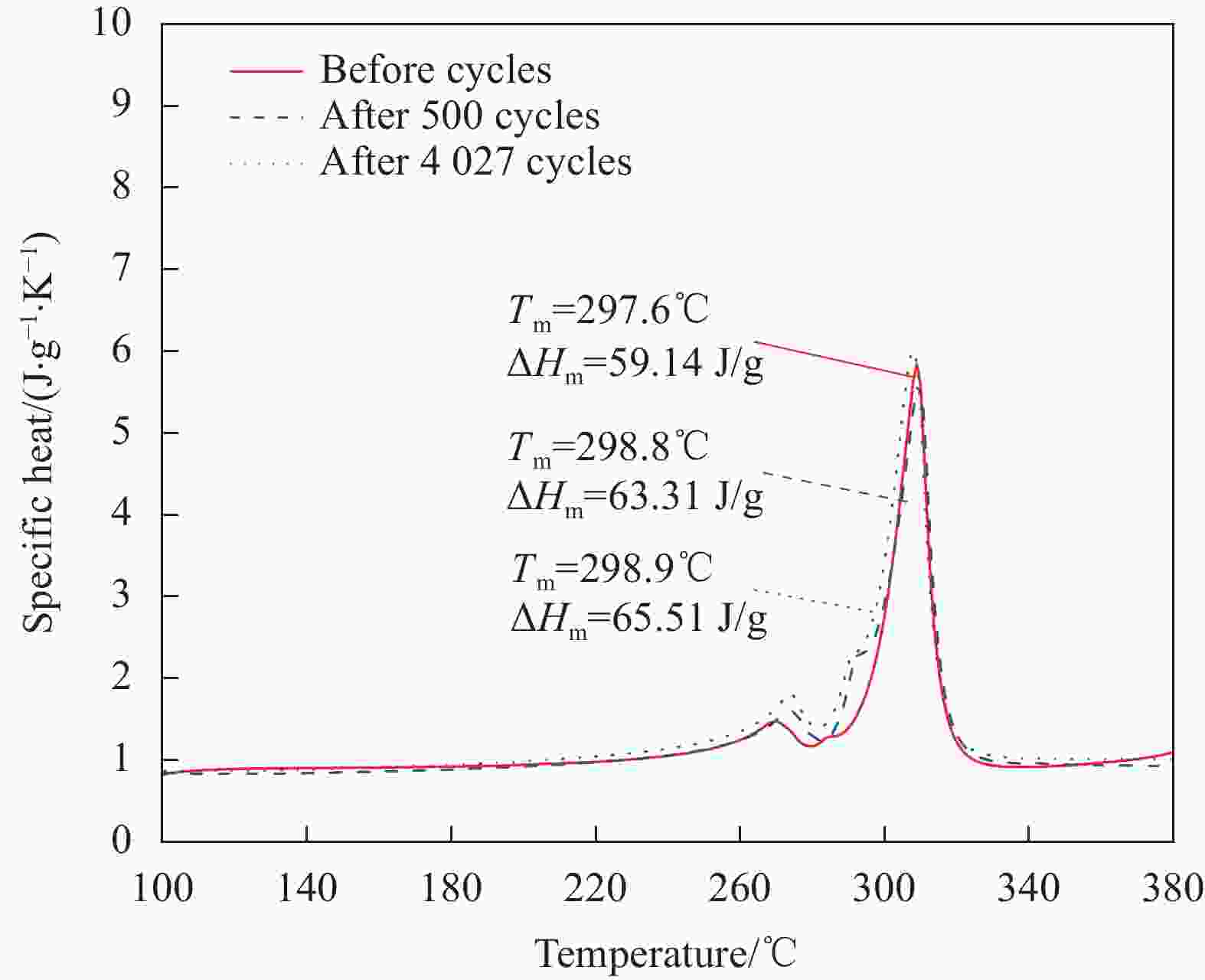
Table 4. Phase change properties of NaNO3/SCA-50 before and after heating/cooling cycles
Number of cycles Onset temperature/℃ Latent heat/(J·g−1) Terminal temperature/℃ Before cycle 297.6 59.14 315.3 500 cycles 298.8 63.31 315.9 4027 cycles 298.9 65.51 314.4 -
[1] 林文珠, 凌子夜, 方晓明, 等. 相变储热的传热强化技术研究进展[J]. 化工进展, 2021, 40(9):5166-5179. doi: 10.16085/j.issn.1000-6613.2021-0460LIN Wenzhu, LING Ziye, FANG Xiaoming, et al. Research progress on heat transfer of phase change material heat storage technology[J]. Chemical Industry and Engineering Progress,2021,40(9):5166-5179(in Chinese). doi: 10.16085/j.issn.1000-6613.2021-0460 [2] MCCORMICK P G, SUEHRCKE H. The effect of intermittent solar radiation on the performance of PV systems[J]. Solar Energy,2018,171:667-674. doi: 10.1016/j.solener.2018.06.043 [3] HAIKARAINEN C, PETTERSSON F, SAXEN H. Optimising the regional mix of intermittent and flexible energy technologies[J]. Journal of Cleaner Production,2019,219:508-517. doi: 10.1016/j.jclepro.2019.02.103 [4] 陈海生, 刘畅, 徐玉杰, 等. 储能在碳达峰碳中和目标下的战略地位和作用[J]. 储能科学与技术, 2021, 10(5):1477-1485. doi: 10.19799/j.cnki.2095-4239.2021.0389CHEN Haisheng, LIU Chang, XU Yujie, et al. The strategic position and role of energy storage under the goal of carbon peak and carbon neutrality[J]. Energy Storage Science and Technology,2021,10(5):1477-1485(in Chinese). doi: 10.19799/j.cnki.2095-4239.2021.0389 [5] LI Q, LI C, DU Z, et al. A review of performance investigation and enhancement of shell and tube thermal energy storage device containing molten salt based phase change materials for medium and high temperature applications[J]. Applied Energy,2019,255:113806. doi: 10.1016/j.apenergy.2019.113806 [6] 李昭, 李宝让, 陈豪志, 等. 相变储热技术研究进展[J]. 化工进展, 2020, 39(12):5066-5085. doi: 10.16085/j.issn.1000-6613.2020-0376LI Zhao, LI Baorang, CHEN Haozhi, et al. State of the art review on phase change thermal energy storage technology[J]. Chemical Industry and Engineering Progress,2020,39(12):5066-5085(in Chinese). doi: 10.16085/j.issn.1000-6613.2020-0376 [7] JIANG F, ZHANG L, SHE X, et al. Skeleton materials for shape-stabilization of high temperature salts based phase change materials: A critical review[J]. Renewable and Sustainable Energy Reviews,2020,119:109539. doi: 10.1016/j.rser.2019.109539 [8] ZHU J, LI R, ZHOU W, et al. Fabrication of Al2O3-NaCl composite heat storage materials by one-step synthesis method[J]. Journal of Wuhan University of Technology (Materials Science),2016,31(5):950-954. doi: 10.1007/s11595-016-1473-x [9] GE Z, YE F, DING Y. Composite materials for thermal energy storage: Enhancing performance through microstructures[J]. ChemSusChem,2014,7:1318-1325. doi: 10.1002/cssc.201300878 [10] LIU R, ZHANG F, SU W, et al. Impregnation of porous mullite with Na2SO4 phase change material for thermal energy storage[J]. Solar Energy Materials and Solar Cells,2015,134:268-274. doi: 10.1016/j.solmat.2014.12.012 [11] YU Q, JIANG Z, CONG L, et al. A novel low-temperature fabrication approach of composite phase change materials for high temperature thermal energy storage[J]. Applied Energy,2019,237:367-377. doi: 10.1016/j.apenergy.2018.12.072 [12] QIN Y, YU X, LENG G, et al. Effect of diatomite content on diatomite matrix based composite phase change thermal storage material[J]. Materials Research Innovations. 2014, 18: 453-456. [13] GHANI S A A, JAMARI S S, ABIDIN S Z. Waste materials as the potential phase change material substitute in thermal energy storage system: A review[J]. Chemical Engineering Communications,2021,208(5):687-707. doi: 10.1080/00986445.2020.1715960 [14] WANG T, ZHANG T, XU G, et al. A new low-cost high-temperature shape-stable phase change material based on coal fly ash and K2CO3[J]. Solar Energy Materials and Solar Cells,2020,206:110328. doi: 10.1016/j.solmat.2019.110328 [15] 王燕, 黄云, 姚华, 等. 太阳盐/钢渣定型复合相变储热材料的制备与性能研究 [J]. 过程工程学报, 2021, 21(3): 332-340.WANG Yan, HUANG Yun, YAO Hua, et al. Fabrication and characterization of form-stable solar salt/steel slag composite phase change material for thermal energy storage[J]. The Chinese Journal of Process Engineering. 2021, 21(3): 332-340(in Chinese). [16] ANAGNOSTOPOULOS A, NAVARRO M E, STEFANIDOU M, et al. Red mud-molten salt composites for medium-high temperature thermal energy storage and waste heat recovery applications[J]. Journal of Hazardous Materials,2021,413:125407. doi: 10.1016/j.jhazmat.2021.125407 [17] 徐涛, 王海洋, 张建良, 等. 兰炭灰熔融特性的计算模型 [J]. 中国冶金, 2018, 28(12): 15-18.XU Tao, WANG Haiyang, ZHANG Jianliang, et al. Calculating model for melting characteristics of semi coke[J]. China Metallurgy, 2018, 28(12): 15-18(in Chinese). [18] YU Q, LU Y, ZHANG C, et al. Preparation and thermal properties of novel eutectic salt/nano-SiO2/ expanded graphite composite for thermal energy storage[J]. Solar Energy Materials and Solar Cells,2020,215:110590. doi: 10.1016/j.solmat.2020.110590 [19] 熊亚选, 药晨华, 宋超宇, 等. 低成本兰炭灰骨架定型相变储热材料的制备及性能研究[J]. 华电技术, 2021, 43(7):62-67. doi: 10.3969/j.issn.1674-1951.2021.07.010XIONG Yaxuan, YAO Chenhua, SONG Chaoyu, et al. Preparation and properties of low-cost phase-change heat storage materials based on semi-coke ash[J]. Huadian Technology,2021,43(7):62-67(in Chinese). doi: 10.3969/j.issn.1674-1951.2021.07.010 [20] XIONG Y, SUN M, WU Y, et al. Effects of synthesis methods on thermal performance of nitrate salt nanofluids for concentrating solar power[J]. Energy & Fuels, 2020, 34(9): 11606-11619. [21] GUO Q, WANG T. Study on preparation and thermal properties of sodium nitrate/silica composite as shape-stabilized phase change material[J]. Thermochimica Acta,2015,613:66-70. doi: 10.1016/j.tca.2015.05.023 -





 下载:
下载: