Research progress in piezoelectric catalysis of barium titanate nanomaterials
-
摘要: 社会快速发展带来巨大经济效益的同时,也带来了一系列生态环境问题,如水污染、大气污染和污染物排放。催化降解被认为是处理各种污染的一种有效策略,相对于传统的光催化,压电催化是近几年提出的一种全新的催化方式。通过压电催化将机械能转化为化学能是解决当前水污染难题的一个有效手段,大量的压电材料被应用于压电催化降解的研究,其中BaTiO3基纳米粉体作为一种典型的压电材料,因具有成本低、压电活性强等优点,引起了研究者的广泛关注。本文首先对压电催化的理论和起源进行了概述,列举了一些常用的压电催化材料并针对其压电催化应用进行举例。围绕BaTiO3介绍了其基本结构、纳米BaTiO3粉体的常用制备方法和在压电催化领域的应用及一些典型的改性方法。最后对BaTiO3基纳米粉体在压电催化领域的未来发展趋势进行了展望。Abstract: The rapid development of society has brought huge economic benefits, but also brought a series of ecological environment problems, such as water pollution, air pollution and pollutant discharge. Catalytic degradation is considered as an effective strategy to deal with various kinds of pollution. Compared with traditional photocatalysis, piezoelectric catalysis is a new catalytic method proposed in recent years. Through the piezoelectric catalytic convert mechanical energy into chemical energy is an effective means of solving the water pollution problem, large numbers of piezoelectric materials have been applied in the research of catalytic degradation of piezoelectric, including BaTiO3 nano powder as a kind of typical piezoelectric material, because of low cost, the advantages of strong piezoelectric activity, caused the wide attention of researchers. In this paper, the theory and origin of piezoelectric catalysis are summarized, some commonly used piezoelectric catalysis materials are listed and their applications are illustrated. Around BaTiO3, the basic structure, common preparation methods of nano-BaTiO3 powder, application in piezoelectric catalysis and some typical modification methods are introduced. Finally, the future development trend of BaTiO3-based nano-powders in piezoelectric catalysis field is prospected.
-
Key words:
- perovskite /
- barium titanate /
- piezoelectric catalysis /
- degradation /
- modified method
-
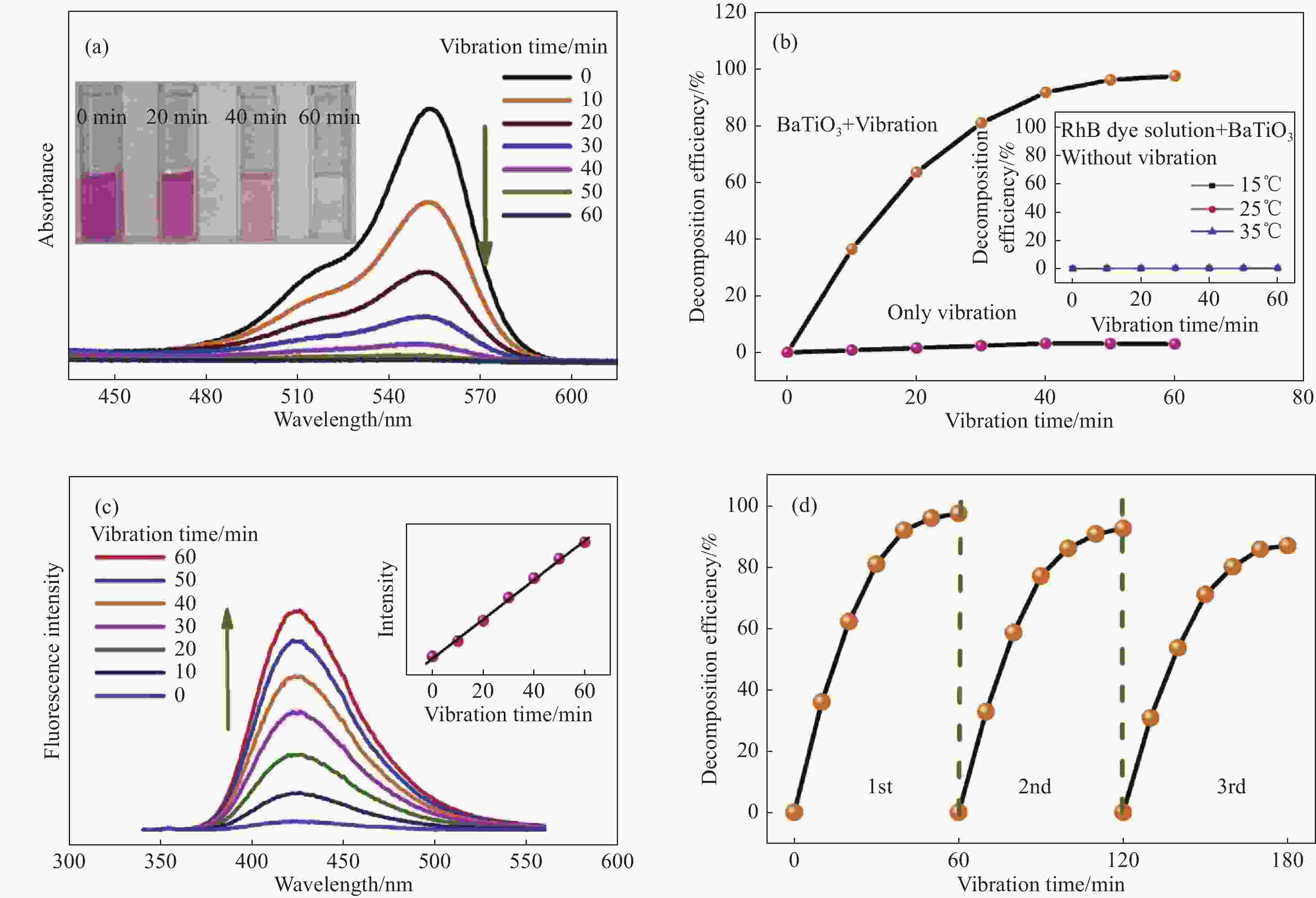
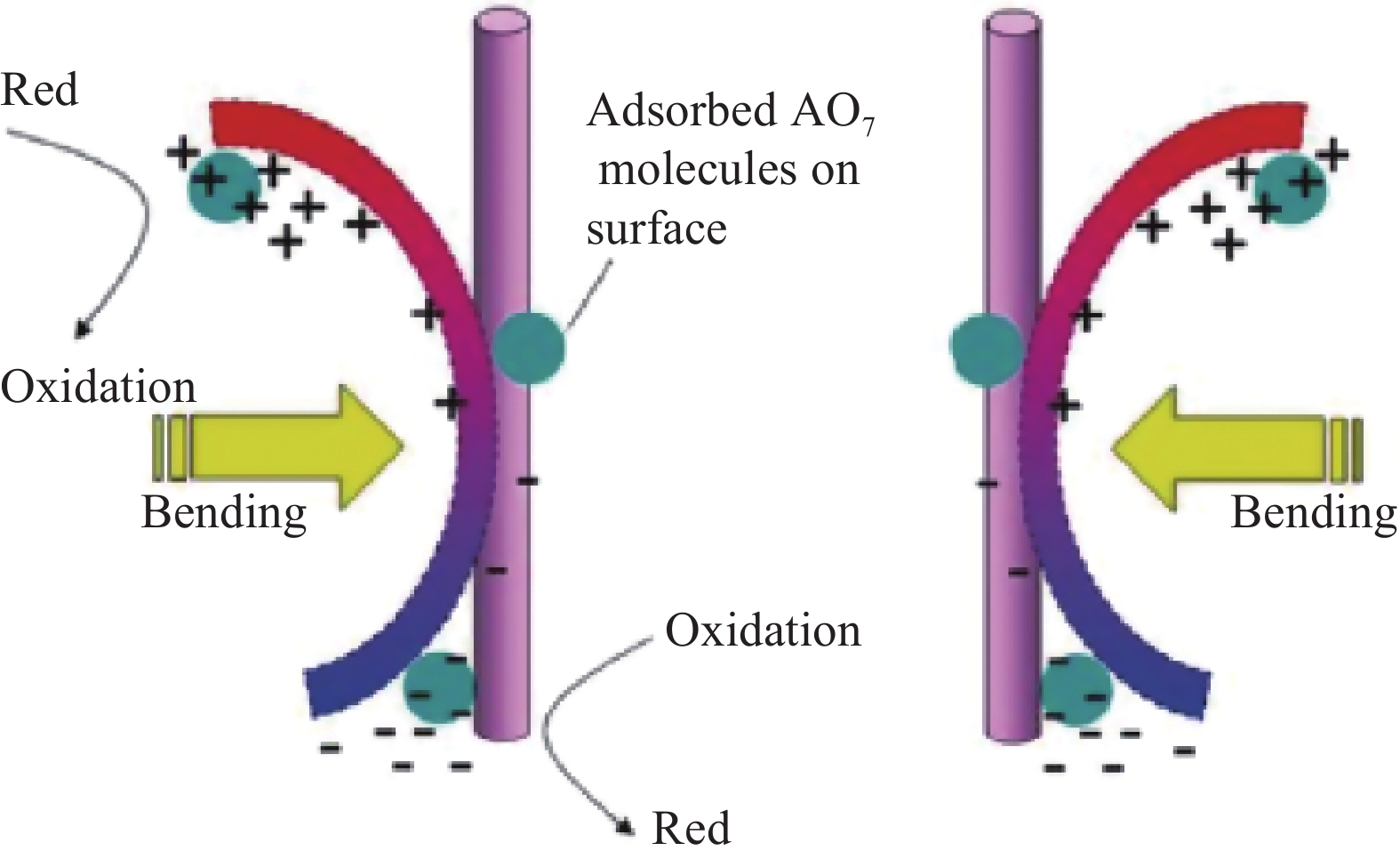
图 4 (a) 不同振动时间下罗丹明B(RhB)的UV-vis吸收光谱(插图是RhB的振动-催化分解照片);(b) 不同条件下RhB的分解效率(插图是在一定温度下保持60 min无振动的情况下加入BaTiO3的RhB染料分解率);(c) 2-羟基对苯二甲酸在不同振动时间下捕获BaTiO3纳米纤维诱导的•OH的荧光光谱(插图是荧光强度对振动时间的响应的线性拟合);(d) 回收BaTiO3纳米纤维用于RhB的振动催化分解[45]
Figure 4. (a) UV-vis absorbance spectra of Rhodamine B (RhB) under different vibration time (Illustration shows the vibro-catalytic decomposition of RhB); (b) Decomposition efficiency of RhB under different conditions (Illustration shows the decomposition rate of RhB dye with BaTiO3 added when no vibration is maintained for 60 min at a certain temperature); (c) Fluorescence spectra of 2-hydroxyterephthalic acid for trapping the •OH induced by BaTiO3 nanofibers for different vibration time (Inset is the linear fitting of the response of fluorescence intensity on the vibration time); (d) Recycling BaTiO3 nanofiber catalysts for the vibration-catalytic decomposition of RhB[45]
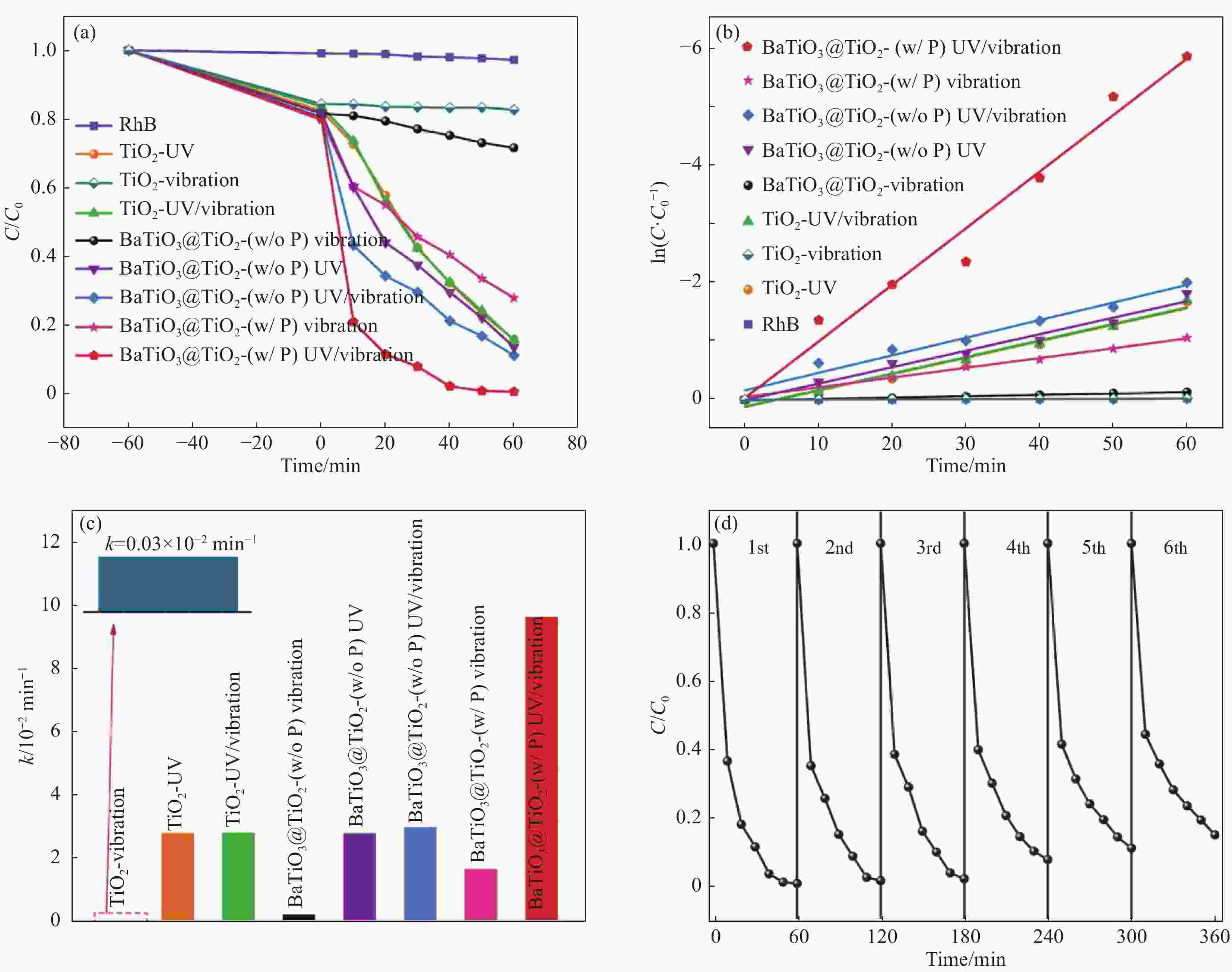
图 5 (a) 在紫外光照射或超声作用下TiO2纳米纤维(NFs)和BaTiO3@TiO2 NFs的催化降解效率随时间的变化及其相应的动力学曲线;(b) 伪一级动力学线性拟合;(c) 不同条件下TiO2 NFs和BaTiO3@TiO2 NFs降解RhB的降解速率常数;(d) BaTiO3@TiO2 NFs在协同催化RhB下的循环稳定性[66]
Figure 5. (a) Catalytic degradation efficiencies of RhB as a function of time and their corresponding kinetic curves for TiO2 nanofibers (NFs) and BaTiO3@TiO2 NFs under ultraviolet light irradiation or ultrasonic; (b) Linear fittings of pseudo-first-order kinetics; (c) Comparisons the corresponding apparent rate constants for degradation of RhB by TiO2 NFs and BaTiO3@TiO2 NFs under various catalytic conditions; (d) Cycling stability of BaTiO3@TiO2 NFs for synergetic piezo-photocatalysis of RhB[66]
w/o—Without; w/—With; P—Polarization; k—Degradation rate
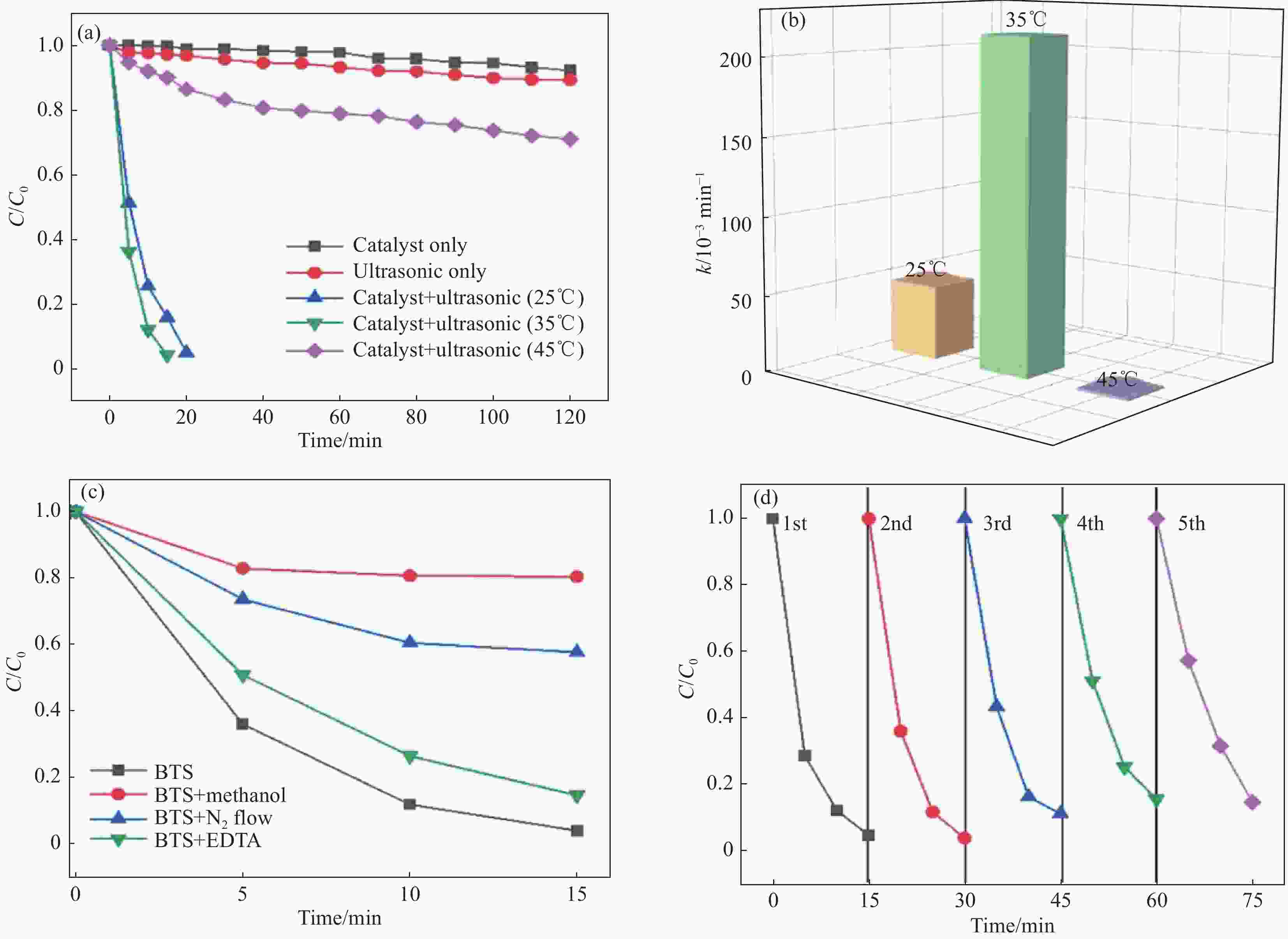
图 9 不同条件下BaTi0.89Sn0.11O3纳米颗粒对RhB的压电催化活性 (a)、BaTi0.89Sn0.11O3纳米颗粒的反应速率常数 (b)、35℃下的捕获实验 (c)和BaTi0.89Sn0.11O3纳米颗粒在35℃下降解RhB的回收能力 (d)[79]
Figure 9. Piezocatalytic activities of RhB with BaTi0.89Sn0.11O3 nanoparticles (a), comparison of reaction rate at different working temperatures (b) under different conditions, trapping experiments for the decomposition of RhB operated at 35℃ (c) and recycling ability of BaTi0.89Sn0.11O3 for the degradation of RhB at 35℃ (d)[79]
EDTA—Ethylene diamine tetraacetic acid; BTS—BaTi0.89Sn0.11O3
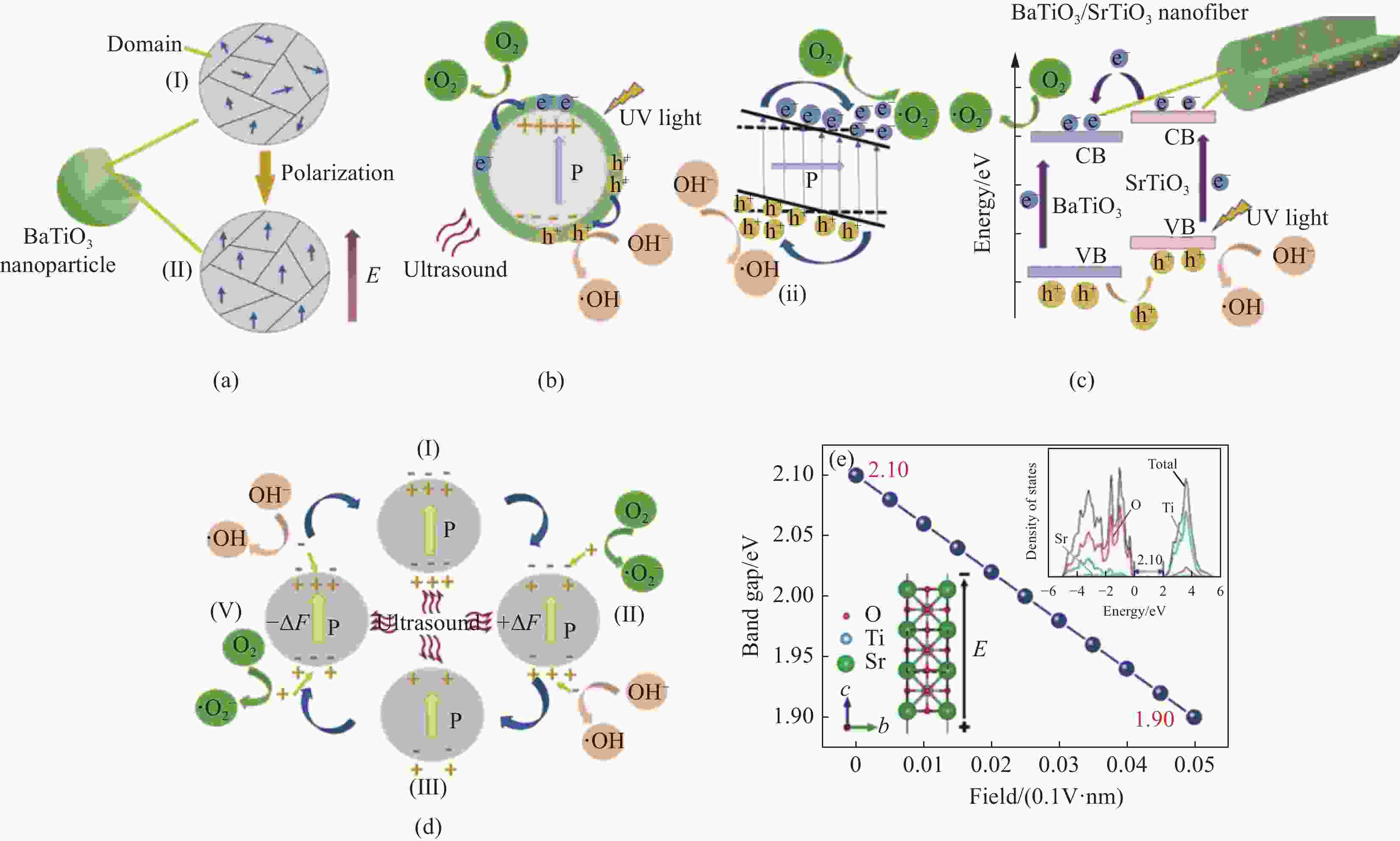
图 10 (a) BaTiO3/SrTiO3 NFs的催化降解机制示意图;(b) 直流电场作用下BaTiO3/SrTiO3 NFs极化过程示意图;(c) BaTiO3/SrTiO3 NFs的压电光催化过程原理图和超声波照射下BaTiO3/SrTiO3异质结构的能带图;(d) 紫外光照射下异质结构BaTiO3/SrTiO3 NFs中的电子-空穴对分离和转移示意图;(e) 钛酸锶的带隙作为外加电场的函数(插图是钛酸锶的原子结构(左)和计算出的态密度(右))[82]
Figure 10. Diagram of catalytic degradation mechanism of BaTiO3/SrTiO3 NFs(a), Schematic for polarization process of BaTiO3/SrTiO3 NFs under DC electric field(b), Schematic for the piezo-photocatalytic process of BaTiO3/SrTiO3 NFs and the energy band diagram of BaTiO3/SrTiO3 heterostructure under both UV irradiation and ultrasonic (c), Schematic of the electron–hole pairs separation and transfer in heterostructured BTO/STO NFs under UV light irradiation. Principle scheme of the piezo- catalysis of BaTiO3/SrTiO3 NFs during action of ultrasonic (d), Band gap of SrTiO3 as a function of the applied electric field, insets are the atomic configurations (left) and the calculated density of states for SrTiO3 (right) (e) [82]
E—Electric field
表 1 不同形貌BaTiO3的催化性能
Table 1. Catalytic properties of BaTiO3 with different morphologies
Catalysts Dye species Catalytic method Degradation rate/min−1 Refs. BaTiO3 powder RhB (10 mg/L) Ultrasonic 0.0012 [68] BaTiO3 powder RhB (10 mg/L) Ultraviolet light 0.0096 [68] BaTiO3 nanofibers RhB (7.5 mg/L) Ultrasonic 0.0736 [69] BaTiO3 nanoparticles RhB (5 mg/L) Ultrasonic 0.0025 [67] BaTiO3 nanowires MO (5 mg/L) Ultrasonic 0.0152 [57] BaTiO3 nanowires RhB (5 mg/L) Ultrasonic 0.0339 [67] -
[1] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature,1972,238(5358):37-38. [2] XU X L, XIAO L B, WU Z, et al. Harvesting vibration energy to piezo-catalytically generate hydrogen through Bi2WO6 layered-perovskite[J]. Nano Energy,2020,78:105351. doi: 10.1016/j.nanoen.2020.105351 [3] FENG J X, SUN J X, LIU X S, et al. Enhancement and mechanism of nano-BaTiO3 piezocatalytic degradation of tricyclazole by co-loading Pt and RuO2[J]. Environmental Science: Nano,2019,6:2241-2252. doi: 10.1039/C9EN00367C [4] YANG B, WU C, WANG J W, et al. When C3N4 meets BaTiO3: Ferroelectric polarization plays a critical role in building a better photocatalyst[J]. Ceramics International,2020,46:4248-4255. doi: 10.1016/j.ceramint.2019.10.145 [5] ZHU P, CHEN Y, SHI J L. Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity[J]. Advanced Materials,2020,32(29):2001976. doi: 10.1002/adma.202001976 [6] NIE Q, XIE Y F, MA J M, et al. High piezo-catalytic activity of ZnO/Al2O3 nanosheets utilizing ultrasonic energy for wastewater treatment[J]. Journal of Cleaner Production,2020,242:118532. doi: 10.1016/j.jclepro.2019.118532 [7] WANG B, ZHANG Q, HE J Q, et al. Co-catalyst-free large ZnO single crystal for high-efficiency piezocatalytic hydrogen evolution from pure water[J]. Journal of Energy Che-mistry,2022,65:304-311. doi: 10.1016/j.jechem.2021.06.004 [8] KANG Z H, KE K H, LIN E Z, et al. Piezoelectric polarization modulated novel Bi2WO6/g-C3N4/ZnO Z-scheme heterojunctions with g-C3N4 intermediate layer for efficient piezo-photocatalytic decomposition of harmful organic pollutants[J]. Journal of Colloid and Interface Science,2022,607:1589-1602. doi: 10.1016/j.jcis.2021.09.007 [9] FENG Y W, LING L L, WANG Y X, et al. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis[J]. Nano Energy,2017,40:481-486. doi: 10.1016/j.nanoen.2017.08.058 [10] ZHOU C, LIU W C, LI H Q, et al. Separable magnetic Fe3O4@MoS2 composite for adsorption and piezo-catalytic degradation of dye[J]. Catalysts,2021,11(11):1403. doi: 10.3390/catal11111403 [11] LI S, ZHAO Z C, YU D F, et al. Few-layer transition metal dichalcogenides (MoS2, WS2, and WSe2) for water splitting and degradation of organic pollutants: Understanding the piezocatalytic effect[J]. Nano Energy,2019,66:104083. doi: 10.1016/j.nanoen.2019.104083 [12] LEI R, GAO F, YUAN J, et al. Free layer-dependent piezoelectricity of oxygen-doped MoS2 for the enhanced piezocatalytic hydrogen evolution from pure water[J]. Applied Surface Science,2022,576:151851. doi: 10.1016/j.apsusc.2021.151851 [13] DONG C Y, FU Y M, ZANG W L, et al. Self-powering/self-cleaning electronic-skin basing on PVDF/TiO2 nanofibers for actively detecting body motion and degrading organic pollutants[J]. Applied Surface Science,2017,416:424-431. doi: 10.1016/j.apsusc.2017.04.188 [14] 赁敦敏, 肖定全, 朱建国, 等. 新型无铅压电陶瓷的研制[J]. 电子元件与材料, 2004, 23(11):13-15. doi: 10.3969/j.issn.1001-2028.2004.11.005LIN D M, XIAO D Q, ZHU J G, et al. The development of a new Lead-free piezoelectric ceramics[J]. Electronic Components and Materials,2004,23(11):13-15(in Chinese). doi: 10.3969/j.issn.1001-2028.2004.11.005 [15] CURIE J, CURIE P. Development by pressure of polar electricity in hemihedral crystals with inclined faces[J]. Bulletin de la Societe Mathematique de France, 1880, 3: 90. [16] HONG K S, XU H F, KONISHI H, et al. Direct water splitting through vibrating piezoelectric microfibers in water[J]. Journal of Physical Chemistry Letters,2010,1(6):997-1002. doi: 10.1021/jz100027t [17] HONG K S, XU H F, KONISHI H, et al. Piezo-electrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers[J]. The Journal of Physical Chemistry C,2012,116(24):13045-13051. doi: 10.1021/jp211455z [18] TU S, GUO Y, ZHANG Y, et al. Piezocatalysis and piezo-photocatalysis: Catalysts classification and modification strategy, reaction mechanism, and practical application[J]. Advanced Functional Materials,2020,30(48):2005158. doi: 10.1002/adfm.202005158 [19] STARR M B, WANG X. Fundamental analysis of piezocatalysis process on the surfaces of strained piezoelectric materials[J]. Scientific Reports,2013,3(1):1-8. [20] LIANG Z, YAN C F, RTIMI S, et al. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook[J]. Applied Catalysis B: Environmental,2019,241:256-269. doi: 10.1016/j.apcatb.2018.09.028 [21] CROSS L, NEWNHAM R. History of ferroelectrics[J]. Ceramics and Civilization,1987,3:289-305. [22] PARK K I, XU S, LIU Y, et al. Piezoelectric BaTiO3 thin film nanogenerator on plastic substrates[J]. Nano Letters,2010,10:4939-4943. doi: 10.1021/nl102959k [23] SCHOFIELD D, BROWN R F. An investigation of some barium titanate compositions for transducer applications[J]. Canadian Journal of Physics,1957,35:594-607. doi: 10.1139/p57-067 [24] KIM H, TORRES F, VILLAGRAN D, et al. 3D printing of BaTiO3/PVDF composites with electric in situ poling for pressure sensor applications[J]. Macromolecular Materials and Engineering,2017,302:1700229. doi: 10.1002/mame.201700229 [25] 郭文哲. 电纺微纳米纤维材料在压电传感器及柔性可拉伸电极中的应用[D]. 青岛: 青岛大学, 2019.GUO W Z. Application of electrospinning micronanofiber materials in piezoelectric sensor and flexible stretchable electrode[D]. Qingdao: Master's thesis of Qingdao University, 2019(in Chinese). [26] KAPPADAN S, GEBREAB T W, THOMAS S, et al. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants[J]. Materials Science in Semiconductor Processing,2016,51:42-47. doi: 10.1016/j.mssp.2016.04.019 [27] CHEN L, JIA Y, ZHAO J, et al. Strong piezocatalysis in barium titanate/carbon hybrid nanocomposites for dye wastewater decomposition[J]. Journal of Colloid and Interface Science,2021,586:758-765. doi: 10.1016/j.jcis.2020.10.145 [28] WANG X D, SUMMERS C J, WANG Z L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays[J]. Nano Letters,2004,4(3):423-426. doi: 10.1021/nl035102c [29] CHOI M Y, CHOI D Y, JIN M J, et al. Mechanically powered transparent flexible charge-generating nanodevices with piezoelectric ZnO nanorods[J]. Advanced Materials,2009,21:2185-2189. doi: 10.1002/adma.200803605 [30] MA J P, REN J, JIA Y M, et al. High efficiency biharvesting light/vibration energy using piezoelectric zinc oxide nanorods for dye decomposition[J]. Nano Energy,2019,62:376-383. doi: 10.1016/j.nanoen.2019.05.058 [31] WANG S S, WU Z, CHEN J, et al. Lead-free sodium niobate nanowires with strong piezo-catalysis for dye wastewater degradation[J]. Ceramics International,2019,45:11703-11708. doi: 10.1016/j.ceramint.2019.03.045 [32] WANG L K, WANG J F, YE C Y, et al. Photodeposition of CoOx nanoparticles on BiFeO3 nanodisk for efficiently piezocatalytic degradation of rhodamine B by utilizing ultrasonic vibration energy[J]. Ultrasonics Sonochemistry,2021,80:105813. doi: 10.1016/j.ultsonch.2021.105813 [33] LIN H, WU Z, JIA Y, et al. Piezoelectrically induced mechano-catalytic effect for degradation of dye wastewater through vibrating Pb(Zr0.52Ti0.48)O3 fibers[J]. Applied Physics Letters,2014,104(16):162907. doi: 10.1063/1.4873522 [34] ZHOU Z H, LIN Y L, ZHANG P A, et al. Hydrothermal fabrication of porous MoS2 and its visible light photocatalytic properties[J]. Materials Letters,2014,131:122-124. doi: 10.1016/j.matlet.2014.05.162 [35] WU J M, CHANG W E, CHANG Y T, et al. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single- and few-layers MoS2 nanoflowers[J]. Advanced Materials,2016,28(19):3718-3725. doi: 10.1002/adma.201505785 [36] CHEN T, MENG J, WU S, et al. Room temperature synthesized BaTiO3 for photocatalytic hydrogen evolution[J]. Journal of Alloys and Compounds,2018,754:184-189. doi: 10.1016/j.jallcom.2018.04.300 [37] DEMIRCIVI P, GULEN B, SIMSEK E, et al. Enhanced photocatalytic degradation of tetracycline using hydrothermally synthesized carbon fiber decorated BaTiO3[J]. Materials Chemistry and Physics,2020,241:122236. doi: 10.1016/j.matchemphys.2019.122236 [38] JIANG B, IOCOZZIA J, ZHAO L, et al. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties[J]. Chemical Society Review,2019,48:1194-1228. doi: 10.1039/C8CS00583D [39] PAN L, SUN S, CHEN Y, et al. Advances in piezo-phototronic effect enhanced photocatalysis and photoelectrocatalysis[J]. Advanced Energy Materials,2020,10:1-25. [40] ZHANG G, LIU G, WANG L, et al. Inorganic perovskite photocatalysts for solar energy utilization[J]. Chemical Society Reviews,2016,45:5951-5984. doi: 10.1039/C5CS00769K [41] ZHANG S W, ZHANG B P, LI S, et al. SPR enhanced photocatalytic properties of Au-dispersed amorphous BaTiO3 nanocomposite thin films[J]. Journal of Alloys and Compounds,2016,654:112-119. doi: 10.1016/j.jallcom.2015.09.053 [42] KUMAR S, SHARMA M, POWAR S, et al. Impact of remnant surface polarization on photocatalytic and antibacterial performance of BaTiO3[J]. Journal of the European Cera-mic Society,2019,39:2915-2922. doi: 10.1016/j.jeurceramsoc.2019.03.029 [43] YADAV A A, HUNGE Y M, MATHE V L, et al. Photocatalytic degradation of salicylic acid using BaTiO3 photocatalyst under ultraviolet light illumination[J]. Journal of Materials Science Materials in Electronics,2018,29:15069-15073. doi: 10.1007/s10854-018-9646-3 [44] CORDERO F. Quantitative evaluation of the piezoelectric response of unpoled ferroelectric ceramics from elastic and dielectric measurements: Tetragonal BaTiO3[J]. Jour-nal of Applied Physics,2018,123(9):94-103. [45] XU X, WU Z, XIAO L, et al. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis[J]. Journal of Alloys and Compounds,2018,762:915-921. doi: 10.1016/j.jallcom.2018.05.279 [46] TANAKA H, MISANO M. Advances in designing perovskite catalysts[J]. Current Opinion in Solid State & Materials Science,2001,5(5):381-387. doi: 10.1016/S1359-0286(01)00035-3 [47] KOWALSKI D, KIUCHI H, MOTOHASHI T, et al. Activation of cataly tically active edge. sharing domains in Ca2FCoO3 for oxygen evolution reaction in highly alkaline media[J]. ACS Applied Materials & Interfaces,2019,11(32):28823-28829. doi: 10.1021/acsami.9b06854 [48] LEE W W, CHUNG W H, HUANG W S, et al. Photocatalytic activity and mechanism of nano-cubic barium titanate prepared by a hydrothermal method[J]. Journal of the Taiwan Institute of Chemical Engineers,2013,44(4):660-669. doi: 10.1016/j.jtice.2013.01.005 [49] BAO N, SHEN L, SRINIVASAN G, et al. Shape-controlled monocrystalline ferroelectric barium titanate nanostructures: From nanotubes and nanowires to ordered nanostructures[J]. Journal of Physical Chemistry C,2008,112(23):8634-8642. doi: 10.1021/jp802055a [50] JIAO H, ZHAO K, MA L, et al. A simple one-step hydrothermal synthesis and photocatalysis of bowl-like BaTiO3 nanoparticles[J]. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry,2016,47(5):647-654. [51] WANG P, FAN C, WANG Y, et al. A dual chelating sol-gel synthesis of BaTiO3 nanoparticles with effective photocatalytic activity for removing humic acid from water[J]. Materials Research Bulletin,2013,48(2):869-877. doi: 10.1016/j.materresbull.2012.11.075 [52] MI L, ZHANG Q, WANG H, et al. Synthesis of BaTiO3 nanoparticles by sol-gel assisted solid phase method and its formation mechanism and photocatalytic activity[J]. Ceramics International,2020,46(8):10619-10633. doi: 10.1016/j.ceramint.2020.01.066 [53] CHEN Y H, CHEN Y D. Kinetic study of Cu(II) adsorption on nanosized BaTiO3 and SrTiO3 photocatalysts[J]. Jour-nal of Hazardous Materials,2011,185(1):168-173. doi: 10.1016/j.jhazmat.2010.09.014 [54] LI H, SUN Y, ZHANG W, et al. Preparation of heterostructured Ag/BaTiO3 nanofibers via electrospinning[J]. Jour-nal of Alloys and Compounds,2010,508(2):536-539. [55] REN P, FAN H, WANG X. Electrospun nanofibers of ZnO/BaTiO3 heterostructures with enhanced photocatalytic activity[J]. Catalysis Communications,2012,25:32-35. doi: 10.1016/j.catcom.2012.04.003 [56] LI J, INUKAI K, TAKAHASHI Y, et al. Synthesis and size control of monodispersed BaTiO3-PVP nanoparticles[J]. Journal of Asian Ceramic Societies,2016,4(4):394-402. doi: 10.1016/j.jascer.2016.09.001 [57] WU J, QIN N, BAO D, et al. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration[J]. Nano Energy,2018,45:44-51. doi: 10.1016/j.nanoen.2017.12.034 [58] CHAROONSUK T, SRIPHAN S, NAWANIL C, et al. Tetragonal BaTiO3 nanowires: A template-free salt-flux-assisted synthesis and its piezoelectric response based on mecha-nical energy harvesting[J]. Journal of Materials Chemistry C,2019,7(27):8277-8286. doi: 10.1039/C9TC01622H [59] XUE P, WU H, XIA W, et al. Molten salt synthesis of BaTiO3 nanorods: Dielectric, optical properties and structural characterizations[J]. Journal of the American Ceramic Society,2018,3:1508-1563. [60] LIU X F, XIAO L Y, ZHANG Y, et al. Signifificantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye[J]. Journal of Materiomics,2020,6:256-262. doi: 10.1016/j.jmat.2020.03.004 [61] LI P C, WU J, WU Z, et al. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles[J]. Nano Energy,2019,63:103832. doi: 10.1016/j.nanoen.2019.06.028 [62] 刘海波, 阎建辉. 钛酸钡的制备及光催化性能研究[J]. 湖南理工学院学报, 2007, 20(4):76-79.LIU H B, YAN J H. Preparation and photocatalytic properties of Barium titanate[J]. Journal of Hunan Institute of Science and Technology,2007,20(4):76-79(in Chinese). [63] 赵锐. 改性静电纺高分子纳米纤维对水中典型污染物的吸附研究[D]. 长春: 吉林大学, 2018.ZHAO R. The adsorption of modified electrospinning polymer nanofibers to typical pollutants in water[D]. Changchun: Jilin University, 2018(in Chinese). [64] YOUSEF A, BROOKS R M, ABDELKAREEM M A, et al. Electrospun NiCu nanoalloy decorated on carbon nanofibers as chemical stable electrocatalyst for methanol oxidation[J]. ECS Electrochemistry Letters,2015,4(9):F51-F55. doi: 10.1149/2.0091509eel [65] WEN S, LIANG M, ZOU J, et al. Synthesis of a SiO2 nano-fibre confined Ni catalyst by electrospinning for the CO2 reforming of methane[J]. Journal of Materials Chemistry A,2015,3(25):13299-13307. doi: 10.1039/C5TA01699A [66] WU J R, WANG W W, TIAN Y, et al. Piezotronic effect boosted photocatalytic performance of heterostructured BaTi3/TiO2 nanofibers for degradation of organic pollutants[J]. Nano Energy,2020,77:105122. doi: 10.1016/j.nanoen.2020.105122 [67] YU C Y, TAN M X, LI Y, et al. Ultrahigh piezocatalytic capability in eco-friendly BaTiO3 nanosheets promoted by 2D morphology engineering[J]. Journal of Colloid and Interface Science,2021,596:288-296. doi: 10.1016/j.jcis.2021.03.040 [68] CUI Y, BRISCOE J, DUNN S. Effect of ferroelectricity on solar-light-driven photocatalytic activity of BaTiO3—Influence on the carrier separation and stern layer formation[J]. Chemistry of Materials,2013,25(21):4215-4223. doi: 10.1021/cm402092f [69] LIU D, JIN C, SHAN F, et al. Synthesizing BaTiO3 nanostructures to explore morphological influence, kinetics, and mechanism of piezocatalytic dye degradation[J]. ACS Applied Materials & Interfaces,2020,12(15):17443-17451. doi: 10.1021/acsami.9b23351 [70] RAN J R, ZHANG J, YU J G, et al. Earth-abundant cocatalysts for semiconductorbased photocatalytic water splitting[J]. Chemistry Society Review,2014,43(22):7787. doi: 10.1039/C3CS60425J [71] HISATOMI T, KUBOTA J, DOMEN K. Recent advances in semiconductors for photocatalytic and photoelectroche-mical water splitting[J]. Chemistry Society Review,2014,43(22):7520-7535. doi: 10.1039/C3CS60378D [72] YANG L, LUO S, YUE L, et al. High efficient photocatalytic degradation of p-nitrophenol on a unique Cu2O/TiO2 p-n heterojunction network catalyst[J]. Environmental Science & Technology,2010,44(19):7641-7646. [73] WANG Y P, YANG H, SUN X F, et al. Preparation and photocatalytic application of ternary n-BaTiO3/Ag/p-AgBr heterostructured photocatalysts for dye degradation[J]. Materials Research Bulletin,2020,124:110754. doi: 10.1016/j.materresbull.2019.110754 [74] LV J X, CHEN X L, CHEN S S, et al. A visible light induced ultrasensitive photoelectrochemical sensor based on Cu3Mo2O9/BaTiO3 p-n heterojunction for detecting oxytetracycline[J]. Journal of Electroanalytical Chemistry,2019,842:161-167. doi: 10.1016/j.jelechem.2019.04.070 [75] ZHOU L P, DAI S Q, XU S, et al. Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants[J]. Applied Catalysis B: Environmental,2021,291:120019. doi: 10.1016/j.apcatb.2021.120019 [76] YU J, WANG S, LOW J, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics,2013,15(39):16883-16890. doi: 10.1039/c3cp53131g [77] JIAO D Y, CHEN F M, WANG S F, et al. Preparation and study of photocatalytic performance of a novel Z-scheme heterostructured SnS2/BaTiO3 composite[J]. Vacuum,2021,186:110052. doi: 10.1016/j.vacuum.2021.110052 [78] YANG B, CHEN H B, YANG Y D, et al. Insights into the tribo-/pyro-catalysis using Sr-doped BaTiO3 ferroelectric nanocrystals for efficient water remediation[J]. Chemical Engineering Journal,2021,416:128986. doi: 10.1016/j.cej.2021.128986 [79] ZHAO Q, XIAO H Y, FU G H, et al. Highly-efficient piezocatalytic performance of nanocrystalline BaTi0.89Sn0.11O3 catalyst with Tc near room temperature[J]. Nano Energy,2021,85:106028. doi: 10.1016/j.nanoen.2021.106028 [80] 郝亮, 张慧娜, 闫建成, 等. 氧空位缺陷对光催化活性的影响及其机制[J]. 天津科技大学报, 2018, 33(5):1-13, 72.HAO L, ZHANG H N, YAN J C, et al. Effect of oxygen vacancy defects on photocatalytic activity and its mechanism[J]. Journal of Tianjin University of Science & Technology,2018,33(5):1-13, 72(in Chinese). [81] WANG P L, LI X Y, FAN S Y, et al. Impact of oxygen vacancy occupancy on piezo-catalytic activity of BaTiO3 nanobelt[J]. Applied Catalysis B: Environmental,2020,279:119340. doi: 10.1016/j.apcatb.2020.119340 [82] LIU X T, SHEN X F, SA B S, et al. Piezotronic-enhanced photocatalytic performance of heterostructured BaTiO3/SrTiO3 nanofibers[J]. Nano Energy,2021,89:106391. doi: 10.1016/j.nanoen.2021.106391 [83] 杨腾祥, 申国栋, 钱利江, 等. 外电场极化银-钛酸钡/涤纶织物制备及其光催化性能[J]. 纺织学报, 2022, 43(2):189-195.YANG T X, SHEN G D, QIAN L J, et al. Preparation of silver-barium titanate/polyester fabric and its photocatalytic properties[J]. Textile Journal,2022,43(2):189-195(in Chinese). -






 下载:
下载: