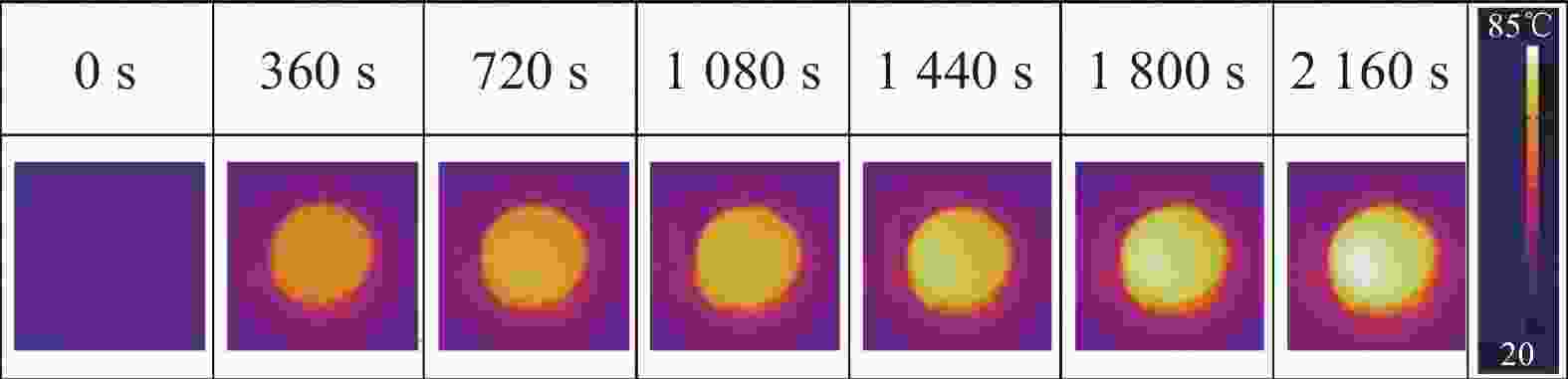
Thermal storage performance of shape stabilized phase change materials with high thermal conductivity derived from ZIF-67 etched via tannic acid
-
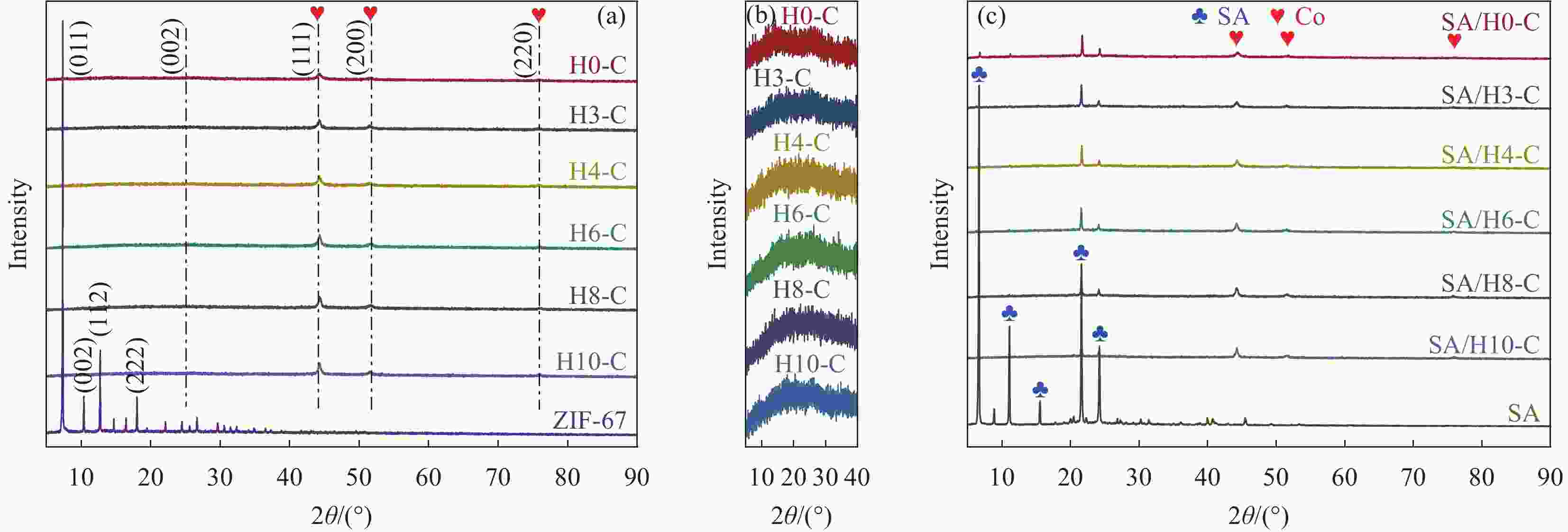
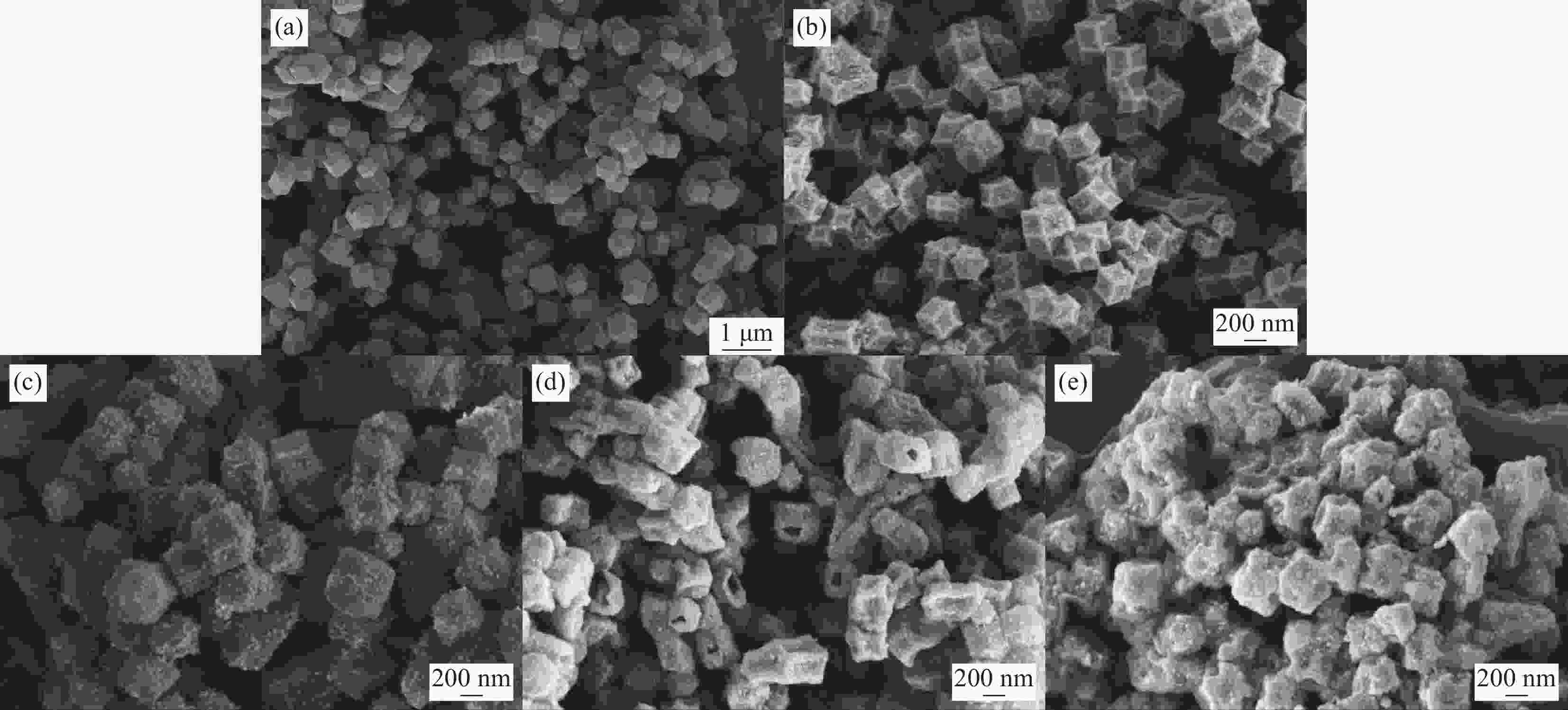
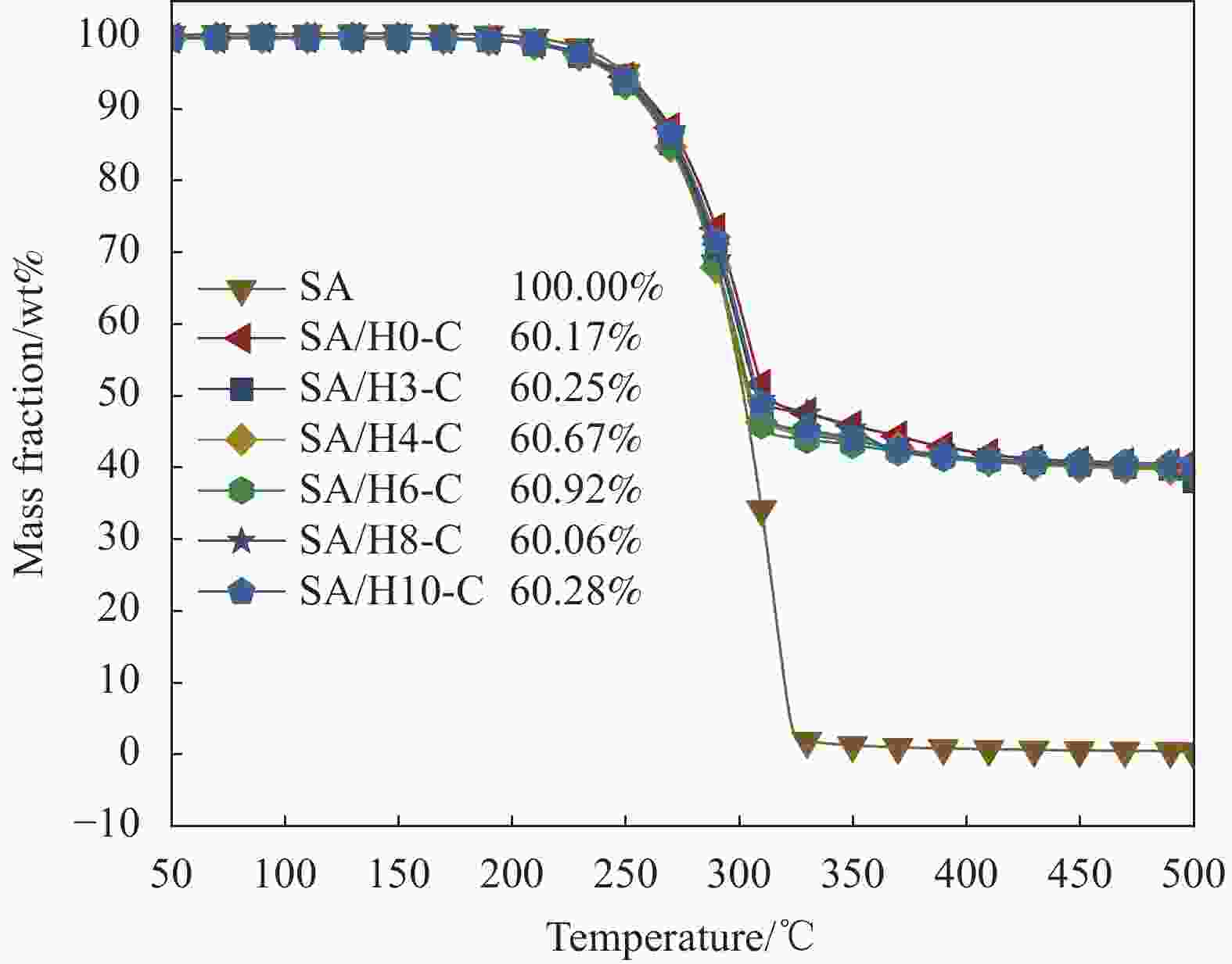
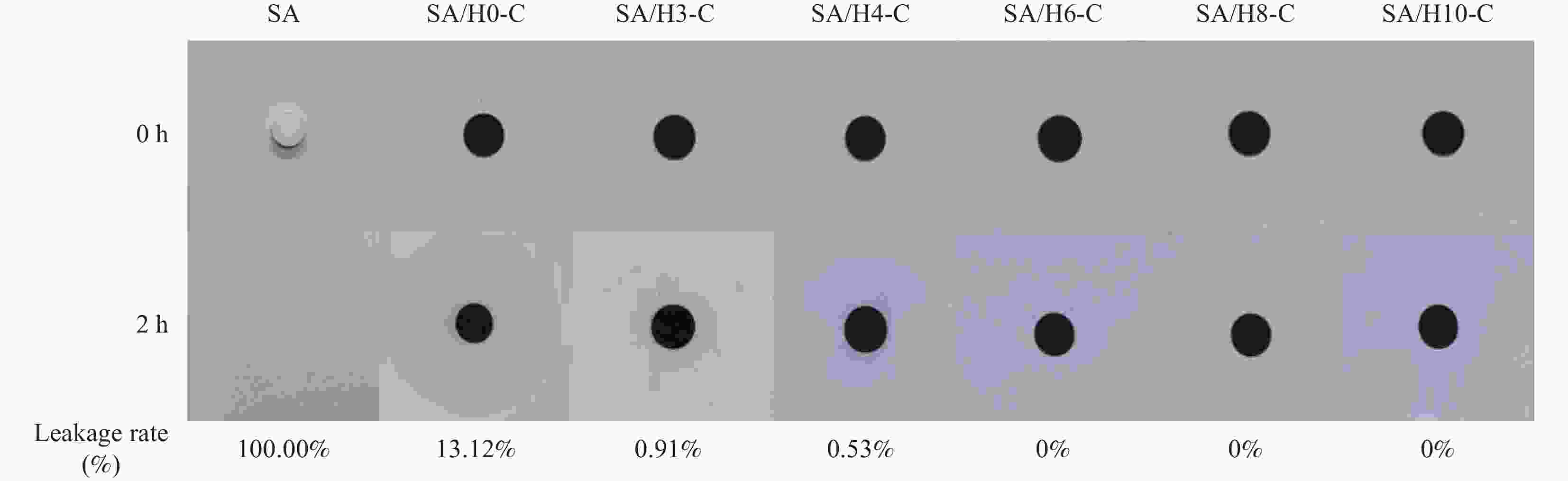
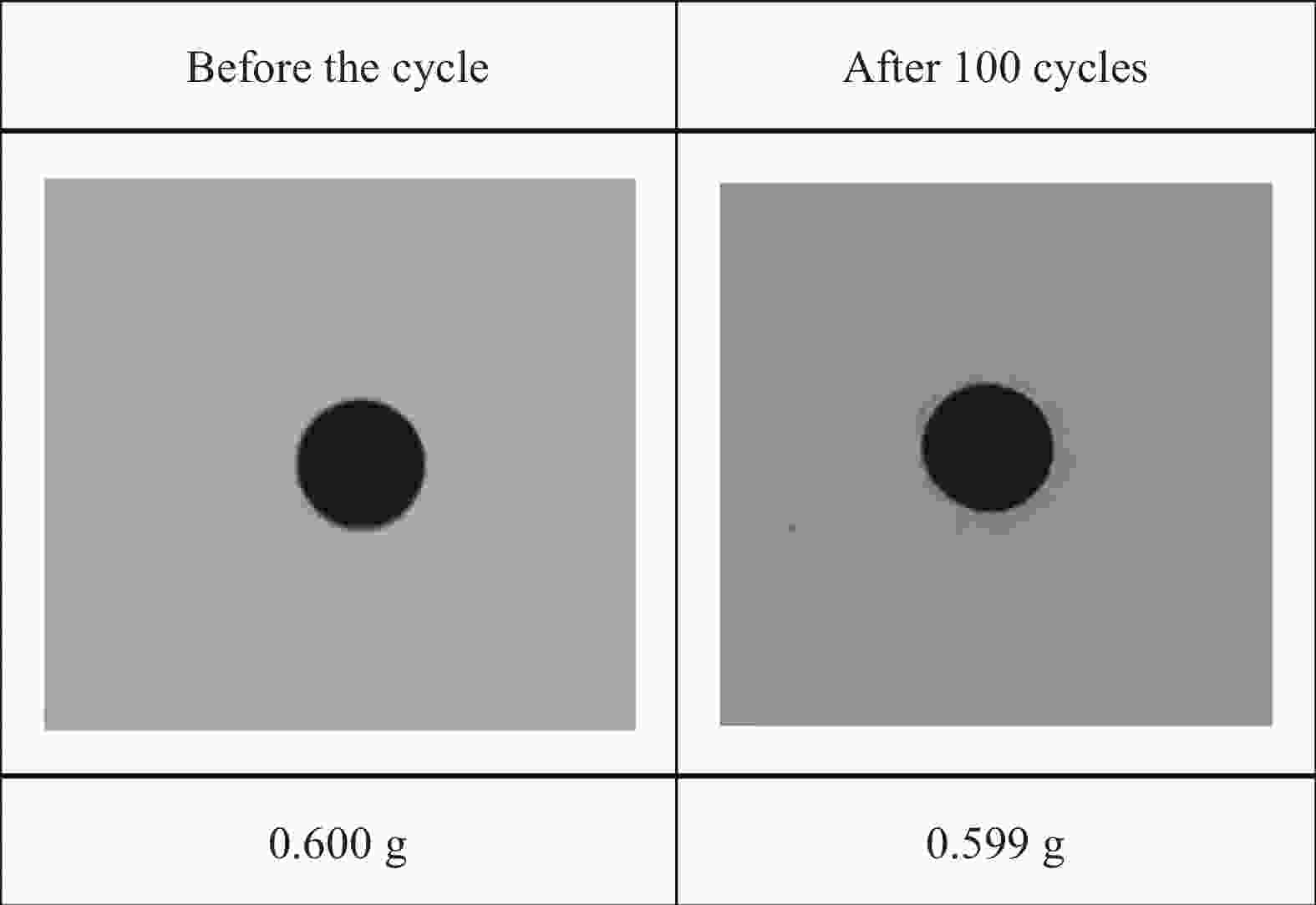
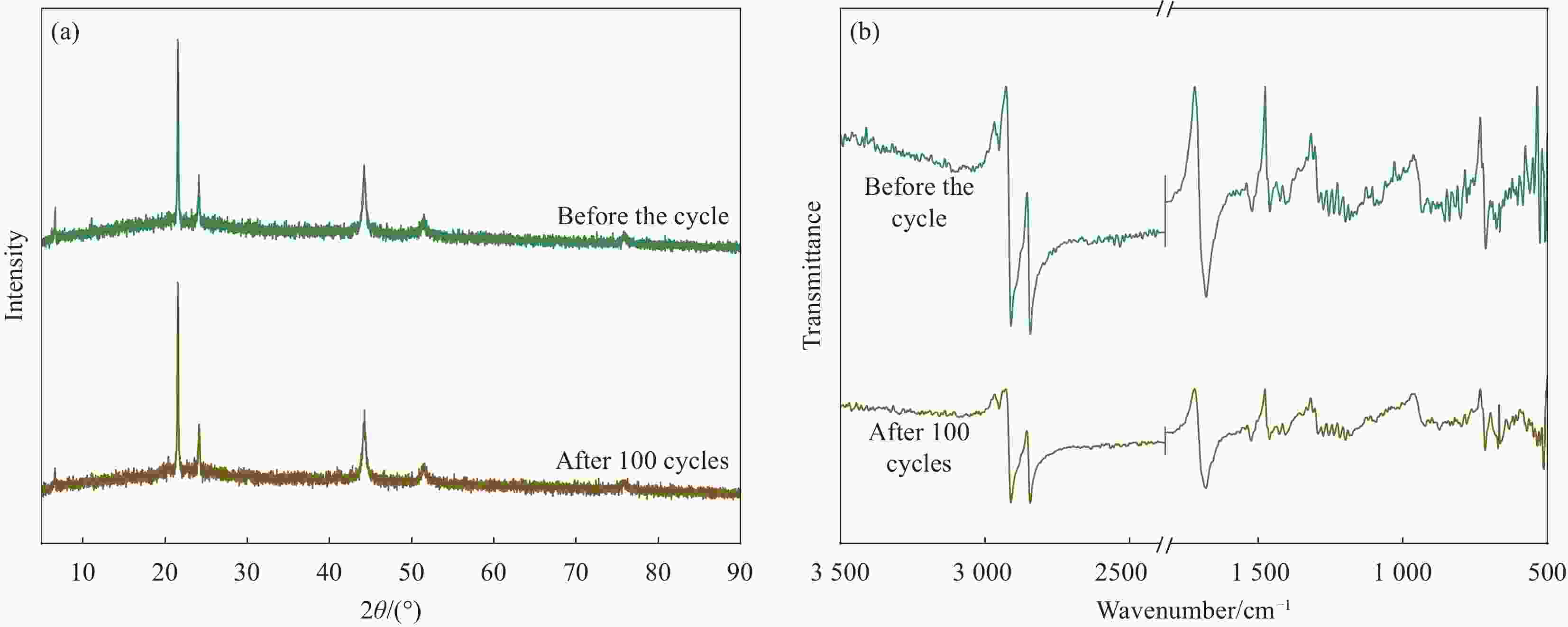
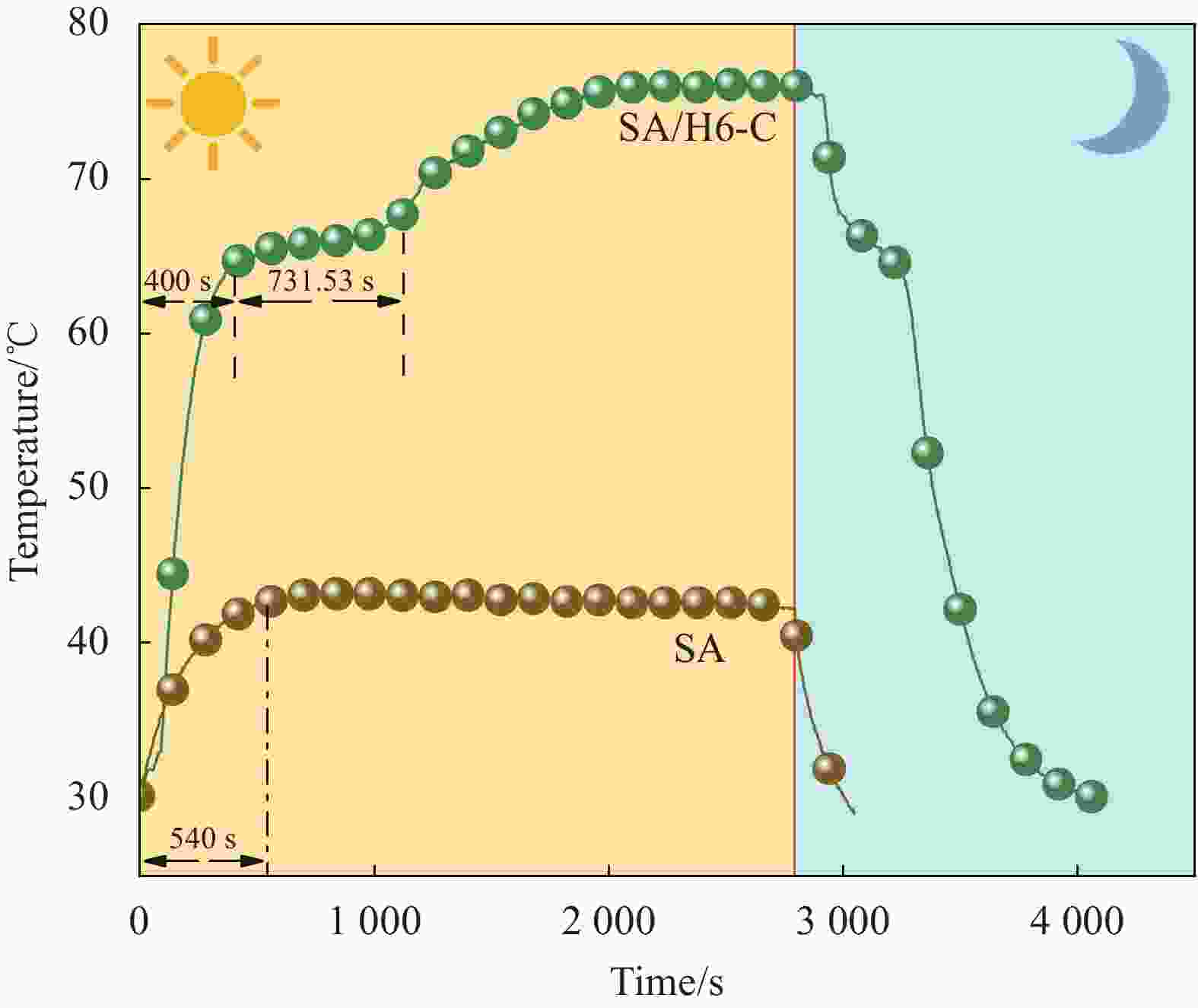
摘要: 为解决有机固-液相变材料(PCMs)导热系数低和相变易泄漏的难题,利用丹宁酸刻蚀ZIF-67制备碳基骨架作为支撑体(HX-C),硬脂酸(SA)为相变芯材,采用真空熔融吸附法构筑导热增强型定形相变材料(SA/HX-C)。为评估其储热能力,对热稳定性、储热性能、导热系数、定形能力及光热转换能力进行研究。同时,借助氮气等温吸附-脱附、傅里叶红外光谱、X-射线衍射和扫描电子显微镜进行表征。结果表明:丹宁酸刻蚀ZIF-67可实现对其碳化衍生物的扩孔作用,提高SA/HX-C的定形能力。所制备的SA/HX-C具有良好的储热性能、导热能力及光热转换性能。其中,刻蚀时间为6 min的复合相变材料(SA/H6-C)的储热效率可达80.84%,光热转化效率高达76.29%,导热系数(0.461 W/(m·K))相比于SA提高了156.11%。SA/H6-C在相变过程中无任何形貌变化和泄漏,重复循环储/放热100次后仍然具有良好的储热能力。Abstract: To solve the defects of low thermal conductivity and leakage of organic solid-liquid phase change materials (PCMs), ZIF-67 was etched by tannin acid to obtain the carbon-based supports (HX-C), stearic acid (SA) was the phase change material and then used to prepare the enhanced thermal conductivity PCMs (SA/HX-C) via vacuum melting adsorption method. In detail, thermal stability, heat storage property, thermal conductivity, shape stability and photo-thermal conversion were investigated to evaluate the thermal storage performance. Meanwhile, characterizations of nitrogen isothermal adsorption-desorption, FTIR, XRD and SEM were conducted. Results revealed that tannic acid can expand the pore size of carbonized ZIF-67 derivatives, thus enhancing the shape stability. The obtained SA/HX-C own favorable heat storage property, thermal conductivity, and photo-thermal conversion. Among them, etching time of 6 min for PCMs (SA/H6-C) exhibits high thermal storage efficiency of 80.84% and photo-thermal conversion of 76.29%. Thermal conductivity is strengthened to 0.461 W/(m·K), which is 156.11% higher than that of SA. No leakage and shape change are observed for SA/H6-C during phase transition, it still shows good thermal storage performance even after recycling 100 times.
-
Key words:
- phase change thermal storage /
- shape stability /
- ZIF-67 /
- photo-thermal conversion /
- stearic acid
-
表 1 SA/HX-C制备参数
Table 1. Preparation parameters of SA/HX-C
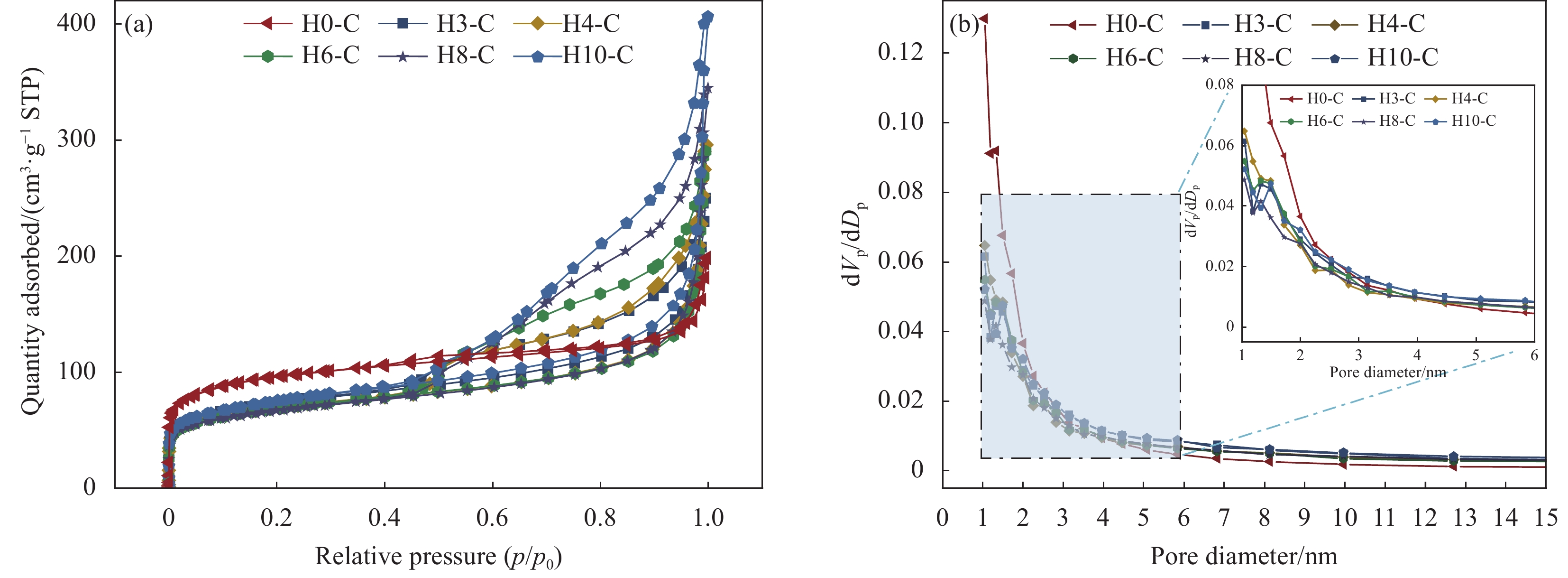
Precursor Etching time/min Support Carbonization temperature/℃ Composite PCM SA mass fraction/wt% ZIF-67 0 H0-C 700 SA/H0-C 60 ZIF-H3 3 H3-C SA/H3-C ZIF-H4 4 H4-C SA/H4-C ZIF-H6 6 H6-C SA/H6-C ZIF-H8 8 H8-C SA/H8-C ZIF-H10 10 H10-C SA/H10-C Notes: PCM—Phase change material; SA—Stearic acid. 表 2 HX-C的比表面积、孔容和平均孔径
Table 2. Surface area, pore volume and average pore diameter of HX-C
Sample Surface area/(m2·g−1) Pore volume/(cm3·g−1) Average pore diameter/nm H0-C 357.63 0.240 2.63 H3-C 259.74 0.337 6.02 H4-C 247.11 0.381 7.43 H6-C 249.13 0.386 7.46 H8-C 238.53 0.432 9.43 H10-C 267.30 0.498 9.69 表 3 SA和SA/HX-C的热性能
Table 3. Thermal properties of SA and SA/HX-C
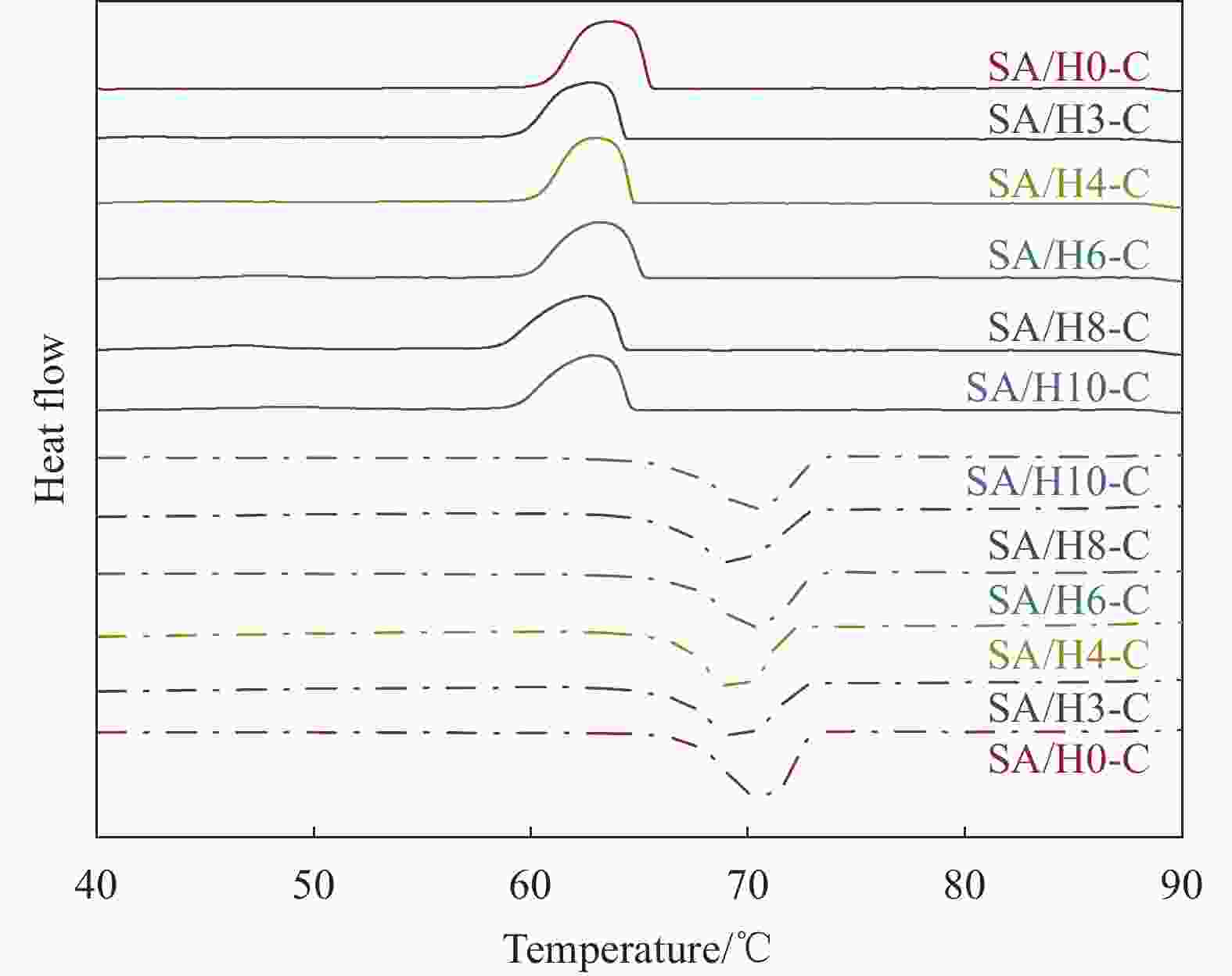
Sample Tm/Tf /℃ ΔHm/ΔHf/(J·g−1) ΔT/℃ λ/(W·(m·K)−1) SA 68.53/65.01 220.28/221.48 3.52 0.180 SA/H0-C 67.72/67.12 109.52/110.48 0.60 0.452 SA/H3-C 67.53/66.70 106.80/101.43 0.77 0.453 SA/H4-C 67.53/66.84 109.34/103.21 0.69 0.457 SA/H6-C 67.38/66.70 109.93/104.35 0.68 0.461 SA/H8-C 67.25/66.55 109.09/100.27 0.70 0.456 SA/H10-C 67.17/66.32 109.67/99.80 0.75 0.458 Notes: Tm, Tf and ΔT—Melting temperature, solidification temperature and supercooling; ΔHm and ΔHf—Latent heat of melting and latent heat of solidification; λ—Thermal conductivity. 表 4 SA/H6-C循环储/放热100次前后的热性能
Table 4. Thermal properties of SA/H6-C before and after 100 cycles of storage/exhaust heat
Cycle time Tm/Tf/℃ ΔHm/ΔHf/(J·g−1) ΔT/℃ Before the cycle 67.38/66.70 109.93/104.35 0.68 After 100 cycles 67.39/66.71 109.25/103.72 0.68 -
[1] XIA R, ZHANG W, YANG Y, et al. Transparent wood with phase change heat storage as novel green energy storage composites for building energy conservation[J]. Journal of Cleaner Production,2021,296:126598. doi: 10.1016/j.jclepro.2021.126598 [2] NALLUSAMY N, SAMPATH S, VELRAJ R. Experimental investigation on a combined sensible and latent heat storage system integrated with constant/varying (solar) heat sources[J]. Renewable Energy,2007,32(7):1206-1227. doi: 10.1016/j.renene.2006.04.015 [3] 吴韶飞, 闫霆, 蒯子函, 等. 高导热膨胀石墨/棕榈酸定形复合相变材料的制备及储热性能研究[J]. 化工学报, 2019, 70(9):3553-3564.WU Shaofei, YAN Ting, KUAI Zihan, et al. Preparation and thermal energy storage properties of high heat conduction expanded graphite/palmitic acid form-stable phase change materials[J]. CIESC Journal,2019,70(9):3553-3564(in Chinese). [4] 翟天尧, 李廷贤, 仵斯, 等. 高导热膨胀石墨/硬脂酸定形相变储能复合材料的制备及储/放热特性[J]. 科学通报, 2018, 63(7):674-683. doi: 10.1360/N972017-00831ZHAI Tianyao, LI Tingxian, WU Si, et al. Preparation and thermal performance of form-stable expanded graphite/stearic acid composite phase change materials with high thermal conductivity[J]. Chinese Science Bulletin,2018,63(7):674-683(in Chinese). doi: 10.1360/N972017-00831 [5] CHENG X, LI G, YU G, et al. Effect of expanded graphite and carbon nanotubes on the thermal performance of stearic acid phase change materials[J]. Journal of Materials Science,2017,52(20):12370-12379. doi: 10.1007/s10853-017-1350-9 [6] YANG X, GUO Z, LIU Y, et al. Effect of inclination on the thermal response of composite phase change materials for thermal energy storage[J]. Applied Energy,2019,238:22-33. doi: 10.1016/j.apenergy.2019.01.074 [7] WU S, LI T X, YAN T, et al. High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage[J]. International Journal of Heat and Mass Transfer,2016,102:733-744. doi: 10.1016/j.ijheatmasstransfer.2016.06.066 [8] HEKIMOĞLU G, SARI A, KAR T, et al. Walnut shell derived bio-carbon/methyl palmitate as novel composite phase change material with enhanced thermal energy storage properties[J]. Journal of Energy Storage,2021,35:102288. doi: 10.1016/j.est.2021.102288 [9] LI C, ZHAO X, ZHANG B, et al. Stearic acid/copper foam as composite phase change materials for thermal energy storage[J]. Journal of Thermal Science,2020,29(2):492-502. doi: 10.1007/s11630-020-1272-8 [10] KHADIRAN T, HUSSEIN M Z, ZAINAL Z, et al. Activated carbon derived from peat soil as a framework for the preparation of shape-stabilized phase change material[J]. Energy,2015,82:468-478. doi: 10.1016/j.energy.2015.01.057 [11] WANG X, CHENG X, LI D, et al. Preparation a three-dimensional hierarchical graphene/stearic acid as a phase change materials for thermal energy storage[J]. Materials Research Express,2020,7(9):95506. doi: 10.1088/2053-1591/abb69e [12] MA X, LIU F, HELIAN Y, et al. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review[J]. Energy Conversion and Management,2021,229:113760. doi: 10.1016/j.enconman.2020.113760 [13] LI H, LIU F, MA X, et al. An efficient basic heterogeneous catalyst synthesis of magnetic mesoporous Fe@C support SrO for transesterification[J]. Renewable Energy,2020,149:816-827. doi: 10.1016/j.renene.2019.12.118 [14] ZHANG H, NAI J, YU L, et al. Metal-organic-framework-based materials as platforms for renewable energy and environmental applications[J]. Joule,2017,1(1):77-107. doi: 10.1016/j.joule.2017.08.008 [15] CRAVILLON J, NAYUK R, SPRINGER S, et al. Controlling zeolitic imidazolate framework nano- and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering[J]. Chemistry of Materials,2011,23(8):2130-2141. doi: 10.1021/cm103571y [16] HU Y, SONG X, ZHENG Q, et al. Zeolitic imidazolate framework-67 for shape stabilization and enhanced thermal stability of paraffin-based phase change materials[J]. RSC Advances,2019,9(18):9962-9967. doi: 10.1039/C9RA00874H [17] CHEN X, GAO H, XING L, et al. Nanoconfinement effects of N-doped hierarchical carbon on thermal behaviors of organic phase change materials[J]. Energy Storage Materials,2019,18:280-288. doi: 10.1016/j.ensm.2018.08.024 [18] FU Y, ZHEN L, ZHOU B, et al. New strategy of synthesizing zeolitic imidazolate framework-67 with hierarchical pores for heat storage[J]. Materials Letters,2021,293:129722. [19] ZHANG W, JIANG X, ZHAO Y, et al. Hollow carbon nanobubbles: Monocrystalline MOF nanobubbles and their pyrolysis[J]. Chemical Science,2017,8(5):3538-3546. doi: 10.1039/C6SC04903F [20] QIAN T, LI J, MIN X, et al. Integration of pore confinement and hydrogen-bond influence on the crystallization behavior of C18 PCMs in mesoporous silica for form-stable phase change materials[J]. ACS Sustainable Chemistry and Engineering,2018,6(1):897-908. doi: 10.1021/acssuschemeng.7b03267 [21] LI D, CHENG X, LI Y, et al. Effect of MOF derived hierarchical Co3O4/expanded graphite on thermal performance of stearic acid phase change material[J]. Solar Energy,2018,171:142-149. doi: 10.1016/j.solener.2018.06.062 [22] WEI H, XIE X, LI X, et al. Preparation and characterization of capric-myristic-stearic acid eutectic mixture/modified expanded vermiculite composite as a form-stable phase change material[J]. Applied Energy,2016,178:616-623. doi: 10.1016/j.apenergy.2016.06.109 [23] WANG X, ZHONG W, LI Y. Nanoscale Co-based catalysts for low-temperature CO oxidation[J]. Catalysis Science and Technology,2015,5(2):1014-1020. doi: 10.1039/C4CY01147C [24] ZHANG Q, LIU J. Sebacic acid/CNT sponge phase change material with excellent thermal conductivity and photo-thermal performance[J]. Solar Energy Materials and Solar Cells,2018,179:217-222. doi: 10.1016/j.solmat.2017.11.019 [25] ADVINCULA P A, DE LEON A C, RODIER B J, et al. Accommodating volume change and imparting thermal conductivity by encapsulation of phase change materials in carbon nanoparticles[J]. Journal of Materials Chemistry A,2018,6(6):2461-2467. doi: 10.1039/C7TA09664J [26] ZHANG N, YUAN Y, YUAN Y, et al. Effect of carbon nanotubes on the thermal behavior of palmitic-stearic acid eutectic mixtures as phase change materials for energy storage[J]. Solar Energy,2014,110:64-70. doi: 10.1016/j.solener.2014.09.003 [27] ZHANG X, LIN Q, LUO H, et al. Three-dimensional graphitic hierarchical porous carbon/stearic acid composite as shape-stabilized phase change material for thermal energy storage[J]. Applied Energy,2020,260:114278. doi: 10.1016/j.apenergy.2019.114278 [28] YUAN Y, ZHANG N, LI T, et al. Thermal performance enhancement of palmitic-stearic acid by adding graphene nanoplatelets and expanded graphite for thermal energy storage: A comparative study[J]. Energy,2016,97:488-497. doi: 10.1016/j.energy.2015.12.115 [29] ATINAFU D G, DONG W, HUANG X, et al. Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity[J]. Applied Energy,2018,211:1203-1215. doi: 10.1016/j.apenergy.2017.12.025 [30] DING J, WU X, SHEN X, et al. A promising form-stable phase change material composed of C/SiO2 aerogel and palmitic acid with large latent heat as short-term thermal insulation[J]. Energy,2020,210:118478. doi: 10.1016/j.energy.2020.118478 [31] LI A, WANG J, DONG C, et al. Core-sheath structural carbon materials for integrated enhancement of thermal conductivity and capacity[J]. Applied Energy,2018,217:369-376. doi: 10.1016/j.apenergy.2017.12.106 [32] ATINAFU D G, DONG W, HOU C, et al. A facile one-step synthesis of porous N-doped carbon from MOF for efficient thermal energy storage capacity of shape-stabilized phase change materials[J]. Materials Today Energy,2019,12:239-249. doi: 10.1016/j.mtener.2019.01.011 [33] LU A, LI W, SALABAS E, et al. Low temperature catalytic pyrolysis for the synthesis of high surface area, nanostructured graphitic carbon[J]. Chemistry of Materials,2006,18(8):2086-2094. doi: 10.1021/cm060135p [34] QIAN T, ZHU S, WANG H, et al. Comparative study of single-walled carbon nanotubes and graphene nanoplatelets for improving the thermal conductivity and solar-to-light conversion of PEG-infiltrated phase-change material composites[J]. ACS Sustainable Chemistry and Engineering,2018,7(2):2446-2458. -






 下载:
下载: