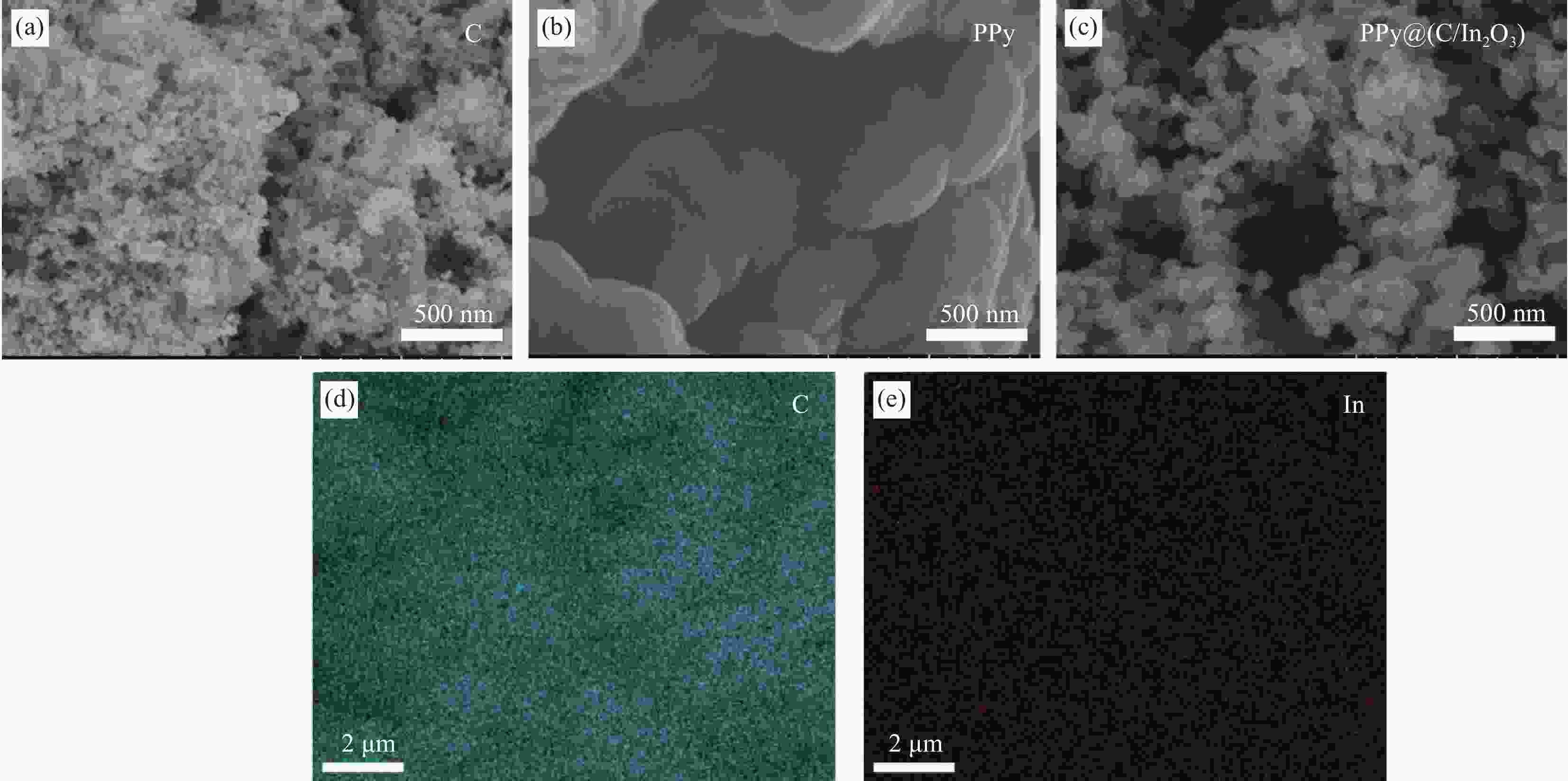
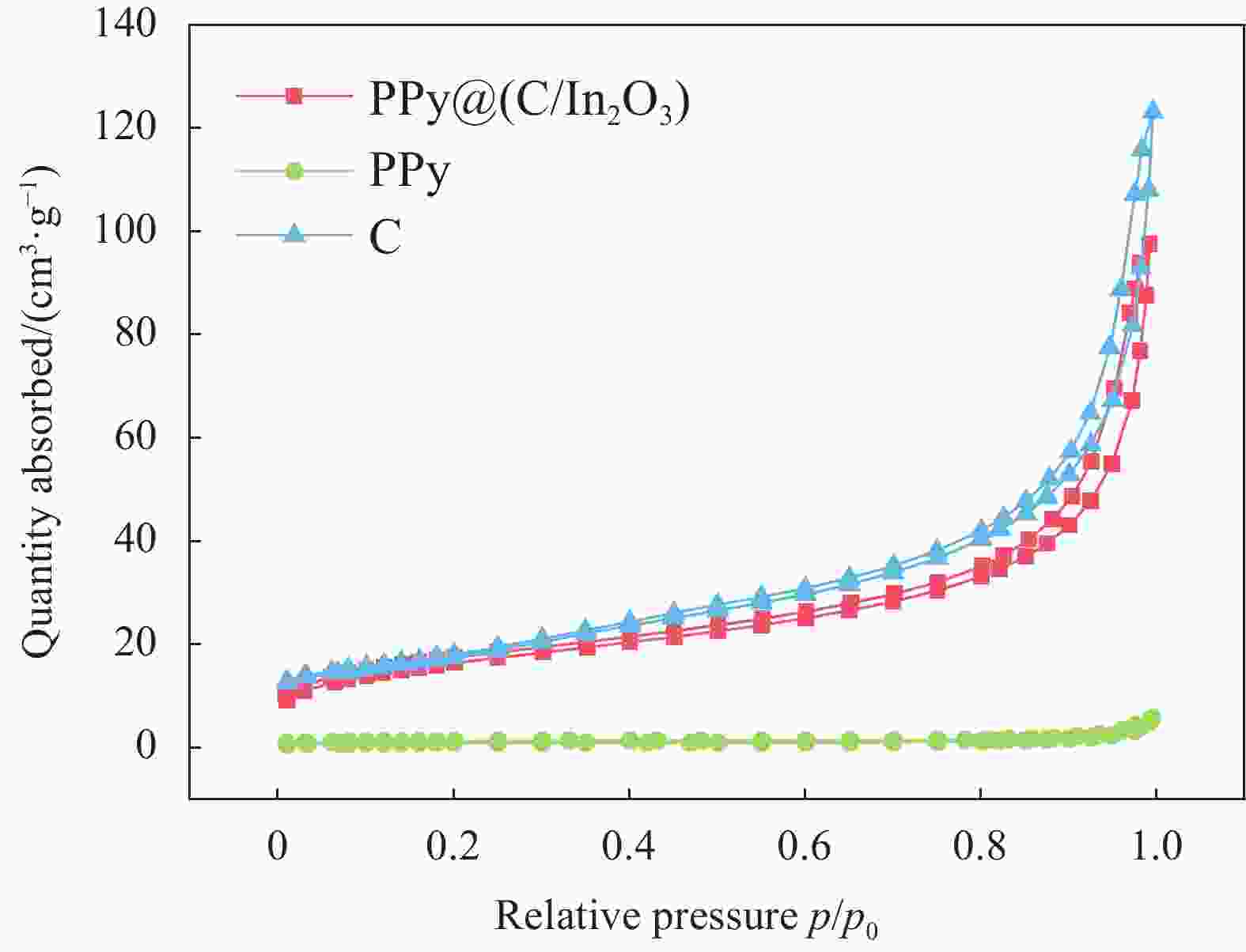
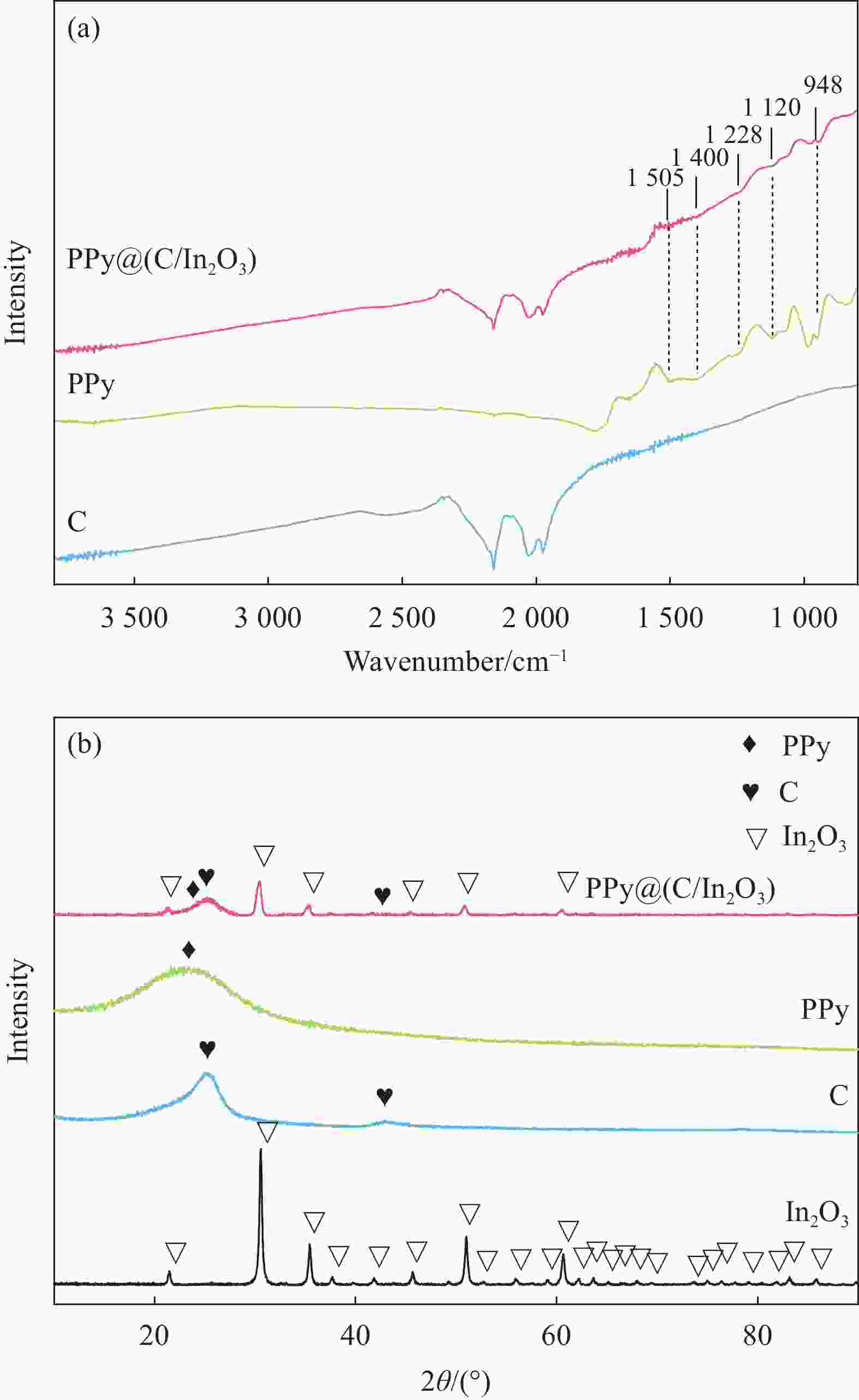
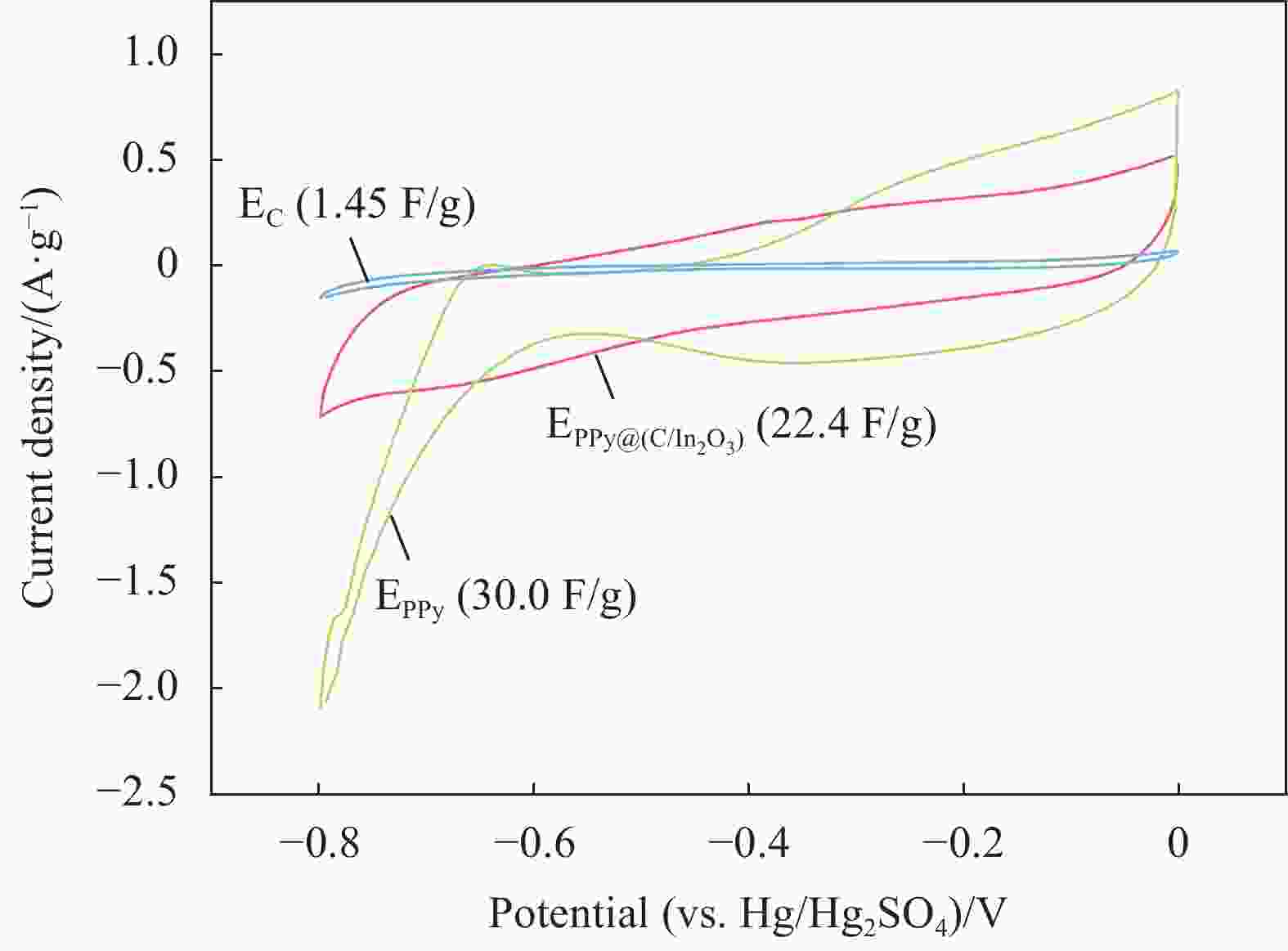
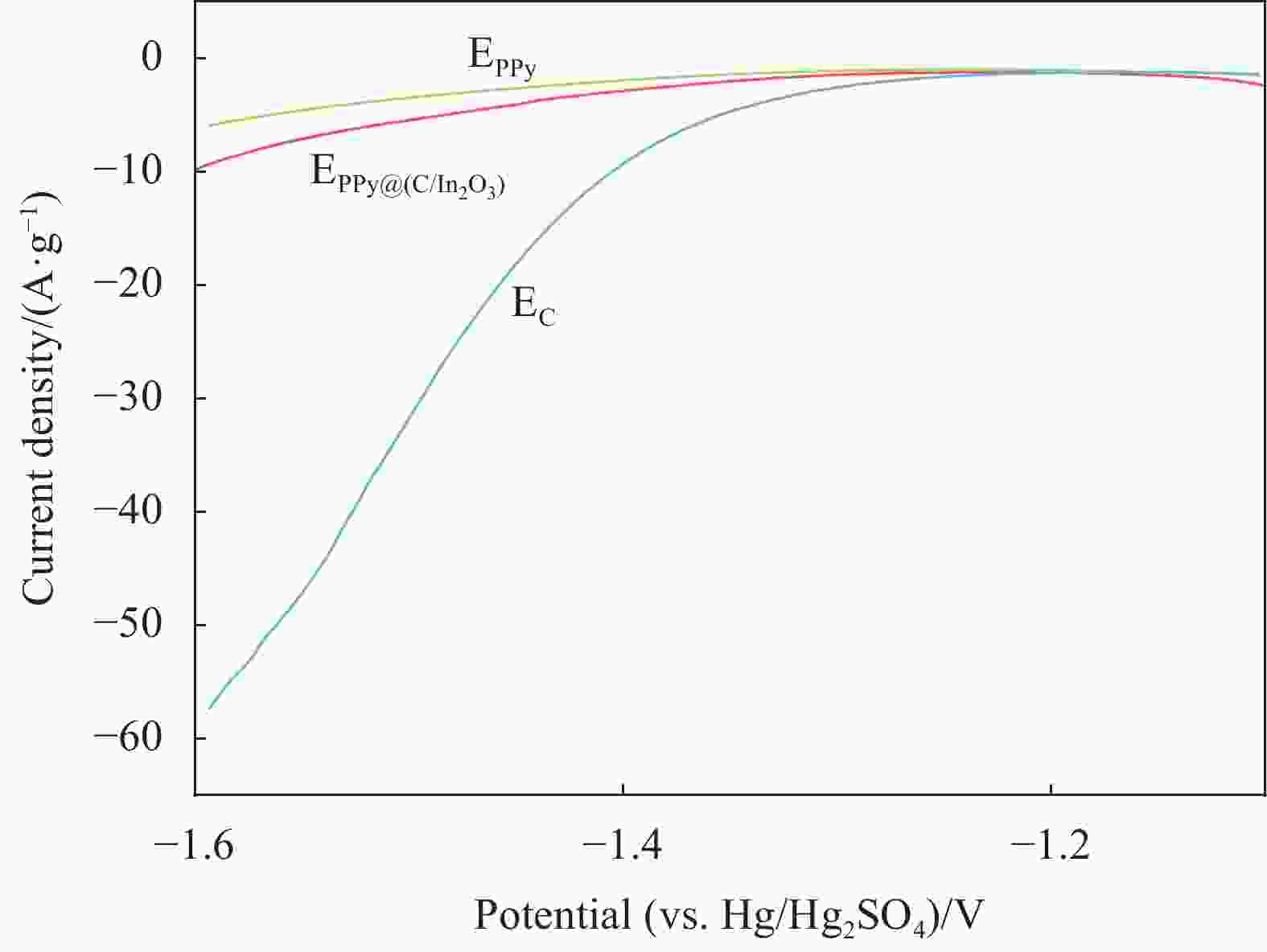
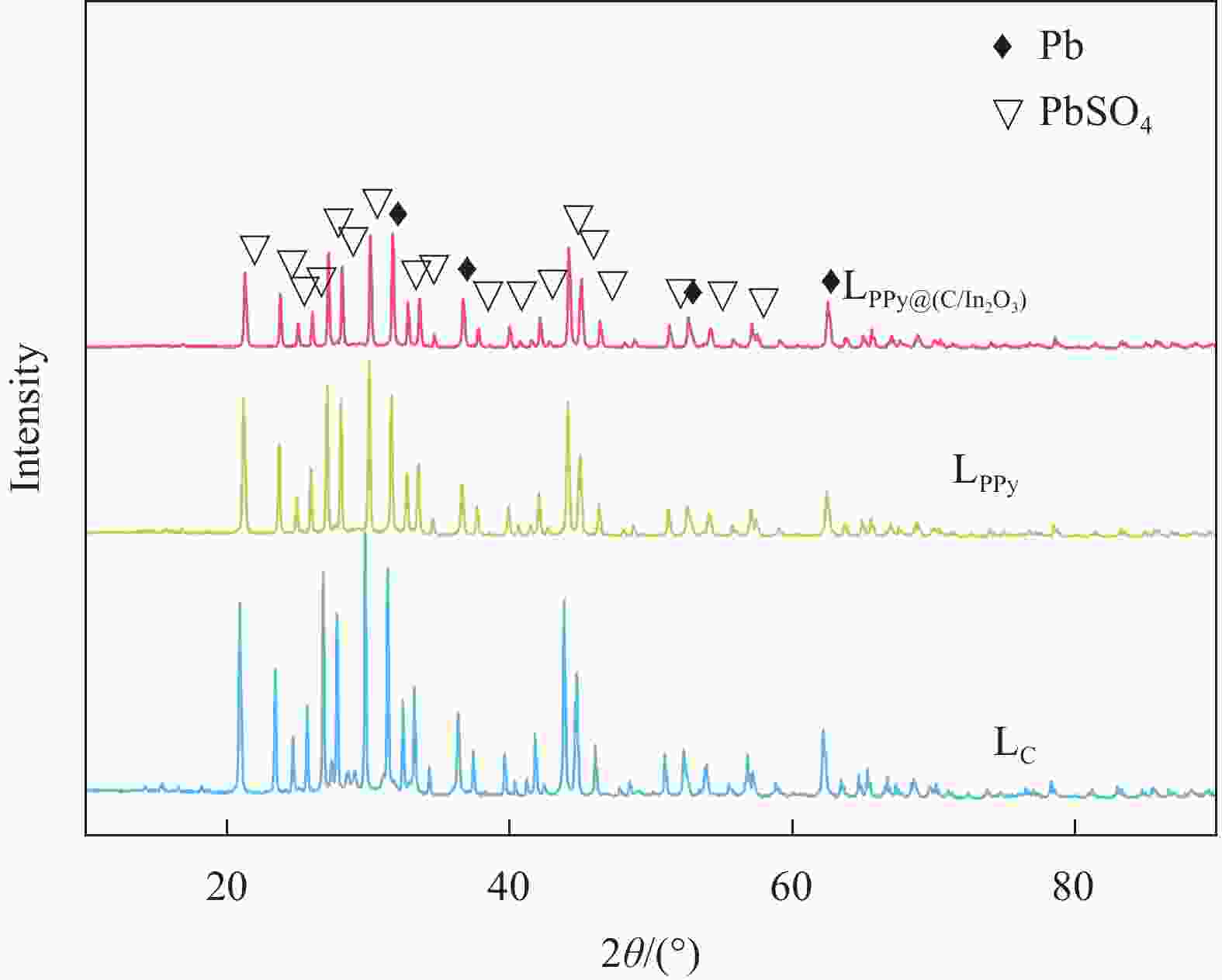
Preparation of polypyrrole coated with conductive carbon black/indium oxide composite and its application in lead-acid batteries
-
摘要: 为了改善铅酸电池负极不可逆硫酸盐化及析氢问题,通过原位化学聚合的方法制备导电炭黑/氧化铟表面包覆聚吡咯[PPy@(C/In2O3)]复合材料,采用SEM、FTIR、BET和XRD等表征手段分别对复合材料的微观形貌和结构进行分析;通过循环伏安法(CV)和线性扫描法(LSV)测试了复合材料的电化学性能。最后,将PPy@(C/In2O3)复合材料添加到铅酸电池负极活性材料中,探究PPy@(C/In2O3)对铅酸电池高倍率部分荷电状态(HRPSoC)循环寿命及放电容量的影响。结果表明:PPy@(C/In2O3)保留了导电炭黑的基本结构特征,具有较大比表面积;同时具有较高析氢过电位及较大比容量。当将PPy@(C/In2O3)复合材料添加到铅酸电池负极活性材料中,不仅可以降低负极板内阻抑制电池的负极硫酸盐化问题,而且可以减弱电池负极析氢问题,在提高铅酸电池放电容量同时,显著提高了铅酸电池高倍率部分荷电状态循环寿命。最终,含有PPy@(C/In2O3)的负极板的铅酸电池显示出了优异的HRPSoC循环寿命,较空白组电池循环寿命提高了1.78倍。
-
关键词:
- 聚吡咯包覆导电炭黑/氧化铟复合材料 /
- 硫酸盐化 /
- 析氢 /
- 高倍率部分荷电状态 /
- 铅酸电池
Abstract: In order to improve the irreversible sulfation and hydrogen evolution of the negative electrode of lead-acid batteries, in this study, the olypyrrole coated with conductive carbon black/indium oxide composite [PPy@(C/In2O3)] were prepared by in-situ oxidation polymerization on C/In2O3. The composite materials were characterized by SEM, FTIR, BET and XRD. The electrochemical performance of the composites was analyzed by CV and LSV. Finally, the PPy@(C/In2O3) composite materials were added in the negative active material of lead-acid batteries. The effect of composite materials on the high-rate partial-state-of-charge (HRPSoC) performance of lead-acid batteries was investigated. The results show that the PPy@(C/In2O3) retain the structural feature of C, and have larger specific surface area than PPy, and have higher hydrogen evolution over-potential and capacitance than C. When PPy@(C/In2O3) composite materials were added to the negative active material of the lead-acid batteries, it can not only reduce the internal resistance of the negative plate and inhibit the negative sulfation problem of the batteries, but also reduce the hydrogen evolution problem of the negative electrode of the batteries. At the same time, the discharge capacity significantly improves the cycle life of the lead-acid batteries under the HRPSoC operation. Finally, the lead-acid batteries containing the negative plate of PPy@(C/In2O3) show excellent HRPSoC cycle life which increased by 1.78 times compared with the cycle life of the blank battery. -
图 10
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $ 、LPPy及LC的Nyquist图Figure 10. Nyquist plots of test batteries of
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $ , LPPy and LC${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $, LPPy and LC—Lead-acid battery prepared by PPy@(C/In2O3), PPy and C additive
图 12
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $ 、LPPy及LC充放电电压在1 A·s条件下随高倍率部分荷电状态(HRPSoC)循环寿命的变化Figure 12. Change of end-of-charge/discharge voltage as a function of the high-rate partial-state-of-charge (HRPSoC) cycle life for
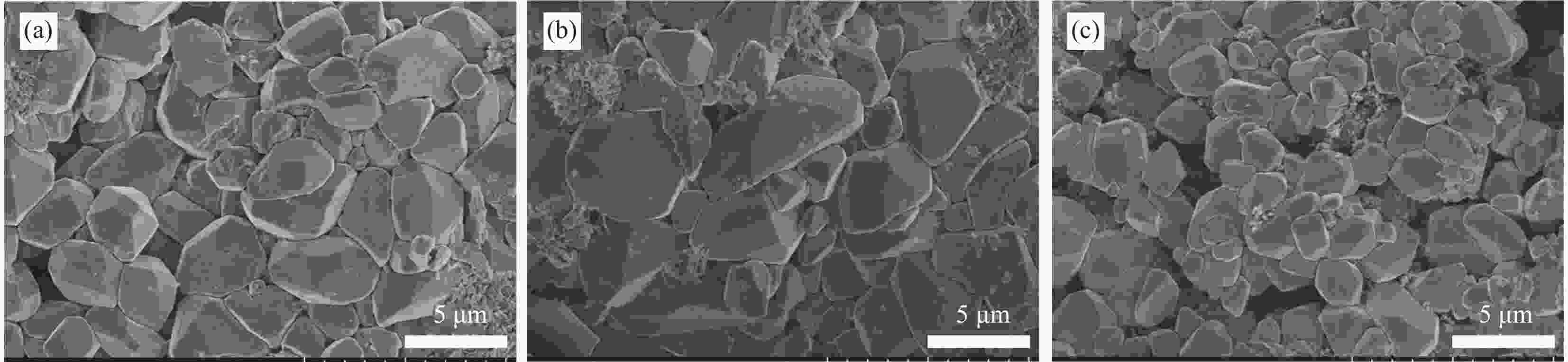
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $ , LPPy and LC at 1 A·s图 13 LC (a)、LPPy (b) 及
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}}$ (c) HRPSoC循环测试结束后负极板的SEM图像Figure 13. SEM images of negative plates after the HRPSoC cycle of LC (a), LPPy (b) and
${{\text{L}}_{\text{PPy@(C/I}{{\text{n}}_{\text{2}}}{{\text{O}}_{\text{3}}}\text{)}}} $ (c) -
[1] BALLANTYNE A D, HALLETT J P, RILEY D J, et al. Lead acid battery recycling for the twenty-first century[J]. Royal Society Open Science,2018,5(5):171368. doi: 10.1098/rsos.171368 [2] YANG J, HU C, WANG H, et al. Review on the research of failure modes and mechanism for lead-acid batteries[J]. International Journal of Energy Research,2017,41(3):336-352. doi: 10.1002/er.3613 [3] LAM L T, HAIGH N P, PHYLAND C G, et al. Failure mode of valve-regulated lead-acid batteries under high-rate partial-state-of-charge operation[J]. Journal of Power Sources,2004,133(1):126-134. doi: 10.1016/j.jpowsour.2003.11.048 [4] LAM L T, LOUEY R, HAIGH N P, et al. VRLA ultrabattery for high-rate partial-state-of-charge operation[J]. Journal of Power Sources,2007,174(1):16-29. doi: 10.1016/j.jpowsour.2007.05.047 [5] HARIPRAKASH B, GAFFOOR S A, SHUKLA A K. Lead-acid batteries for partial-state-of-charge applications[J]. Jour-nal of Power Sources,2009,191(1):149-153. doi: 10.1016/j.jpowsour.2008.12.081 [6] MOSELEY P T, NELSON R F, HOLLENKAMP A F. The role of carbon in valve-regulated lead-acid battery technology[J]. Journal of Power Sources,2006,157(1):3-10. doi: 10.1016/j.jpowsour.2006.02.031 [7] FURUKAWA J, TAKADA T, MONMA D, et al. Further demonstration of the VRLA-type ultra-battery under medium-HEV duty and development of the flooded-type ultra-battery for micro-HEV applications[J]. Journal of Power Sources,2010,195(4):1241-1245. doi: 10.1016/j.jpowsour.2009.08.080 [8] JIANG H, ZHAO T, MA J, et al. Ultrafine manganese dioxide nanowire network for high-performance supercapacitors[J]. Chemical Communications,2011,47(4):1264-1266. doi: 10.1039/C0CC04134C [9] PAVLOV D, NIKOLOV P. Capacitive carbon and electrochemical lead electrode systems at the negative plates of lead-acid batteries and elementary processes on cycling[J]. Journal of Power Sources,2013,242(Complete):380-399. [10] BODEN D P, LOOSEMORE D V, SPENCE M A, et al. Optimization studies of carbon additives to negative active material for the purpose of extending the life of VRLA batteries in high-rate partial-state-of-charge operation[J]. Journal of Power Sources,2010,195(14):4470-4493. doi: 10.1016/j.jpowsour.2009.12.069 [11] NAKAMURA K, SHIOMI M, TAKAHASHI K, et al. Failure modes of valve-regulated lead/acid batteries[J]. Journal of Power Sources,1996,59(1-2):153-157. doi: 10.1016/0378-7753(95)02317-8 [12] RUETSCHI P. Aging mechanisms and service life of lead-acid batteries[J]. Journal of Power Sources,2004,127(1-2):33-44. doi: 10.1016/j.jpowsour.2003.09.052 [13] SUBBURAJ A S, PUSHPAKARAN B N, BAYNE S B. Overview of grid connected renewable energy based battery projects in USA[J]. Renewable & Sustainable Energy Reviews,2015,45:219-234. [14] FERNANDEZ M, VALENCIANO J, TRINIDAD F, et al. The use of activated carbon and graphite for the development of lead-acid batteries for hybrid vehicle applications[J]. Journal of Power Sources,2010,195(14):4458-4469. doi: 10.1016/j.jpowsour.2009.12.131 [15] PAVLOV D, NIKOLOV P, ROGACHEV T. Influence of expander components on the processes at the negative plates of lead-acid cells on high-rate partial-state-of-charge cycling. Part II. Effect of carbon additives on the processes of charge and discharge of negative plates[J]. Journal of Power Sources,2010,195(14):4444-4457. doi: 10.1016/j.jpowsour.2009.12.132 [16] PAVLOV D, NIKOLOV P, ROGACHEV T. Influence of carbons on the structure of the negative active material of lead-acid batteries and on battery performance[J]. Journal of Power Sources,2011,196(11):5155-5167. doi: 10.1016/j.jpowsour.2011.02.014 [17] XIANG J Y, DING P, ZHANG H, et al. Beneficial effects of activated carbon additives on the performance of negative lead-acid battery electrode for high-rate partial-state-of-charge operation[J]. Journal of Power Sources,2013,241:150-158. doi: 10.1016/j.jpowsour.2013.04.106 [18] BANERJEE A, ZIV B, SHILINA Y, et al. Single-wall carbon nanotube doping in lead-acid batteries: A new horizon[J]. ACS Applied Materials& Interfaces,2017,9(4):3634-3643. [19] SWOGGER S W, EVERILL P, DUBEY D P, et al. Discrete carbon nanotubes increase lead acid battery charge acceptance and performance[J]. Journal of Power Sources,2014,261:55-63. [20] BULOCK K R, MAHATO B K, WRUCK W J. Use of conductive materials to enhance lead-acid battery formation[J]. Journal of the Electrochemical Society,1991,138(12):3545. doi: 10.1149/1.2085456 [21] LI Z, CHEN B, WANG D. Effects of electrochemically active carbon and indium (III) oxide in negative plates on cycle performance of valve-regulated lead-acid batteries during high-rate partial-state-of-charge operation[J]. Journal of Power Sources,2013,231:34-38. [22] 郎笑石. 高倍率储能Pb-C超级电池负极的研究 [D]. 哈尔滨: 哈尔滨工业大学, 2011.LANG Xiaoshi. Study on the negative electrode of high rated energy storage Pb-C super battery[D]. Haerbin: Harbin Institute of Technology, 2011(in Chinese). [23] ZHOU W, LU L, CHEN D, et al. Construction of high surface potential polypyrrole nanorods with enhanced antibacterial properties[J]. Journal of Materials Chemistry B, 2018, 19: 3128-3135. [24] WANG F, LV X, ZHANG L, et al. Construction of vertically aligned PPy nanosheets networks anchored on MnCo2O4 nanobelts for high-performance asymmetric supercapacitor[J]. Journal of Power Sources,2018,393:169-176. [25] WANG J G, WEI B, KANG F. Facile synthesis of hierarchical conducting polypyrrole nanostructures via a reactive template of MnO2 and their application in supercapacitors[J]. RSC Advances,2013,4(1):199-202. [26] 候辰, 吕志, 肖湘, 等. 原位包覆导电聚吡咯的Li1.26Fe0.22Mn0.52O2富锂铁锰基正极材料的制备及电化学性能的提高[J]. 无机化学学报, 2021, 37(5):875-885. doi: 10.11862/CJIC.2021.110HOU Chen, LV Zhi, XIAO Xiang, et al. Preparation and improvement of electrochemical performance of Li1.26Fe0.22Mn0.52O2 Fe-Mn based Li-rich cathode materials in-situ coated with conductive polypyrrole[J]. Chinese Journal of Inorganic Chemistry,2021,37(5):875-885(in Chinese). doi: 10.11862/CJIC.2021.110 [27] CHEN C, LIU Y, CHEN Y, et al. Effect of polyaniline-modified lignosulfonate added to the negative active material on the performance of lead-acid battery[J]. Electrochimic Acta,2020,338:135859. doi: 10.1016/j.electacta.2020.135859 [28] 朱嫦娥, 任丽, 王立新, 等. 炭黑吸附聚合制备聚吡咯/炭黑导电复合材料[J]. 复合材料学报, 2005, 22(3):45-48. doi: 10.3321/j.issn:1000-3851.2005.03.009ZHU Chang‘e, REN Li, WANG Lixin, et al. Preparation of polypyrrole/carbon black conducting composites by adsorption polymerization[J]. Acta Materiae Compositae Sinica,2005,22(3):45-48(in Chinese). doi: 10.3321/j.issn:1000-3851.2005.03.009 [29] 杨丽佳. 聚吡咯基电极材料的制备及电化学性能研究[D]. 镇江: 江苏科技大学, 2019.YANG Lijia. Preparation and electrochemical properties of polypyrrole-based electrode materials[D]. Zhenjiang: Jiangsu University of Science and Technology, 2019(in Chinese). [30] BORSTEL D V, HOOGESTRAAT G, ZIECHMANN W. Efficiency of lignosulfonates and humic-related substances as expanders in negative electrodes of the lead/acid system[J]. Journal of Power Sources,1994,50(1-2):131-140. doi: 10.1016/0378-7753(93)01892-L -






 下载:
下载: