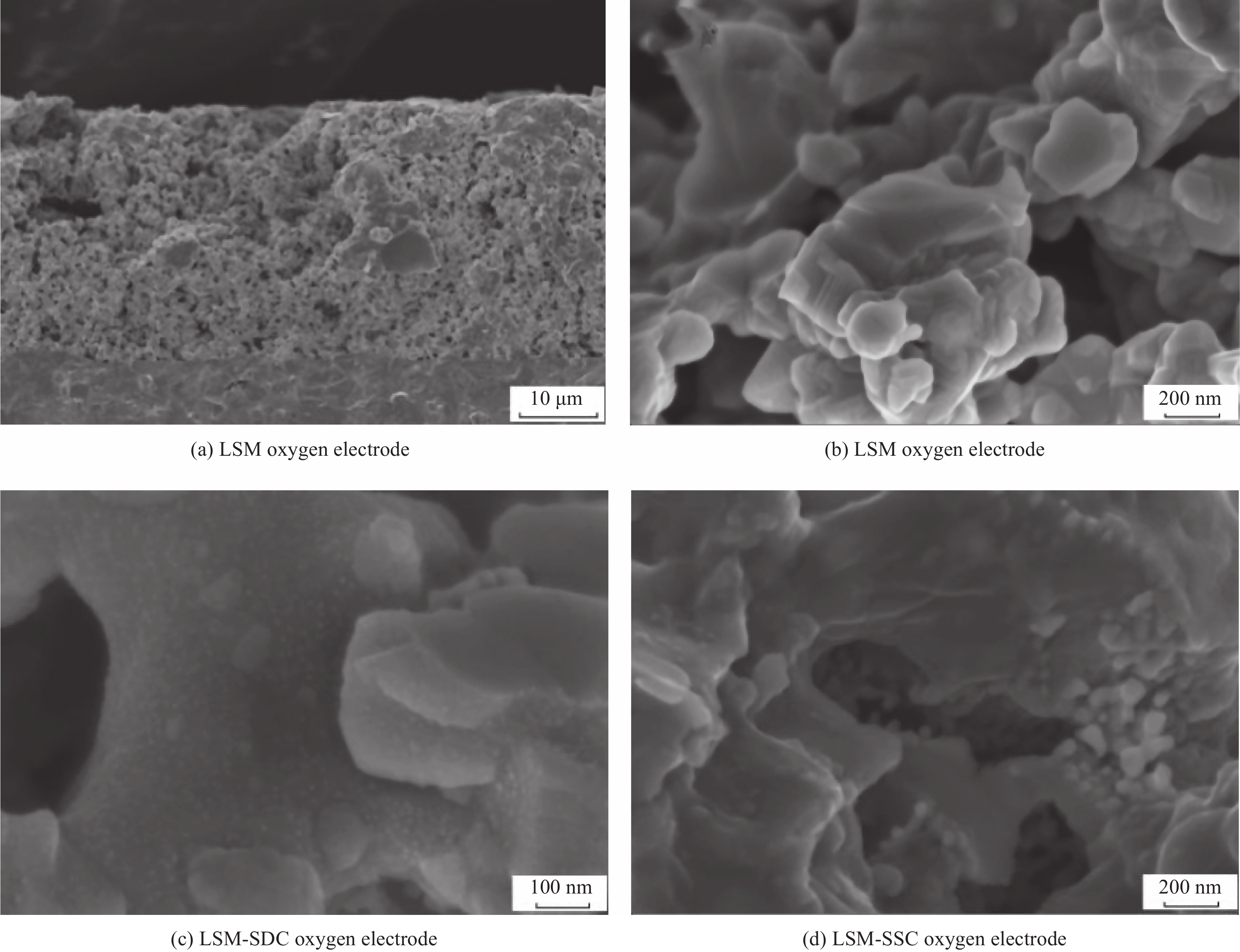
Performance optimization of La0.65Sr0.35MnO3 oxygen electrode based on alternate infiltration method
-
摘要: 氢能以其高效、清洁、可再生的优点成为化石能源的有效替代者,而可逆固体氧化物电池(RSOC)既可利用氢气输出电能,也可电解H2O产生氢气,对其研究具有十分重要的意义。本文对RSOC的氧电极进行了研究,在La0.65Sr0.35MnO3(LSM)氧电极的基础上,采用溶液交替浸渍法将Sm0.2Ce0.8O1.9(SDC)和Sm0.5Sr0.5CoO3−δ(SSC)纳米粒子引入LSM氧电极中。800℃时,交替浸渍1次的LSM-SDC-SSC1氧电极的极化电阻为0.49 Ω·cm2,是纯LSM电极(1.12 Ω·cm2)的43%。SDC和SSC的浸渍顺序对电极形貌和性能的影响随着浸渍次数的增加逐渐减弱,交替浸渍2次的LSM-SDC-SSC2氧电极具有最低的极化过电位和极化电阻。800°C时,Ni-(Y2O3)0.08(ZrO2)0.92(YSZ)/YSZ/LSM-SDC-SSC2单电池在固体氧化物燃料电池(SOFC)模式下的最大功率密度为870 mW·cm−2,是纯LSM电池的6.3倍,在固体氧化物电解池(SOEC)模式下的最大电解电流密度为−1150 mA·cm−2,具有良好的可逆电池输出性能。
-
关键词:
- 储能 /
- 可逆固体氧化物电池 /
- La0.65Sr0.35MnO3(LSM)氧电极 /
- 交替浸渍法 /
- 复合氧电极
Abstract: Hydrogen energy has become an effective substitute for fossil energy due to its advantages of high efficiency, cleanliness and renewability. Reversible solid oxide cell (RSOC) can use hydrogen to output electricity or electrolyze H2O to produce hydrogen, which has very important research significance. The performance optimization of La0.65Sr0.35MnO3 (LSM) oxygen electrode was studied in this research. Sm0.2Ce0.8O1.9 (SDC) and Sm0.5Sr0.5CoO3−δ (SSC) nanoparticles were introduced into the LSM oxygen electrode. The polarization resistance of the one-time alternate LSM-SDC-SSC1 oxygen electrode at 800°C is 0.49 Ω·cm2, which is 43% of the LSM oxygen electrode. The effect of infiltration sequence of SDC and SSC on the morphology and properties of the electrode decrease with the increase of impregnation time. The two-time alternate LSM-SDC-SSC2 oxygen electrode show the lowest polarization overpotential and polarization resistance. The Ni-(Y2O3)0.08(ZrO2)0.92 (YSZ)/YSZ/LSM-SDC-SSC2 single cell obtain a maximum power density of 870 mW·cm−2 in solid oxide fuel cell (SOFC) mode and a maximum electrolysis current density of −1150 mA·cm−2 in the solid oxide electrolytic cell (SOEC) mode at 800°C, which show good reversible cell performance. -
图 5 不同浸渍顺序的氧电极在不同温度下的极化阻抗谱图
Figure 5. Impedance spectra of oxygen electrodes with different infiltration sequences at different temperatures
LSM-SSC-SDC1 and LSM-SSC-SDC2 infiltration SSC first, then SDC, alternate infiltration times are 1 and 2, respectively; LSM-SDC-SSC1 and LSM-SDC-SSC2 infiltration SDC first, then SSC, the alternate infiltration times are 1 and 2 times, respectively
表 1 不同浸渍顺序的氧电极在不同温度下的极化电阻
Table 1. Polarization resistances of oxygen electrodes with different infiltration sequences at different temperatures
Oxygen electrode Polarization resistance Rp/(Ω·cm2) 650℃ 700℃ 750℃ 800℃ LSM-SSC-SDC1 3.263 1.536 0.907 0.551 LSM-SSC-SDC2 0.954 0.436 0.247 0.153 LSM-SDC-SSC1 2.639 1.307 0.806 0.481 LSM-SDC-SSC2 0.988 0.372 0.249 0.179 -
[1] HÖÖK M, TANG X. Depletion of fossil fuels and anthropogenic climate change—A review[J]. Energy Policy,2013,52:797-809. doi: 10.1016/j.enpol.2012.10.046 [2] PANWAR N L, KAUSHIK S C, KOTHARI S. Role of renewable energy sources in environmental protection: A review[J]. Renewable and Sustainable Energy Reviews,2011,15(3):1513-1524. doi: 10.1016/j.rser.2010.11.037 [3] JIANG S P. Challenges in the development of reversible solid oxide cell technologies: A mini review[J]. Asia-Pacific Journal of Chemical Engineering,2016,11(3):386-391. doi: 10.1002/apj.1987 [4] GIORGIO D, DESIDERI U. Potential of reversible solid oxide cells as electricity storage system[J]. Energies,2016,9(8):662. doi: 10.3390/en9080662 [5] WENDEL C H, BRAUN R J. Design and techno-economic analysis of high efficiency reversible solid oxide cell systems for distributed energy storage[J]. Applied Energy,2016,172:118-131. doi: 10.1016/j.apenergy.2016.03.054 [6] AI DAROUKH M, TIETZ F, SEBOLD D, et al. Post-test analysis of electrode-supported solid oxide electrolyser cells[J]. Ionics,2015,21(4):1039-1043. doi: 10.1007/s11581-014-1273-2 [7] THE D, GRIESHAMMER S, SCHROEDER M, et al. Microstructural comparison of solid oxide electrolyser cells operated for 6100 h and 9000 h[J]. Journal of Power Sources,2015,275:901-911. doi: 10.1016/j.jpowsour.2014.10.188 [8] KIM J, JI H I, DASARI H P, et al. Degradation mechanism of electrolyte and air electrode in solid oxide electrolysis cells operating at high polarization[J]. International Journal of Hydrogen Energy,2013,38(3):1225-1235. doi: 10.1016/j.ijhydene.2012.10.113 [9] CHEN K. Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells[J]. International Journal of Hydrogen Energy,2011,36(17):10541-10549. doi: 10.1016/j.ijhydene.2011.05.103 [10] CHEN K, HYODO J, AI N, et al. Boron deposition and poisoning of La0.8Sr0.2MnO3 oxygen electrodes of solid oxide electrolysis cells under accelerated operation conditions[J]. International Journal of Hydrogen Energy,2016,41(3):1419-1431. doi: 10.1016/j.ijhydene.2015.11.013 [11] WEI B, CHEN K, ZHAO L, et al. Chromium deposition and poisoning at La0.6Sr0.4Co0.2Fe0.8O3-δ oxygen electrodes of solid oxide electrolysis cells[J]. Physical Chemistry Che-mistry Physics,2015,17(3):1601-1609. doi: 10.1039/C4CP05110F [12] HEIDARI D, JAVADPOUR S, CHAN S H. Optimization of BSCF-SDC composite air electrode for intermediate temperature solid oxide electrolyzer cell[J]. Energy Conversion and Management,2017,136:78-84. doi: 10.1016/j.enconman.2017.01.007 [13] BI J, YANG S, ZHONG S, et al. An insight into the effects of B-site transition metals on the activity, activation effect and stability of perovskite oxygen electrodes for solid oxide electrolysis cells[J]. Journal of Power Sources,2017,363:470-479. doi: 10.1016/j.jpowsour.2017.07.118 [14] IM H N, JEON S Y, LIM D K. et al. Steam/CO2 Co-electrolysis performance of reversible solid oxide cell with La0.6Sr0.4Co0.2Fe0.8O3-δ-Gd0.1Ce0.9O2-δ oxygen electrode[J]. Journal of the Electrochemical Society,2014,162(1):54-59. [15] ZUO X, CHEN Z, GUAN C, et al. Molten salt synthesis of high-performance, nanostructured La0.6Sr0.4FeO3-δ oxygen electrode of a reversible solid oxide cell[J]. Materials,2020,13(10):2267. doi: 10.3390/ma13102267 [16] ZHANG L, LIU M, HUANG J, et al. Improved thermal expansion and electrochemical performances of Ba0.6Sr0.4Co0.9Nb0.1O3−δ–Gd0.1Ce0.9O1.95 composite cathodes for IT-SOFCs[J]. international Journal of Hydrogen Energy,2014,39(15):7972-7979. doi: 10.1016/j.ijhydene.2014.03.055 [17] CARPANESE M P, CLEMATIS D, BRETEI A, et al. Understanding the electrochemical behaviour of LSM-based SOFC cathodes. Part I—Experimental and electrochemical[J]. Solid State Ionics,2017,301:106-115. doi: 10.1016/j.ssi.2017.01.007 [18] MOGENSEN M B. Materials for reversible solid oxide cells[J]. Current Opinion in Electrochemistry,2020,21:265-273. doi: 10.1016/j.coelec.2020.03.014 [19] YUN B H, KIM K J, JOH D W, et al. Highly active and durable double-doped bismuth oxide-based oxygen electrodes for reversible solid oxide cells at reduced tempera-tures[J]. Journal of Materials Chemistry A,2019,7(36):20558-20566. doi: 10.1039/C9TA09203J [20] SHIMADA H, FUJIMAKI Y, FUJISHIRO Y. Highly active and durable La0.4Sr0.6MnO3−δ and Ce0.8Gd0.2O1.9 nanocompo-site electrode for high-temperature reversible solid oxide electrochemical cells[J]. Ceramics International,2020,46(11):19617-19623. doi: 10.1016/j.ceramint.2020.05.030 [21] KIM J H, SONG R H, KIM J H, et al. Co-synthesis of nano-sized LSM-YSZ composites with enhanced electrochemical property[J]. Journal of Solid State Electrochemistry,2007,11(10):1385-1390. doi: 10.1007/s10008-007-0317-1 [22] TAN Y, GAO S, XIONG C Y, et al. Nano-structured LSM-YSZ refined with PdO/ZrO2 oxygen electrode for intermediate temperature reversible solid oxide cells[J]. International Journal of Hydrogen Energy,2020,45(38):19823-19830. doi: 10.1016/j.ijhydene.2020.05.116 [23] ZHANG S L, WANG H, LU M Y, et al. Electrochemical performance and stability of SrTi0.3Fe0.6Co0.1O3-δ infiltrated La0.8Sr0.2MnO3Zr0.92Y0.16O2-δ oxygen electrodes for intermediate-temperature solid oxide electrochemical cells[J]. Journal of Power Sources,2019,426:233-241. doi: 10.1016/j.jpowsour.2019.04.044 [24] AKBARI Z, BABAEI A. Electrochemical performance of La0.8Sr0.2MnO3 oxygen electrode promoted by Ruddlesden-popper structured La2NiO4[J]. Journal of the American Ceramic Society,2019,103(2):1332-1342. [25] SHAHROKHI S, BABAEI A, ZAMANI C. Electrochemical performance and stability of LNC-infiltrated (La, Sr) MnO3 oxygen electrode/AIP conference proceedings[J]. AIP Publishing LLC,2018,1920(1):020020. [26] FAN H, HAN M. Electrochemical performance and stabi-lity of Sr-doped LaMnO3-infiltrated yttria stabilized zirconia oxygen electrode for reversible solid oxide fuel cells[J]. International Journal of Coal Science & Technology,2014,1(1):56-61. [27] CHEN K, AI N. Enhanced electrochemical performance and stability of (La, Sr)MnO3-(Gd, Ce)O2 oxygen electrodes of solid oxide electrolysis cells by palladium infiltration[J]. International Journal of Hydrogen Energy,2012,37(2):1301-1310. doi: 10.1016/j.ijhydene.2011.10.015 [28] DING D, GONG M, XU C, et al. Electrochemical characteristics of samaria-doped ceria infiltrated strontium-doped LaMnO3 cathodes with varied thickness for yttria-stabi-lized zirconia electrolytes[J]. Journal of Power Sources,2011,196(5):2551-2557. doi: 10.1016/j.jpowsour.2010.11.007 [29] REMBELSK D, VIRICELLE J P, COMBEMALE L, et al. Characterization and comparison of different cathode materials for SC-SOFC: LSM, BSCF, SSC, and LSCF[J]. Fuel Cells,2012,12(2):256-264. doi: 10.1002/fuce.201100064 [30] FAN H, HAN M. Electrochemical stability of Sm0.5Sr0.5CoO3−δ-infiltrated YSZ for solid oxide fuel cells/electrolysis cells[J]. Faraday Discussions,2015,182:477-491. doi: 10.1039/C5FD00022J [31] WANG Y, YANG Z, HAN M, et al. Optimization of Sm0.5Sr0.5CoO3−δ-infiltrated YSZ electrodes for solid oxide fuel cell/electrolysis cell[J]. RSC Advances,2016,6(113):112253-112259. doi: 10.1039/C6RA21200J [32] LU C, SHOLKLAPPER T Z, JACOBSON C P, et al. LSM-YSZ cathodes with reaction-infiltrated nanoparticles[J]. Jour-nal of the Electrochemical Society,2006,153(6):A1115-A1119. doi: 10.1149/1.2192733 [33] LEE S, KIM J, SON J W, et al. High performance air electrode for solid oxide regenerative fuel cells fabricated by infiltration of nano-catalysts[J]. Journal of Power Sources,2014,250:15-20. doi: 10.1016/j.jpowsour.2013.10.123 [34] YOON K J, BISWAS M, KIM H J, et al. Nano-tailoring of infiltrated catalysts for high-temperature solid oxide regenerative fuel cells[J]. Nano Energy,2017,36:9-20. doi: 10.1016/j.nanoen.2017.04.024 [35] MEN H J, TIAN N, QU Y M, et al. Improved performance of a lanthanum strontium manganite-based oxygen electrode for an intermediate-temperature solid oxide electrolysis cell realized via ionic conduction enhancement[J]. Ceramics International,2019,45(6):7945-7949. doi: 10.1016/j.ceramint.2019.01.107 [36] SHIMURA K, NISHINO H, KAKINUMA K, et al. Effect of samaria-doped ceria (SDC) interlayer on the performance of La0.6Sr0.4Co0.2Fe0.8O3-δ/SDC composite oxygen electrode for reversible solid oxide fuel cells[J]. Electrochimica Acta,2017,225:114-120. doi: 10.1016/j.electacta.2016.12.100 [37] LEE T H, FAN L, YU C C, et al. A high-performance SDC-infiltrated nanoporous silver cathode with superior thermal stability for low temperature solid oxide fuel cells[J]. Journal of Materials Chemistry A,2018,6(17):7357-7363. doi: 10.1039/C8TA01104D [38] YOU R W, OUYANG J, FU Y P, et al. Characterization of Ce0.8Sm0.2O2−δ-infiltrated La0.8Ca0.2CoO3−δ cathode for solid oxide fuel cells[J]. Ceramics International,2013,39(7):8411-8419. doi: 10.1016/j.ceramint.2013.04.022 [39] BRITO M E, MORISHITA H, YAMADA J, et al. Further improvement in performances of La0.6Sr0.4Co0.2Fe0.8O3-δ ceria composite oxygen electrodes with infiltrated doped ceria nanoparticles for reversible solid oxide cells[J]. Journal of Power Sources,2019,427:293-298. doi: 10.1016/j.jpowsour.2019.04.066 [40] JIANG W, LV Z, WEI B, et al. Sm0.5Sr0.5CoO3–Sm0.2Ce0.8O1.9 composite oxygen electrodes for solid oxide electrolysis cells[J]. Fuel Cells,2014,14(1):76-82. doi: 10.1002/fuce.201300091 [41] JIANG W, WEI B, LV Z, et al. Co-synthesis of Sm0.5Sr0.5CoO3-Sm0.2Ce0.8O1.9 composite cathode with enhanced electrochemical property for intermediate temperature SOFCs[J]. Fuel Cells,2014,14(6):966-972. doi: 10.1002/fuce.201400022 [42] ZHANG Y, HAN M, SUN Z. High performance and stability of nanocomposite oxygen electrode for solid oxide cells[J]. International Journal of Hydrogen Energy, 2020, 45(8): 5554-5564. [43] HANIFI A R, LAGUNA-BERCERO M A, ETSELL T H, et al. The effect of electrode infiltration on the performance of tubular solid oxide fuel cells under electrolysis and fuel cell modes[J]. International Journal of Hydrogen Energy,2014,39(15):8002-8008. doi: 10.1016/j.ijhydene.2014.03.071 [44] IRVINE J T S, NEAGU D, VERBRAEHEN M C, et al. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers[J]. Nature Energy,2016,1:15014. [45] JIANG S P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges[J]. International Journal of Hydrogen Energy,2012,37(1):449-470. doi: 10.1016/j.ijhydene.2011.09.067 -






 下载:
下载: