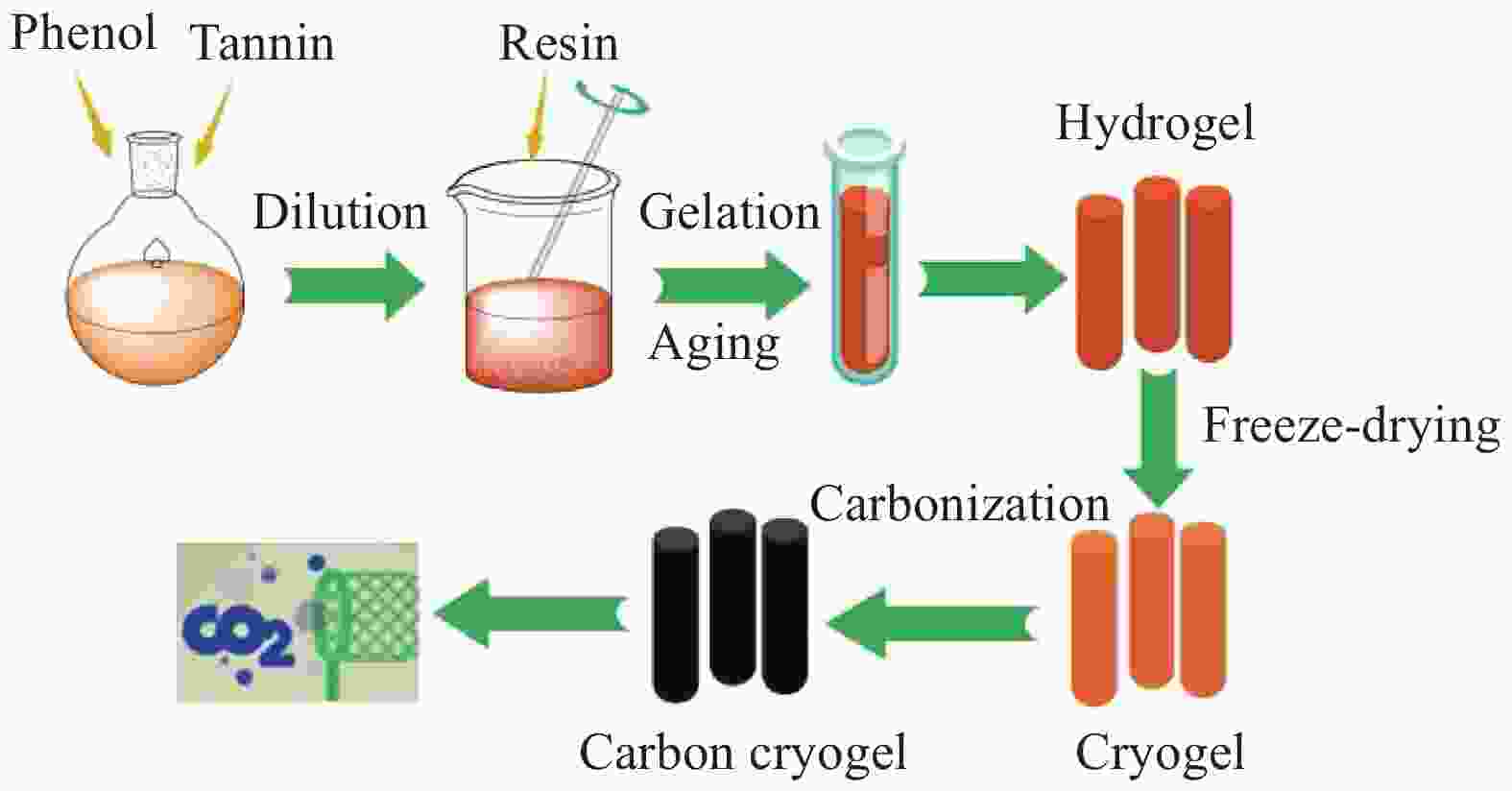
Preparation and CO2 adsorption properties of tannin modified phenolic based carbon cryogels
-
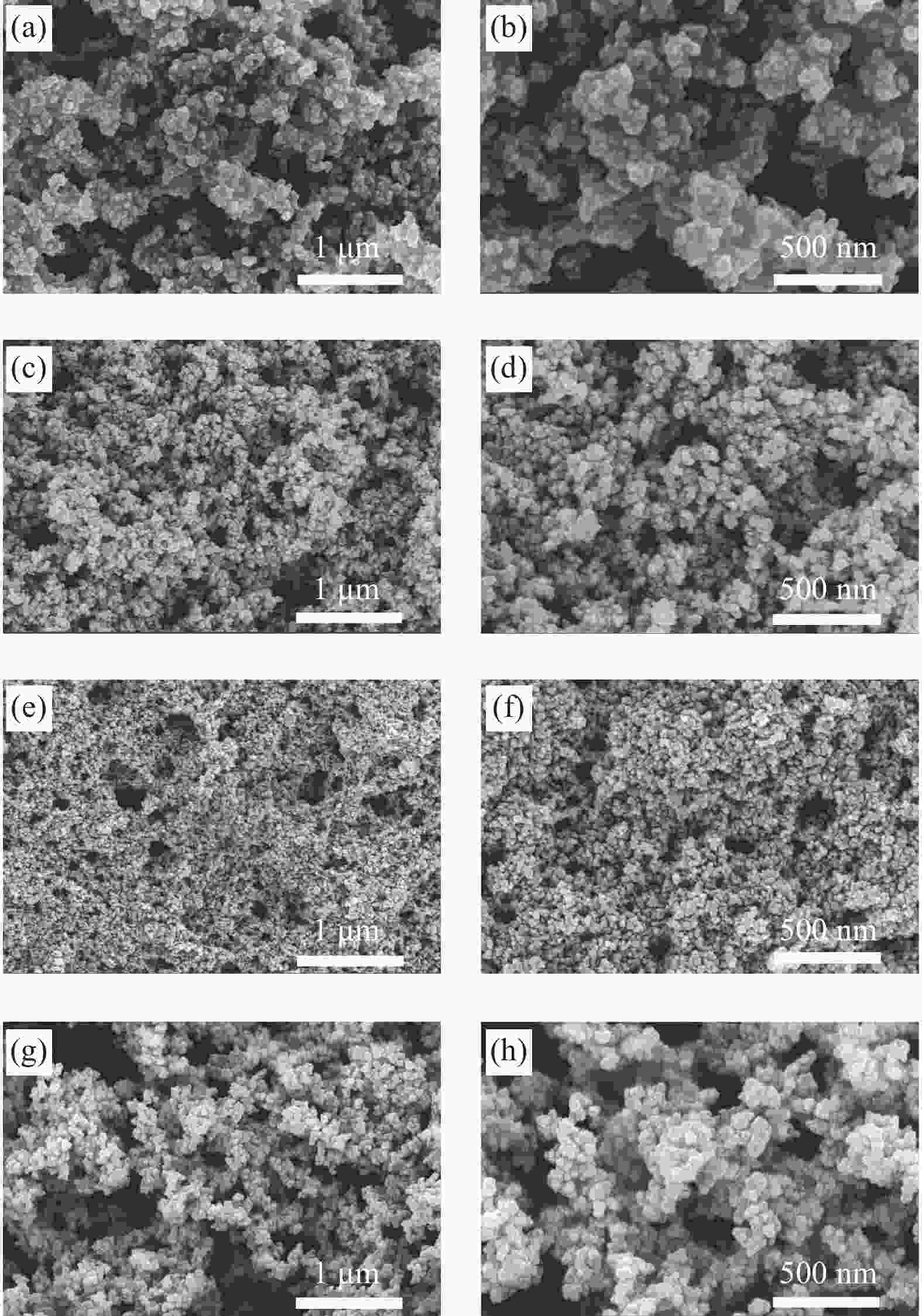
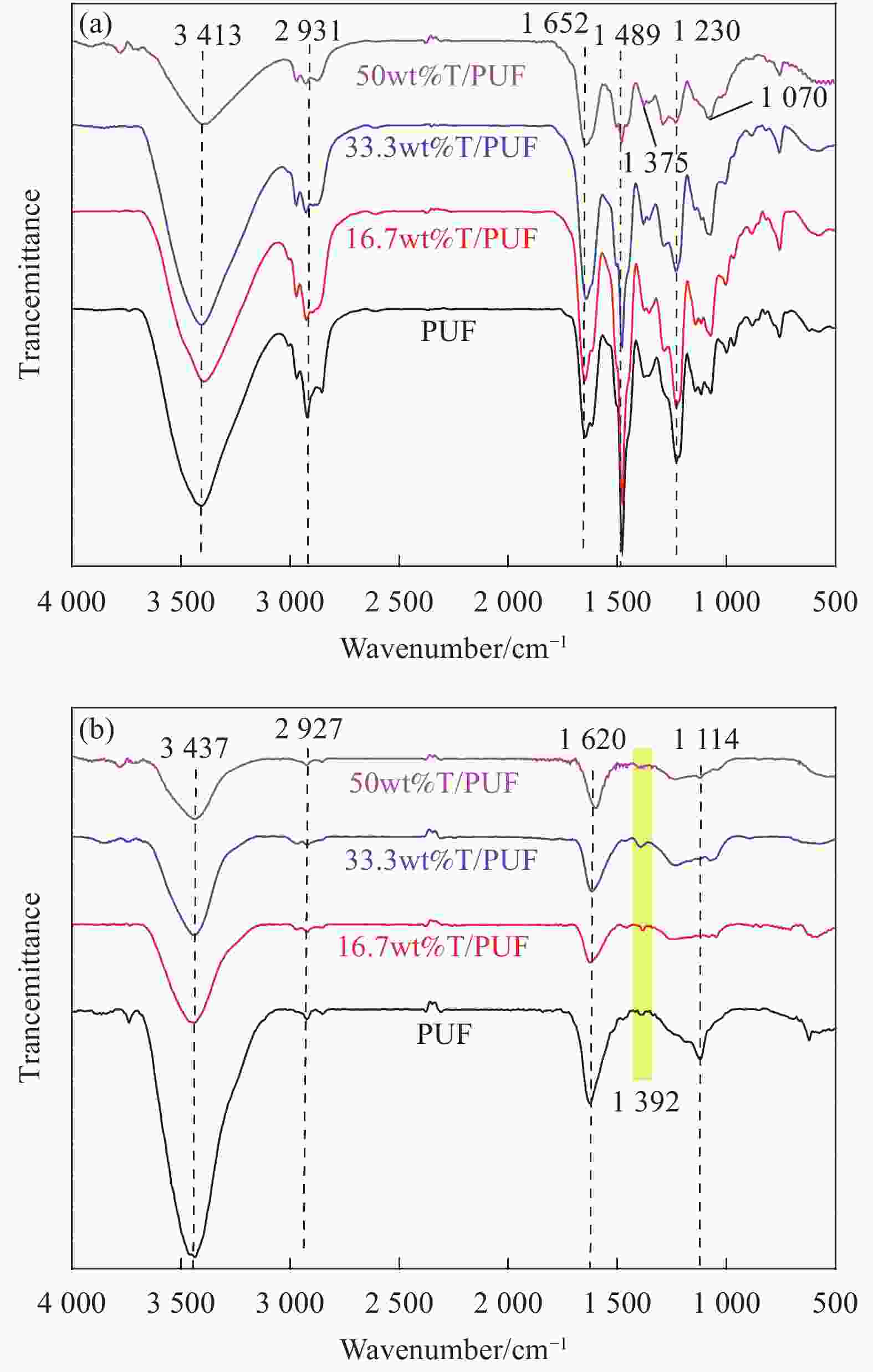
摘要: 基于绿色低成本的单宁所具有的大量反应性羟基,其与醛类反应具有与苯酚或间苯二酚相似的机制。在传统的酚醛树脂基(苯酚-尿素-甲醛)炭气凝胶的基础上,通过添加单宁进行改性,成功制备出新型高效的CO2吸附用酚醛基炭气凝胶。通过扫描电子显微镜(SEM)、傅里叶红外光谱(FTIR)和氮气吸脱附测试对其表面化学和孔隙结构进行了系统表征,同时通过CO2吸脱附测试对其CO2吸附量、选择性吸附及吸附热等进行了研究。结果表明:以绿色可再生的生物质原料单宁对原料进行部分取代,不仅可以显著降低产品成本,还可以明显改善其CO2的吸附性能。当单宁的添加量(15 g)为苯酚用量的50wt%时,样品具有最大的比表面积(1376.31 m2·g−1)和微孔体积(0.55 cm3·g−1),是一种极具潜力的气体吸附材料。其相应的CO2吸附量高达5.36 mmol·g−1,选择性吸附和吸附热则分别为16.84和34.49 kJ·mol−1,性能较未改性的酚醛基炭气凝胶显著改善,同时也优于大部分传统的炭气凝胶材料,这主要归因于其具有较高的比表面积、微孔体积、适宜的孔径分布和良好的三维网络结构。Abstract: Based on the large number of reactive hydroxyl of green and low cost of tannin, its hold the similar mechanism as phenol and resorcinol reacted with formaldehyde. On the basis of carbon cryogels from traditional phenolic resin (phenol-urea-formaldehyde), a new type of carbon cryogels for efficient CO2 capture were successfully prepared by tannin modification. The surface chemistry and pore structure of carbon aerogel were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and nitrogen adsorption and desorption analysis. The adsorption capacity, selectivity and adsorption heat of carbon cryogel were studied by carbon dioxide adsorption and desorption analysis. The results show that the new and efficient phenolic carbon cryogel can be prepared by using green and renewable biomass raw material tannin to partially replace the traditional phenol or resorcinol, which can not only significantly reduce the product cost, but also significantly improve the carbon dioxide adsorption performance. When the addition amount of tannin (15 g) is 50wt% of that of phenol, the sample has the maximum specific surface area (1376.31 m2·g−1) and micropore volume (0.55 cm3·g−1), which is a potential gas adsorption material. The corresponding CO2 adsorption capacity is as high as 5.36 mmol·g−1, and the selective adsorption and adsorption heat are 16.84 and 34.49 kJ·mol−1, respectively. The properties of phenolic carbon aerogels are significantly better than those of unmodified phenolic carbon aerogels, and also better than most of the traditional carbon aerogels. This is mainly attributed to its high specific surface area, micropore volume, suitable pore size distribution and good 3D network structure.
-
Key words:
- tannin /
- phenolic resin /
- carbon cryogels /
- carbon dioxide /
- adsorption /
- nanostructure
-
图 6 0℃下炭气凝胶的CO2吸附等温线(a),计算CO2/N2选择性时压力范围小于20 kPa时CO2和N2吸附的初始斜率(b)和不同吸附量下炭气凝胶的等量吸附热(c)
Figure 6. CO2 adsorption isotherms of carbon cryogels at 0℃ (a), initial slope from CO2 and N2 adsorption in the pressure range of less than 20 kPa for CO2/N2 selectivity calculation (b) and isosteric adsorption heat of carbon cryogels at various adsorption quantities (c)
表 1 单宁改性酚醛基炭气凝胶的配方设计
Table 1. Formulation design of tannin modified phenolic based carbon cryogels
Sample Tannin/g Phenol/g Urea/g PUF 0 30 7.5 16.7wt%T/PUF 5 25 7.5 33.3wt%T/PUF 10 20 7.5 50wt%T/PUF 15 15 7.5 Notes: T—Tannin; PUF—Phenolic carbon aerogel. 表 2 炭气凝胶的孔隙结构参数
Table 2. Pore structure parameters of carbon cryogels
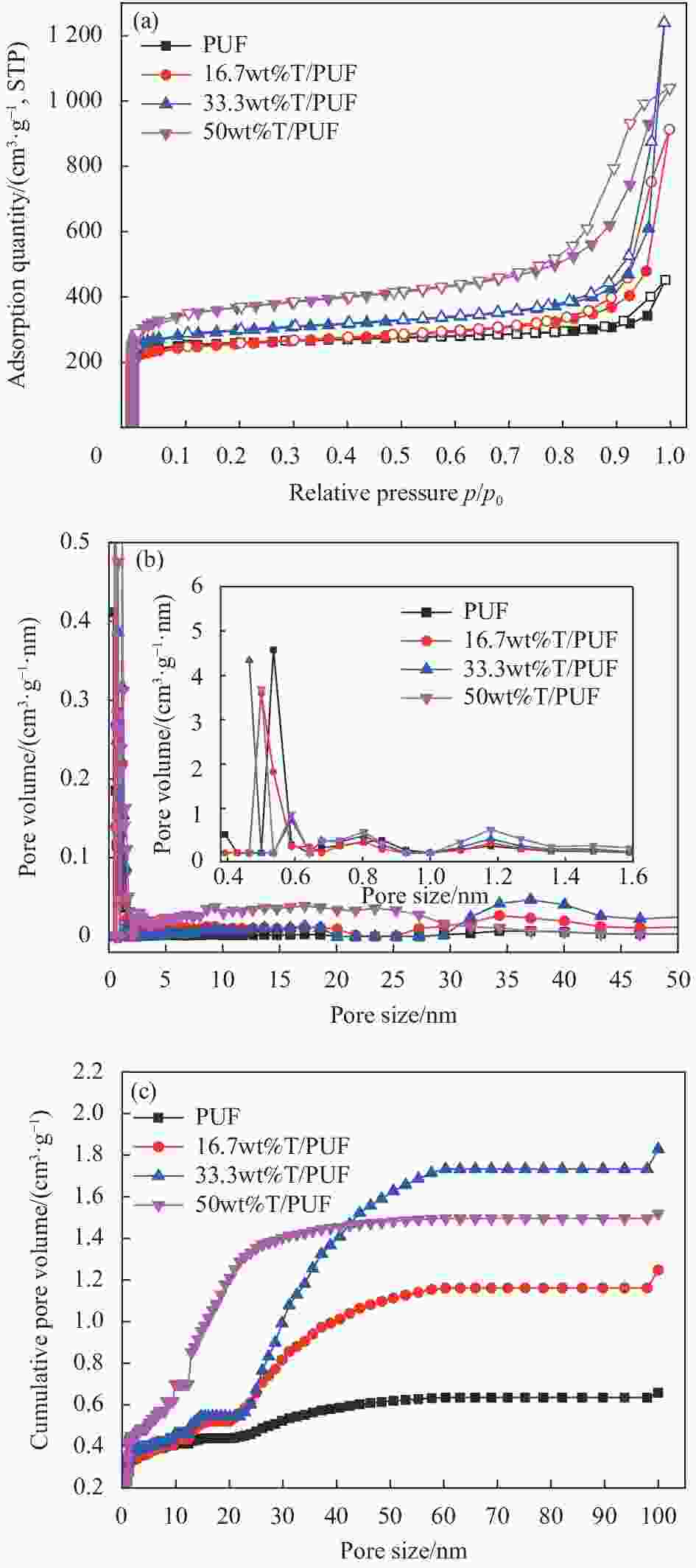
Sample SBET/(m2·g−1) V0.99/(cm3·g−1) VDR/(cm3·g−1) L0/nm VDR/V0.99 V0.99−VDR/(cm3·g−1) PUF 998.95 0.70 0.40 0.64 0.57 0.30 16.7wt%T/PUF 969.84 1.41 0.39 0.68 0.28 1.02 33.3wt%T/PUF 1127.64 1.92 0.45 0.81 0.23 1.47 50wt%T/PUF 1376.31 1.61 0.55 1.14 0.34 1.06 Notes: SBET—Specific surface area; V0.99—Total pore volume; VDR—Micropore volume; L0—Average micropore diameter. 表 3 炭气凝胶的元素相对含量
Table 3. Relative element content of carbon cryogels
Sample Relative element content /at% C N O 16.7wt%T/PUF 93.77 1.39 4.84 33.3wt%T/PUF 91.80 2.10 6.10 50wt%T/PUF 89.44 2.01 8.55 PUF 90.48 2.37 7.15 表 4 不同元素C、N、O的占比
Table 4. Ratio of various C, N, O elements
C/at% N/at% O/at% C1 C2 C3 C4 C5 N1 N2 N3 N4 O1 O2 O3 O4 O5 Binding
energy/eV284.6
±0.1285.6
±0.1287.1
±0.1289.2
±0.1290.1
±0.1398.5
±0.1400.4
±0.2401.4
±0.1403.4
±0.2530.9
±0.5532.5
±0.8534.0
±0.7536.0
±0.3537.9
±0.116.7wt%T/PUF 62.0 7.5 15.6 7.9 7.0 28.5 25.6 25.3 20.6 13.4 56.8 15.3 8.6 5.9 33.3wt%T/PUF 60.0 7.6 16.4 7.9 8.1 25.5 24.6 19.2 30.7 18.6 28.0 36.5 14.9 2.0 50wt%T/PUF 62.3 8.0 17.2 7.7 4.8 17.3 23.4 24.8 34.5 8.0 37.9 40.0 10.5 3.6 PUF 59.4 17.6 11.9 5.8 5.3 27.6 27.3 11.1 34.0 24.8 49.9 17.5 3.3 4.5 表 5 炭气凝胶的CO2吸附量及选择性结果
Table 5. CO2 adsorption and selective values from carbon cryogels
CO2 adsorption/
(mmol·g−1,
100 kPa/0℃)k1 k2 CO2/N2
selective values16.7wt%T/PUF 4.96 13.431 0.996 13.48 33.3wt%T/PUF 5.30 14.144 1.059 13.36 50wt%T/PUF 5.36 12.226 0.726 16.84 PUF 4.61 12.274 1.055 11.63 Note: k1 and k2—Initial slope of the CO2 and N2 isotherms, respectively. -
[1] XING W, LIU C, ZHOU Z, et al. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction[J]. Energy & Environmental Science,2012,5(6):7323-7327. doi: 10.1039/c2ee21653a [2] LIM G, LEE K B, HAM H C. Effect of N-containing functional groups on CO2 adsorption of carbonaceous materials: A density functional theory approach[J]. The Journal of Physical Chemistry C,2016,120(15):8087-8095. doi: 10.1021/acs.jpcc.5b12090 [3] SU F, LU C. CO2 capture from gas stream by zeolite 13X using a dual-column temperature/vacuum swing adsorption[J]. Energy & Environmental Science,2012,5(10):9021-9027. doi: 10.1039/c2ee22647b [4] HAN J, ZHANG L, ZHAO B, et al. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption[J]. Industrial Crops and Products,2019,128:290-297. doi: 10.1016/j.indcrop.2018.11.028 [5] KHAN I U, OTHMAN M H D, ISMAIL A F, et al. Structural transition from two-dimensional ZIF-L to three-dimensional ZIF-8 nanoparticles in aqueous room temperature synthesis with improved CO2 adsorption[J]. Materials Characterization,2018,136:407-416. doi: 10.1016/j.matchar.2018.01.003 [6] ELLO A S, YAPO J A, TROKOUREY A. N-doped carbon aerogels for carbon dioxide (CO2) capture[J]. African Journal of Pure and Applied Chemistry,2013,7(2):61-66. [7] MARQUES L M, CARROTT P J M, CARROTT M M L R. Carbon aerogels used in carbon dioxide capture[J]. Boletín del Grupo Españ ol del Carbón,2016(40):9-12. [8] LIU Q, HAN Y, QIAN X, et al. CO2 adsorption over carbon aerogels the effect of pore and surface properties[J]. Chemistry Select,2019,4(11):3161-3168. [9] KESHAVARZ L, GHAANIM R, MACELROY J M D, et al. A comprehensive review on the application of aerogels in CO2-adsorption: Materials and characterisation[J]. Chemical Engineering Journal,2021,412:128604. doi: 10.1016/j.cej.2021.128604 [10] LI Z, CHEN T, WU X, et al. Nitrogen-containing high surface area carbon cryogel from co-condensed phenol-urea-formaldehyde resin for CO2 capture[J]. Journal of Porous Materials,2018,26(3):847-854. [11] SUN M, BU Y, FENG J, et al. A melamine-formaldehyde-resorcinol aerogel as the sorbent of in-tube solid-phase microextraction[J]. Microchemical Journal,2020,159:105573. doi: 10.1016/j.microc.2020.105573 [12] 李文翠, 郭树才, 朱玉东. 间甲酚甲醛气凝胶炭化工艺的研究[J]. 炭素技术, 2000(1):9-11. doi: 10.3969/j.issn.1001-3741.2000.01.003LI Wencui, GUO Shucai, ZHU Yudong. An investigation on carbonizing process of M-cresol and formaldehyde aerogels[J]. Carbon Techniques,2000(1):9-11(in Chinese). doi: 10.3969/j.issn.1001-3741.2000.01.003 [13] 张倩, 禹筱元, 麦嘉雯, 等. 木质素酚醛基炭气凝胶的制备及电化学性能[J]. 高分子材料科学与工程, 2013, 29(4):152-154.ZHANG Qian, YU Xiaoyuan, MAI Jiawen, et al. Preparation and electrochemical performance of lignin-phenoic carbon aerogels[J]. Polymer Materials Science & Engineering,2013,29(4):152-154(in Chinese). [14] SZCZUREK A, AMARAL-LABAT G, FIERRO V, et al. The use of tannin to prepare carbon gels. Part I: Carbon aerogels[J]. Carbon,2011,49(8):2773-2784. doi: 10.1016/j.carbon.2011.03.007 [15] SZCZUREK A, AMARAL-LABAT G, FIERRO V, et al. The use of tannin to prepare carbon gels:Part II—Carbon cryogels[J]. Carbon,2011,49(8):2785-2794. doi: 10.1016/j.carbon.2011.03.005 [16] AMARAL-LABAT G, GRISHECHKO L I, FIERRO V, et al. Tannin-based xerogels with distinctive porous structures[J]. Biomass and Bioenergy,2013,56:437-445. doi: 10.1016/j.biombioe.2013.06.001 [17] KIM Y H, OGATA T, NAKANO Y. Kinetic analysis of palladium(II) adsorption process on condensed-tannin gel based on redox reaction models[J]. Water Research,2007,41(14):3043-3050. doi: 10.1016/j.watres.2007.02.016 [18] NAKANO Y, TAKESHITA K, TSUTSUMI T. Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel[J]. Water Research,2001,35(2):496-500. doi: 10.1016/S0043-1354(00)00279-7 [19] AMARAL-LABAT G, GRISHECHKO L I, FIERRO V, et al. Unique bimodal carbon xerogels from soft templating of tannin[J]. Materials Chemistry and Physics,2015,149-150:193-201. doi: 10.1016/j.matchemphys.2014.10.006 [20] ROBERTSON C, MOKAYA R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage[J]. Microporous and Mesoporous Materials,2013,179:151-156. doi: 10.1016/j.micromeso.2013.05.025 [21] ADIO S O, GANIYU S A, USMAN M, et al. Facile and efficient nitrogen modified porous carbon derived from sugarcane bagasse for CO2 capture: Experimental and DFT investigation of nitrogen atoms on carbon frameworks[J]. Chemical Engineering Journal,2020,382:122964. doi: 10.1016/j.cej.2019.122964 [22] LI Z L, ZHOU Y L, YAN W, et al. Cost-effective monolithic hierarchical carbon cryogels with nitrogen doping and high-performance mechanical properties for CO2 capture[J]. ACS Applied Materials & Interfaces,2020,12(19):21748-21760. doi: 10.1021/acsami.0c04015 [23] HAO G, LI W, QIAN D, et al. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture[J]. Advanced Materials,2010,22(7):853-857. doi: 10.1002/adma.200903765 [24] FAN X, ZHANG L, ZHANG G, et al. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture[J]. Carbon,2013,61:423-430. doi: 10.1016/j.carbon.2013.05.026 [25] RASINES G, LAVELA P, MACÍAS C, et al. N-doped monolithic carbon aerogel electrodes with optimized features for the electrosorption of ions[J]. Carbon,2015,83:262-274. doi: 10.1016/j.carbon.2014.11.015 [26] LI Y, ZOU B, HU C, et al. Nitrogen-doped porous carbon nanofiber webs for efficient CO2 capture and conversion[J]. Carbon,2016,99:79-89. doi: 10.1016/j.carbon.2015.11.074 [27] XU Y, YANG Z, ZHANG G, et al. Excellent CO2 adsorption performance of nitrogen-doped waste biocarbon prepared with different activators[J]. Journal of Cleaner Production,2020,264:121645. doi: 10.1016/j.jclepro.2020.121645 [28] LIU S, RAO L, YANG P, et al. Superior CO2 uptake on nitrogen doped carbonaceous adsorbents from commercial phenolic resin[J]. Journal of Environmental Sciences,2020,93:109-116. doi: 10.1016/j.jes.2020.04.006 [29] ZHANG W, BAO Y, BAO A. Preparation of nitrogen-doped hierarchical porous carbon materials by a template-free method and application to CO2 capture[J]. Journal of Environmental Chemical Engineering,2020,8(3):103732. doi: 10.1016/j.jece.2020.103732 [30] YUAN X, LI S, JEON S, et al. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis[J]. Journal of Hazardous Materials,2020,399:123010. doi: 10.1016/j.jhazmat.2020.123010 [31] MA X, LI L, ZENG Z, et al. Experimental and theoretical demonstration of the relative effects of O-doping and N-doping in porous carbons for CO2 capture[J]. Applied Surface Science,2019,481:1139-1147. doi: 10.1016/j.apsusc.2019.03.162 [32] RAO L, LIU S, WANG L, et al. N-doped porous carbons from low-temperature and single-step sodium amide activation of carbonized water chestnut shell with excellent CO2 capture performance[J]. Chemical Engineering Journal,2019,359:428-435. doi: 10.1016/j.cej.2018.11.065 [33] WANG Z, GOYAL N, LIU L, et al. N-doped porous carbon derived from polypyrrole for CO2 capture from humid flue gases[J]. Chemical Engineering Journal,2020,396:125376. doi: 10.1016/j.cej.2020.125376 [34] RAO L, MA R, LIU S, et al. Nitrogen enriched porous carbons from D-glucose with excellent CO2 capture performance[J]. Chemical Engineering Journal,2019,362:794-801. doi: 10.1016/j.cej.2019.01.093 -





 下载:
下载: