Preparation and in vitro biological properties of porous ZnO-MgO/hydroxyapatite biocomposites
-
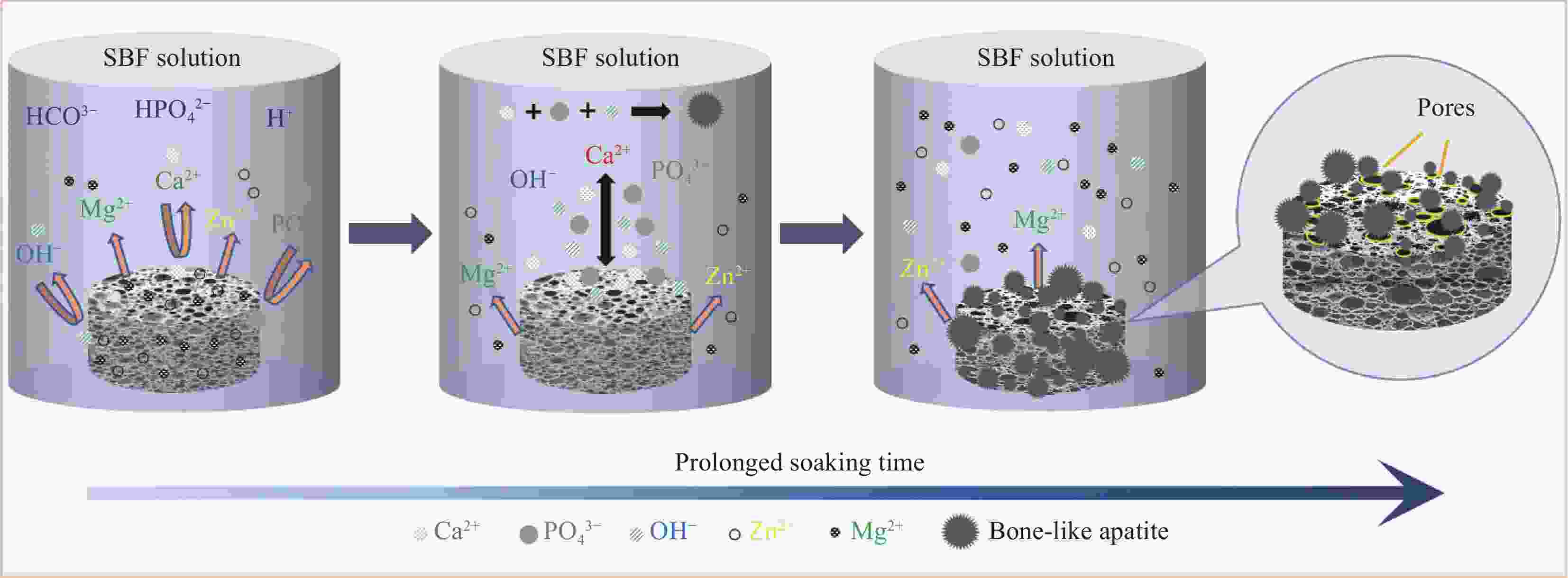
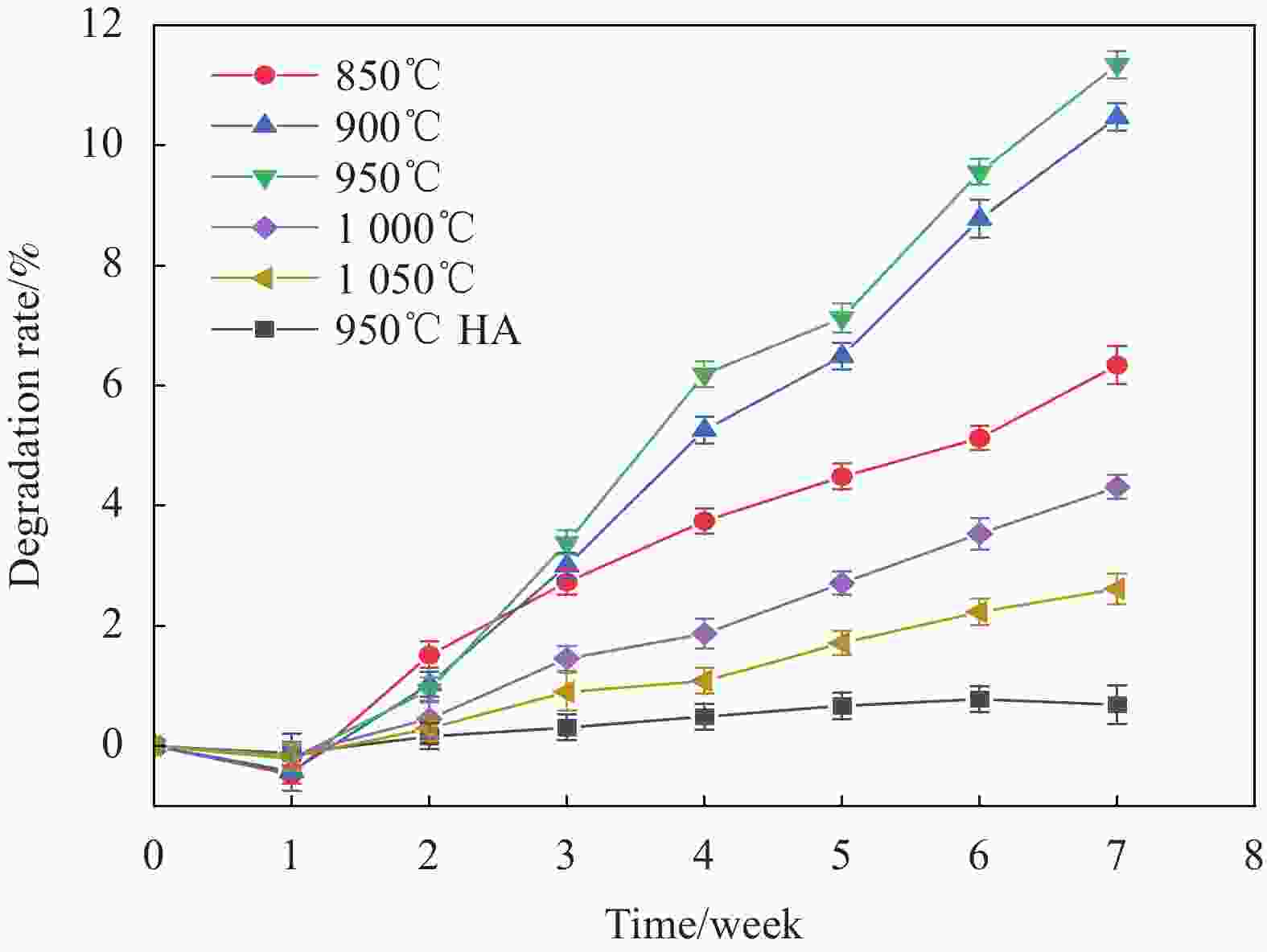
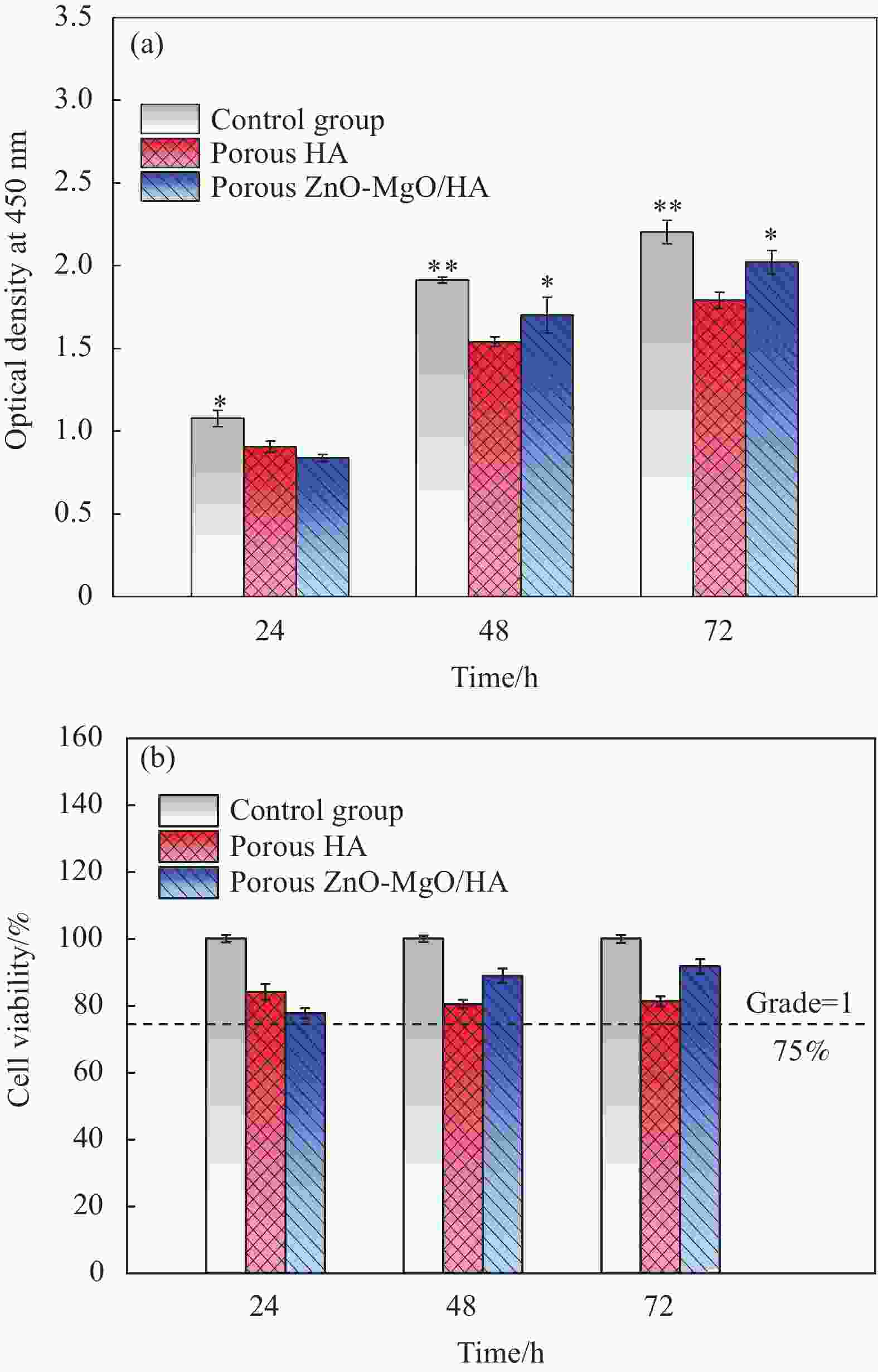
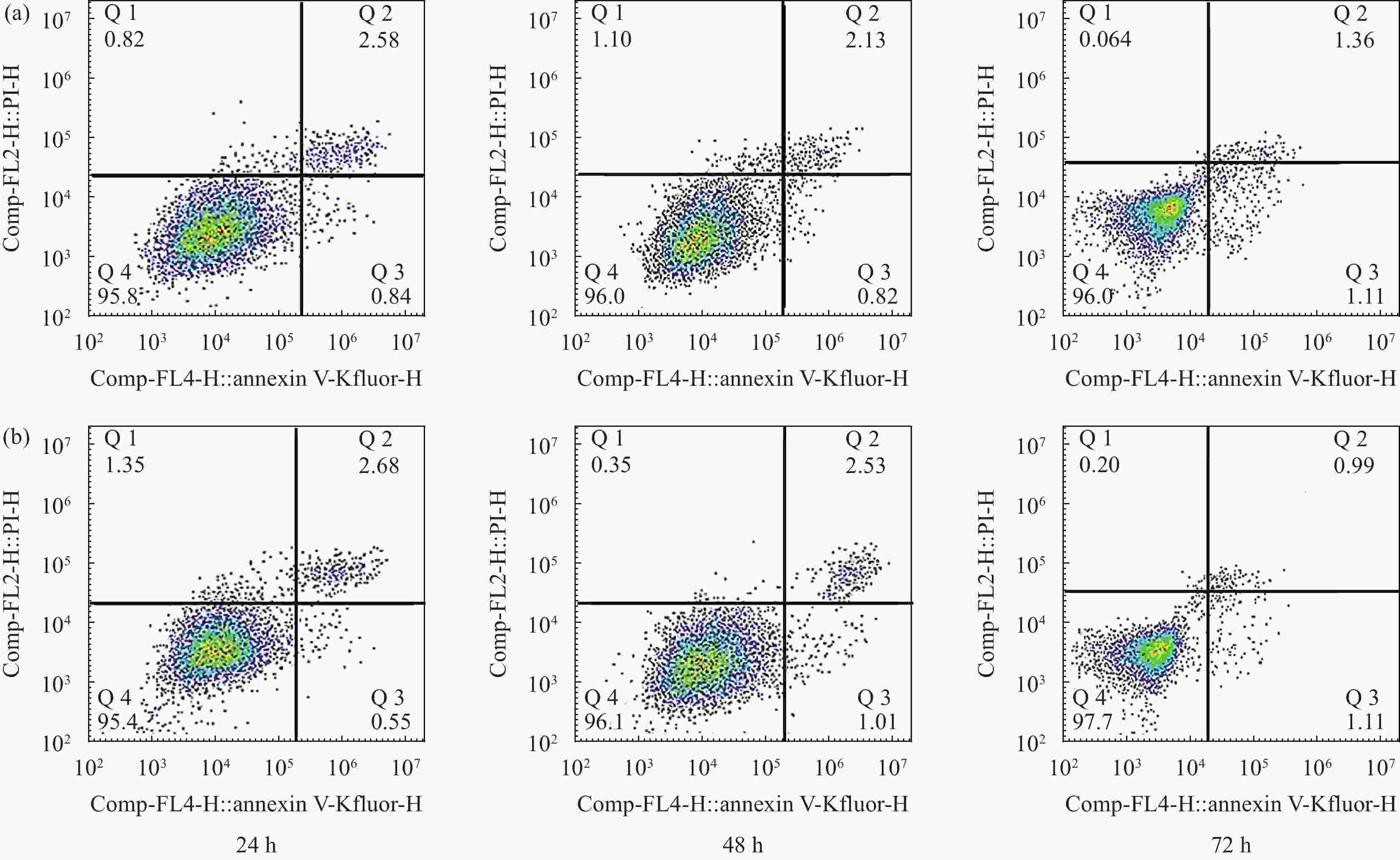
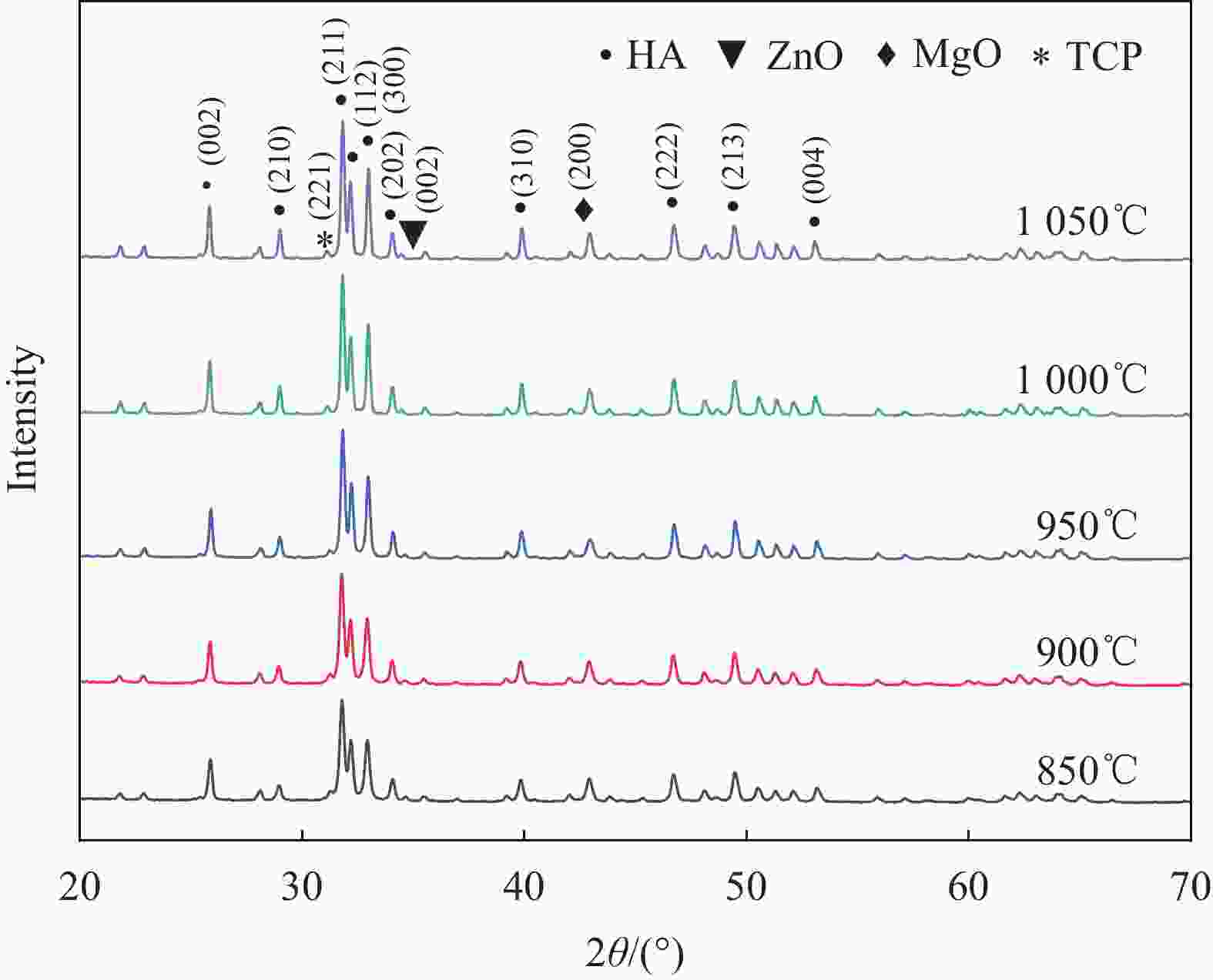
摘要: 为改善多孔羟基磷灰石(HA)生物复合材料的生物活性和成骨诱导能力,本文利用放电等离子烧结(SPS)技术制备了ZnO、MgO质量分数分别为1.3wt%、8.4wt%的多孔ZnO-MgO/HA生物复合材料,研究了不同烧结温度下多孔复合材料微观结构、孔隙特征、体外矿化及降解行为的变化规律并对比分析了活性陶瓷相加入对多孔HA材料体外生物学性能的影响及机制。结果表明:烧结后的多孔复合材料主要由HA相及ZnO、MgO相组成,烧结温度超过950℃后,出现了少量HA分解产物Ca3(PO4)2相;随烧结温度的升高,多孔复合材料孔隙率缓慢下降,孔径尺寸呈逐渐减小的趋势;不同烧结温度下多孔复合材料在模拟体液中均具有良好的类骨磷灰石形成能力,而降解率随温度提高先增大后减小;综合分析,950℃下制备的多孔ZnO-MgO/HA复合材料具有适宜的孔隙特征(孔隙率(34.7±0.2)%,孔径尺寸150~400 μm占比65.5%),同时与多孔HA材料相比,还具有优异的类骨磷灰石形成能力、高的降解率((11.3±0.2)%)和细胞增殖率((91.7±2.1)%)及低的细胞凋亡率((2.3±0.2)%),表明ZnO和MgO活性陶瓷相的加入明显提高了多孔HA材料的成骨诱导能力与生物相容性。Abstract: In order to improve the bioactivity and osteogenic induction ability of porous hydroxyapatite (HA) biocomposites, porous ZnO-MgO/HA biocomposites with the mass fraction of ZnO 1.3wt% and MgO 8.4wt% were prepared by spark plasma sintering (SPS). The changes of microstructure, pore characteristics, in vitro mineralization and degradation behavior of porous composites at different sintering temperatures were studied. The effect of active ceramic phase addition on biological properties of porous HA materials in vitro and its mechanism were analyzed. The results show that the sintered porous composites are mainly composed of HA, ZnO and MgO phase. When the sintering temperature exceeds 950℃, a small amount of HA decomposition product Ca3(PO4)2 phase appears. With the increase of sintering temperature, the porosity of porous composites decreases slowly, and the pore size decreases gradually. The porous composites have good osteoapatite formation ability in simulated body fluid at different sintering temperatures, and the degradation rate increases first and then decreases with the increase of sintering temperature. Comprehensive analysis shows that the porous ZnO-MgO/HA composites prepared at 950℃ have suitable pore characteristics (porosity (34.7±0.2)%, pore size between 150-400 μm accounts for 65.5%). Moreover, the porous HA material has excellent osteogenic ability, high degradation rate ((11.3±0.2)%), high cell proliferation rate ((91.7±2.1)%) and low cell apoptosis rate ((2.3±0.2)%), indicating that the addition of ZnO and MgO active ceramic phase significantly improves the osteogenic induction ability and biocompatibility of porous HA material.
-
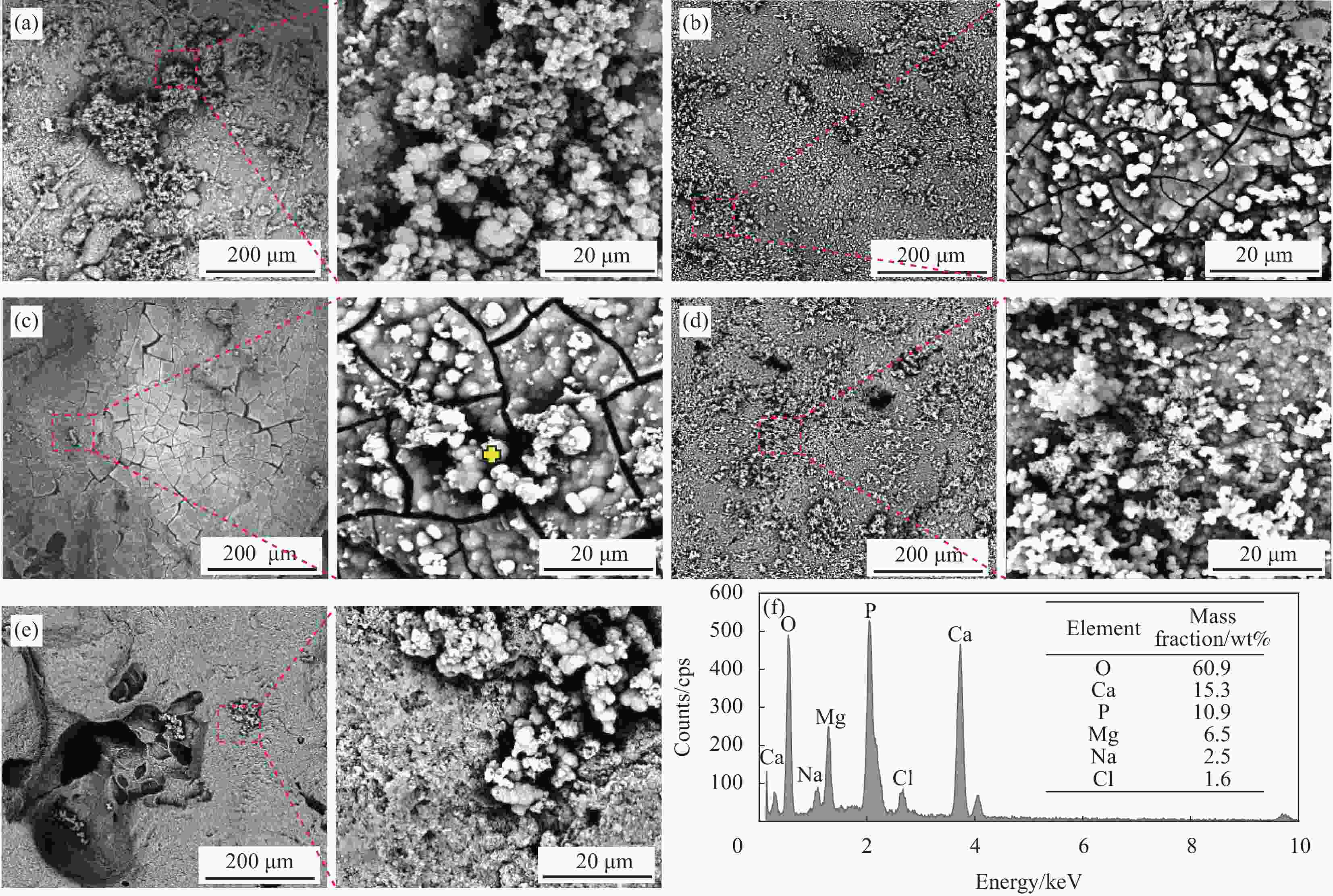
图 3 不同烧结温度下多孔ZnO-MgO/HA复合材料在模拟人工体液溶液中浸泡14天后的表面生物矿化形貌图及EDS点扫描分析图
Figure 3. SEM and EDS point scanning analysis of porous ZnO-MgO/HA composites soaked in simulated artificial body fluid solution for 14 days at different sintering temperatures ((a) 850℃; (b) 900℃ (c); 950℃; (d) 1000℃; (e) 1050℃; (f) EDS point scanning analysis diagram at the marker)
-
[1] 周廉. 中国生物医用材料科学与产业现状及发展战略研究[M]. 北京: 化学工业出版社, 2012.ZHOU Lian. Research on the current situation and development strategy of biomedical materials science and industry in China[M]. Beijing: Chemical Industry Press, 2012(in Chinese). [2] SHAKIR M, JOLLY R, KHAN M S, et al. Nano-hydroxyapatite/chitosan-starch nanocomposite as a novel bone construct: Synthesis and in vitro studies[J]. International Journal of Biological Macromolecules,2015,80:282-292. doi: 10.1016/j.ijbiomac.2015.05.009 [3] NandiNANDI S K, ROY S, MUKHERJEE P, et al. Orthopaedic applications of bone graft & graft substitutes: A review[J]. Indian Journal of Medical Research,2010,132:15-30. [4] AKRAM M, AHMED R, SHAKIR I, et al. Extracting hydroxyapatite and its precursors from natural resources[J]. Journal of Materials Science,2014,49:1461-1475. doi: 10.1007/s10853-013-7864-x [5] RATNAYAKE J T B, MUCALO M, DIAS G J. Substituted hydroxyapatites for bone regeneration: A review of current trends[J]. Journal of Biomedical Materials Research Part B,2017,105B:1285-1299. [6] 侯泽敬, 赵景涛, 李嘉明. 医用钛钼合金的国内外研究概况[J]. 世界有色金属, 2017(10):130-131.HOU Zejing, ZHAO Jingtao, LI Jiaming. Domestic and foreign overview of biomedical porous TiMo alloy[J]. World Nonferrous Metals,2017(10):130-131(in Chinese). [7] 程瑶, 王星星, 汪大林. 硅掺杂纳米羟基磷灰石: 溶解度、抗折和抗压强度解析[J]. 中国组织工程研究, 2016, 20(3):435-440.CHENG Yao, WANG Xingxing, WANG Dalin. Silicon-doped nano-hydroxyapatite: solubility, anti-fracture and compressive strengths[J]. Chinese Journal of Tissue Engineering Research,2016,20(3):435-440(in Chinese). [8] 杨春蓉, 王迎军, 陈晓峰. 纳米羟基磷灰石/胶原/磷酸丝氨酸仿生复合骨组织工程支架材料的制备及表征[J]. 科学通报, 2013, 58(3):267-271. doi: 10.1360/csb2013-58-3-267YANG Chunrong, WANG Yingjun, CHEN Xiaofeng. Preparation and evaluation of biomimetric nano-hydroxyapatite-based composite scaffolds for bone-tissue engineering[J]. Chinese Science Bulletin,2013,58(3):267-271(in Chinese). doi: 10.1360/csb2013-58-3-267 [9] MASTROGIACOMO M, MURAGLIA A, KOMLEV V, et al. Tissue engineering of bone: Search for a better scaffold[J]. Chinese Journal of Tissue Engineering Research,2005,8:277-284. [10] 朱斌, 何远怀, 孟增东, 等. 多孔ZnO/羟基磷灰石生物复合材料的制备与性能[J]. 复合材料学报, 2019, 36(11):2637-2643.ZHU Bin, HE Yuanhuai, MENG Zengdong, et al. Fabrication and properties of porous ZnO/hydroxyapatite biocomposites[J]. Acta Materiae Compositae Sinica,2019,36(11):2637-2643(in Chinese). [11] YANG Y C, CHEN C C, WANG J B, et al. Flame sprayed zinc doped hydroxyapatite coating with antibacterial and biocompatible properties[J]. Ceramic International,2017,43:S829-S835. doi: 10.1016/j.ceramint.2017.05.318 [12] BODHAK S, BOSE S, BANDYOPADHYAY A. Influence of MgO, SrO, and ZnO dopantson electro-thermal polarization behavior and in vitro biological properties of hydroxyapatite ceramics[J]. Journal of the American Ceramic Society,2011,94(4):1281-1288. doi: 10.1111/j.1551-2916.2010.04228.x [13] LALA S, MAITY T N, SINGHA M, et al. Effect of doping (Mg, Mn, Zn) on the microstructure and mechanical properties of spark plasma sintered hydroxyapatites synthesized by mechanical alloying[J]. Ceramics International, 2017, 43(2): 2389-2397. [14] CHEN S, SHI Y, ZHANG X, et al. Biomimetic synthesis of Mg-substituted hydroxyapatite nanocomposites and three-dimensional printing of composite scaffolds for bone regeneration[J]. Journal of Biomedical Materials Research,2019,107(11):2512-2521. doi: 10.1002/jbm.a.36757 [15] YAN Q S. Research progress in spark plasma sintering[J]. Science and Technology Innovation Herald,2016,31(13):36-37. [16] SADEGHI B, CAVALIERE P. Spark plasma sintering: Process fundamentals[M]. Berlin: Springer, 2019. [17] ZHANG J X, LIU K G, ZHOU M L. Development and application of spark plasma sintering[J]. Powder Metallurgy Technology,2002,20(3):129-134. [18] YAO T K, SCOTT S, XIN G Q, et al. Dense iodoapatite ceramics consolidated by low-temperature spark plasma sintering[J]. Journal of the American Ceramic Society,2015,98(12):3733-3739. doi: 10.1111/jace.13867 [19] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 医疗器械生物学评价 第5部分: 体外细胞毒性试验: GB/T 16886.5—2017[S]. 北京: 中国标准出版社, 2017.General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China. Biological evaluation of medical devices—Part 5: In vitro cytotoxicity test: GB/T 16886.5—2017[S]. Beijing: China Standards Press, 2017(in Chinese). [20] 唐旭. SPS制备多孔HA骨修复材料的孔隙特征与力学性能研究[D]. 昆明: 昆明医科大学, 2014.TANG Xu. Study on preparation and biological properties of porous strontium-doped hydroxyapatite bone repair materials prepared by spark plasma sintering[D]. Kunming: Kunming Medical University, 2014(in Chinese). [21] WOODRUFF M, LANGE A, REICHERT C, et al. Bone tissue engineering: From bench tobedside[J]. Materials Today, 2012, 15: 430-435. [22] LOH Q L, CHOONG C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size[J]. Tissue Engineering Part B: Reviews, 2013, 19(6): 486-502. [23] EDWARD F, PAUL H, SPAOWEN M, et al. Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells[J]. Biomaterials, 2003, 24(10): 1745-1750. [24] 林开利. 纳米磷酸钙、硅酸钙及其复合生物与环境材料的制备和性能研究[D]. 上海: 华东师范大学, 2008.LIN Kaili. Preparation and properties of nano-sized calcium phosphate, calcium silicate and their composite biological and environmental materials[D]. Shanghai: East China Normal University, 2008(in Chinese). [25] MALEKI-GHALEH H, SIADATI M H, FALLAH A, et al. Antibacterial and cellular behaviors of novel zinc-doped hydroxyapatite/graphene nanocomposite for bone tissue engineering[J]. International Journal of Molecular Sciences, 2021, 22(17): 9564. [26] 何远怀. 羟基磷灰石/Ti-13Nb-13Zr生物材料的制备和性能研究[D]. 昆明: 昆明理工大学, 2018.HE Yuanhuai. Preparation and properties of hydroxyapatite/Ti-13Nb-13Zr biomaterials[D]. Kunming: Kunming University of Science and Technology, 2018(in Chinese). -





 下载:
下载: