Preparation of palygorskite-Cd0.5Zn0.5S/Zn-Fe LDH composite and its photocatalytic performance
-
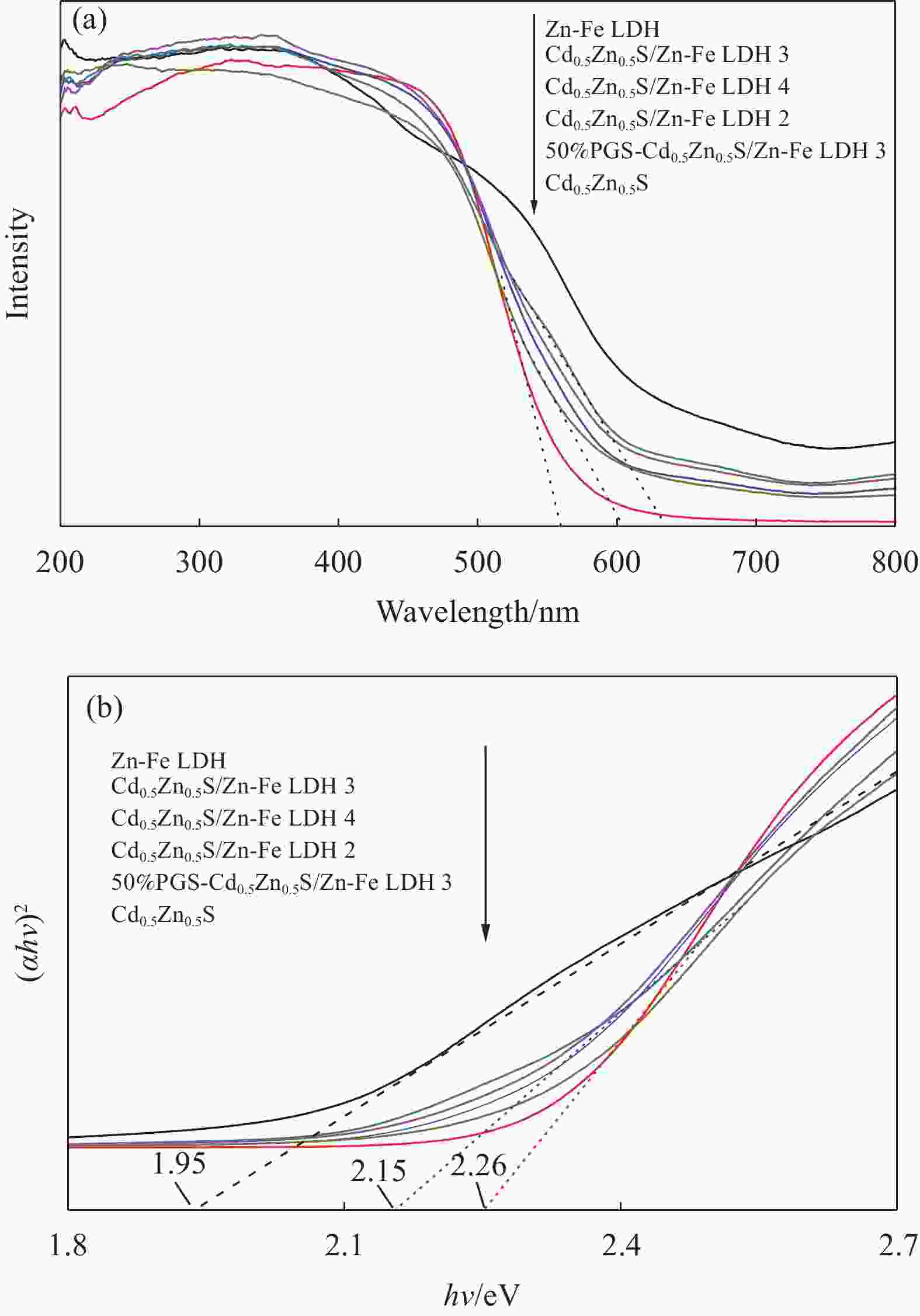
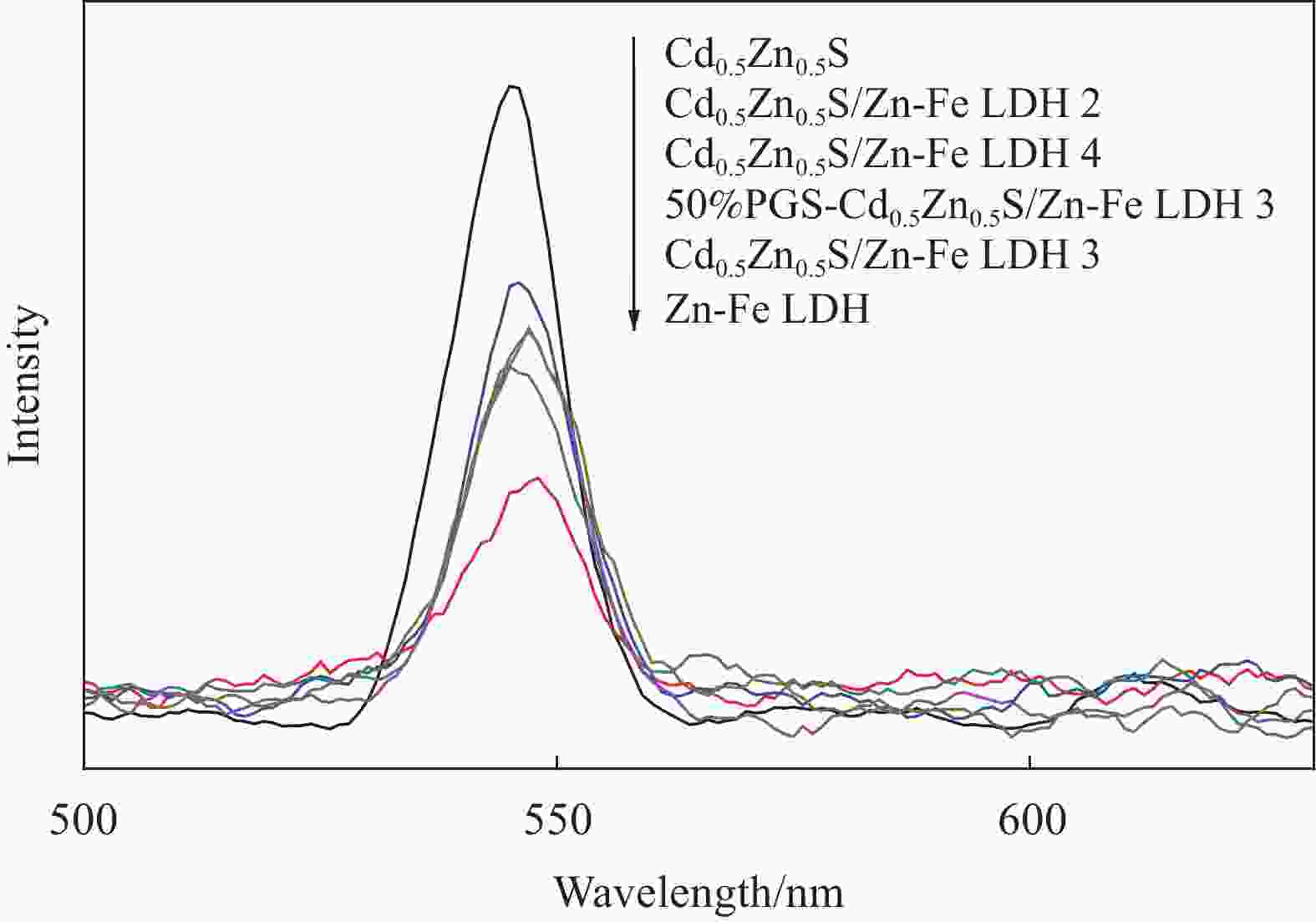
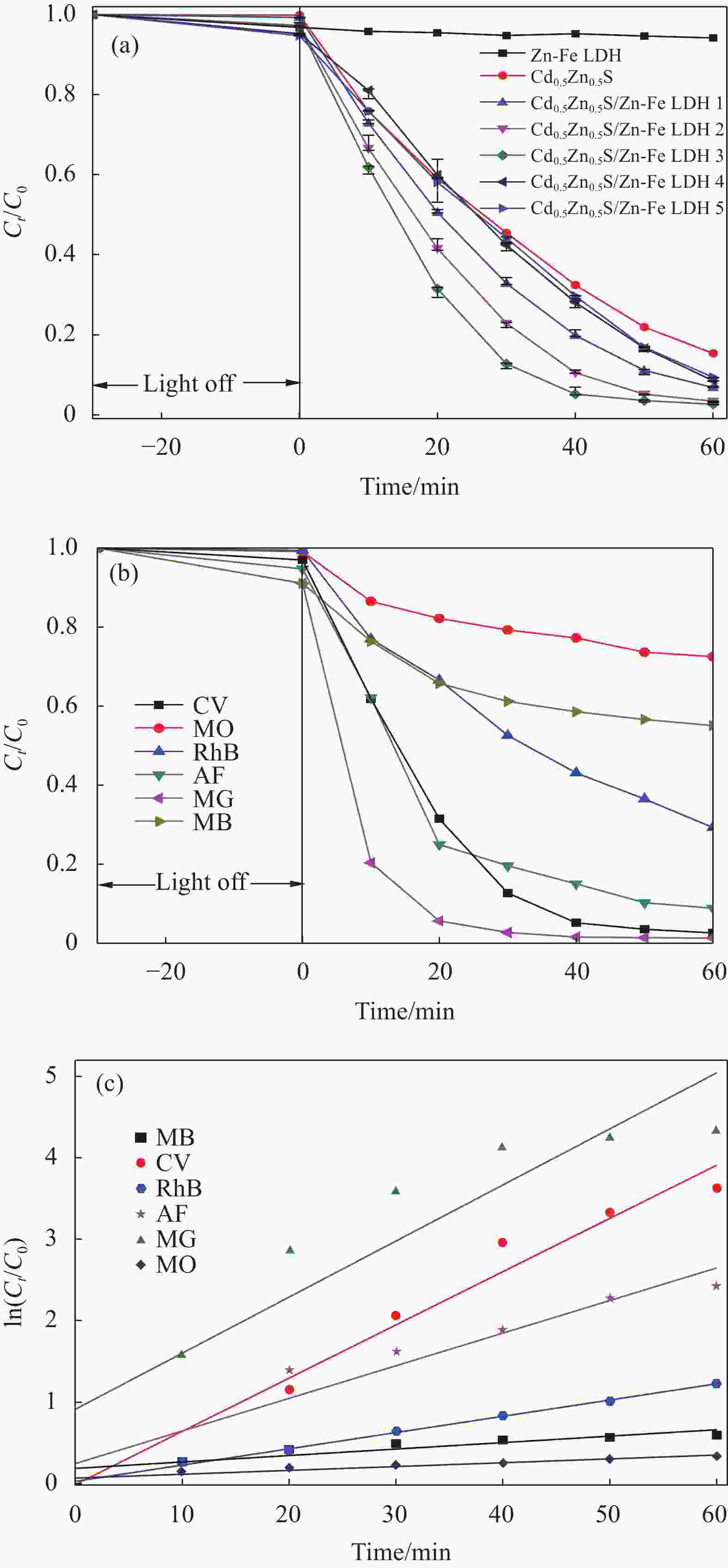
摘要: 为解决Cd0.5Zn0.5S易光腐蚀的缺点,两步水热法制备了坡缕石(PGS)负载Cd0.5Zn0.5S/Zn-Fe 层状双金属氢氧化物(LDH)复合材料(PGS-Cd0.5Zn0.5S/Zn-Fe LDH),通过Zn-Fe LDH和PGS提高光生载流子的分离效率。利用XRD、SEM、TEM、UV-Vis DRS和PL对材料的结构、形貌及光学性能进行了表征。电镜图像显示,片状Zn-Fe LDH表面附着针状PGS与颗粒状Cd0.5Zn0.5S。紫外-可见漫反射光谱表明PGS-Cd0.5Zn0.5S/Zn-Fe LDH吸光区域比Cd0.5Zn0.5S宽,吸收边缘从560 nm红移至605 nm。PGS-Cd0.5Zn0.5S/Zn-Fe LDH在光催化降解结晶紫(CV)中表现出良好的光催化活性,催化活性高于Cd0.5Zn0.5S和Zn-Fe LDH。当PGS 与Cd0.5Zn0.5S/Zn-Fe LDH质量比为50%时,可见光照射60 min, 20 mg PGS-Cd0.5Zn0.5S/Zn-Fe LDH对20 mg/L结晶紫溶液的去除率为97.5%,
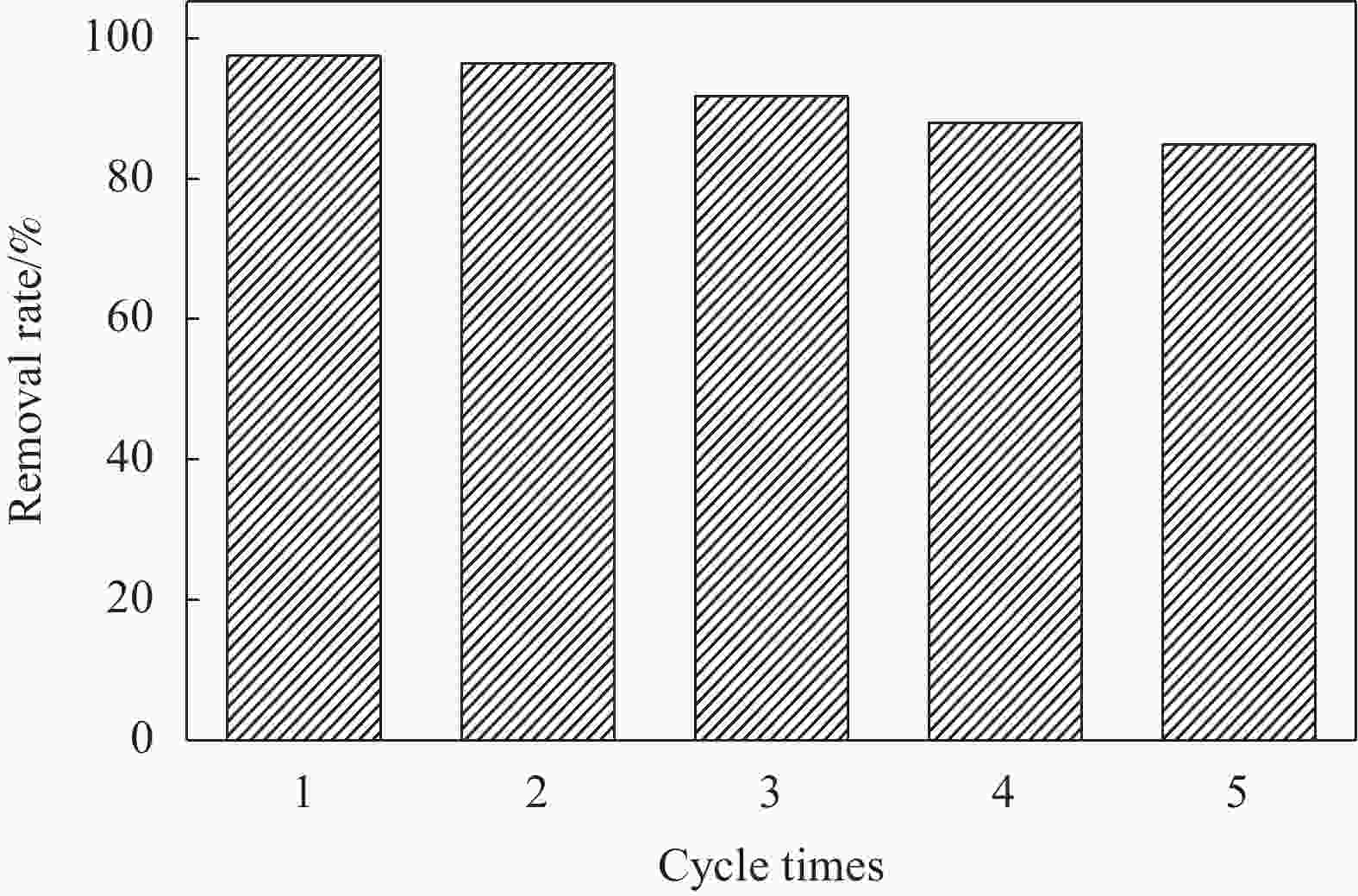
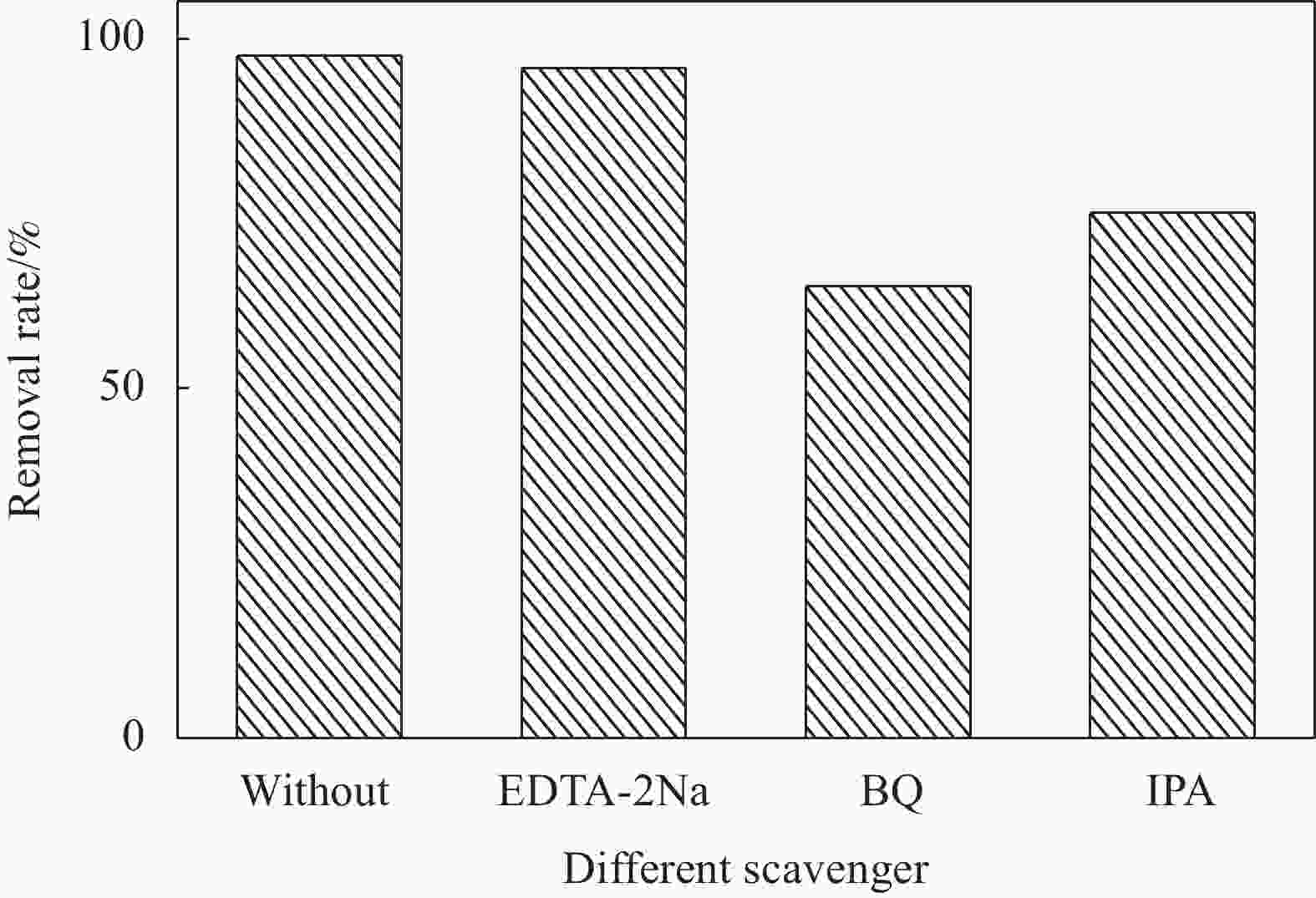
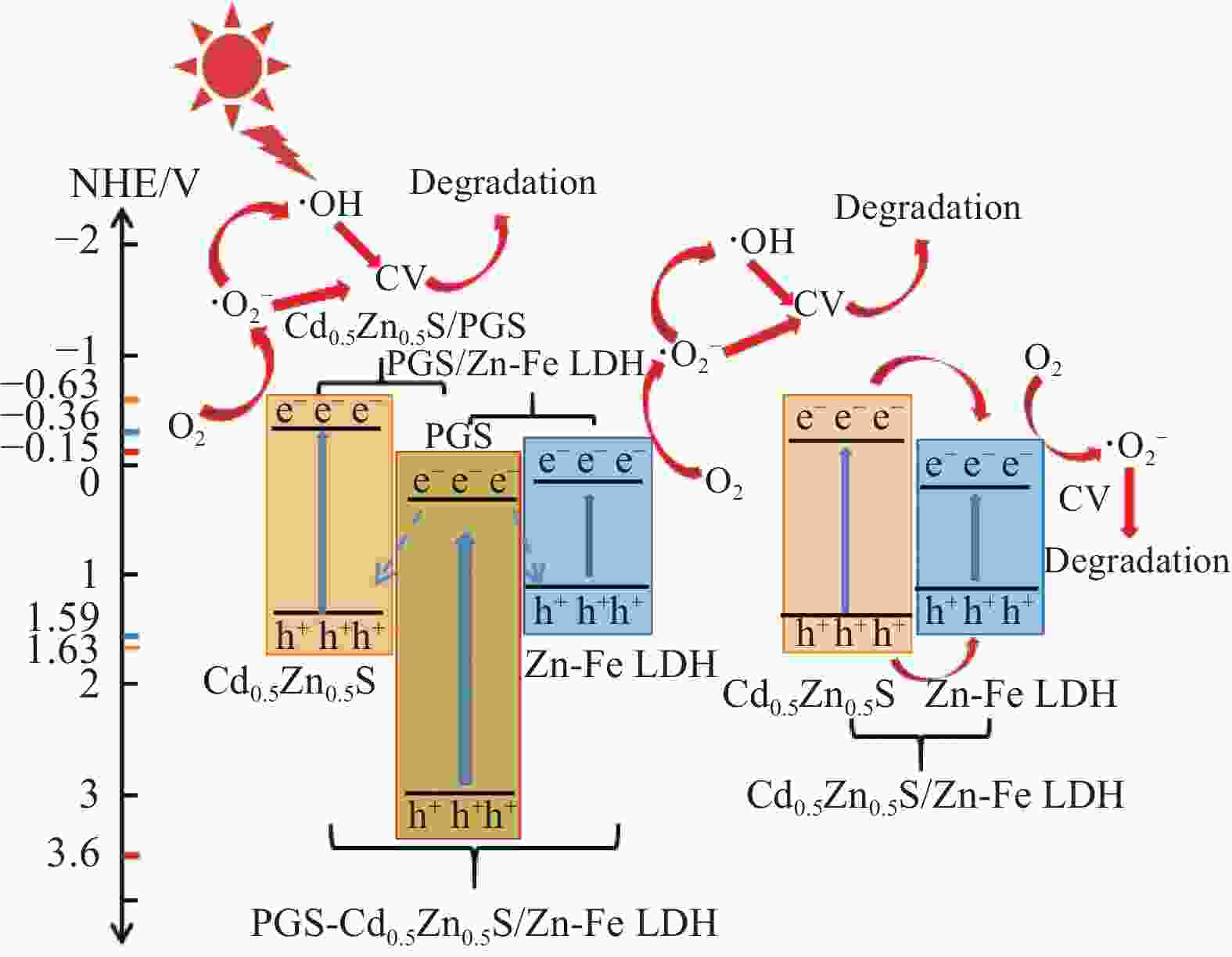
${\text{•}} {\rm{O}}_2^{-}$ 、•OH是光催化降解的主要活性物种,且5次循环实验后仍然保持较高活性。此外,制备的复合材料对孔雀石绿(MG)、酸性品红(AF)、罗丹明B(RhB)、甲基橙(MO)、亚甲基蓝(MB)等染料均表现出较好的光降解效果。-
关键词:
- Cd0.5Zn0.5S/Zn-Fe LDH /
- 坡缕石 /
- 光催化剂 /
- 降解 /
- 染料
Abstract: To solve easy photocorrosion of Cd0.5Zn0.5S, palygorskite (PGS) supported Cd0.5Zn0.5S/Zn-Fe layered double hydroxides (LDH) composites (PGS-Cd0.5Zn0.5S/Zn-Fe LDH) were prepared by a two-step hydrothermal method. The aim is to improve the separation efficiency of photogenerated carriers using Zn-Fe LDH and PGS. Its crystal phase, micromorphology and optical properties were characterized by XRD, SEM, TEM, UV-Vis DRS and PL. The electron microscope images show that the needle-like PGS and graininess Cd0.5Zn0.5S are attached on the surface of Zn-Fe LDH. UV-visible diffuse reflectance spectroscopies confirm that the PGS-Cd0.5Zn0.5S/Zn-Fe LDH has a wider absorption range than Cd0.5Zn0.5S. That absorption range has a red shift from 560 nm to 605 nm. The photocatalytic activity of PGS-Cd0.5Zn0.5S/Zn-Fe LDH is higher than those of Cd0.5Zn0.5S and Zn-Fe LDH in the degradation of crystal violet. The photocatalyst with mass ratio of PGS to Cd0.5Zn0.5S/Zn-Fe LDH of 50%, the removal rate of 20 mg/L crystal violet is 97.5% by 20 mg of PGS-Cd0.5Zn0.5S/Zn-Fe LDH for 60 min.${\text{•}}{\rm{O}}_2^{-} $ and •OH groups are the main active species in photodegradatlon reaction. The photocatalytic activity of composite is well retained after five cycles. Meanwhile, the composite shows good photocatalytic activity in the degradations of various dyes including malachite green (MG), acid fuchsin (AF), Rhodamine B (RhB), methyl orange (MO) and methylene blue (MB).-
Key words:
- Cd0.5Zn0.5S/Zn-Fe LDH /
- palygorskite /
- photocatalyst /
- degradation /
- dye
-
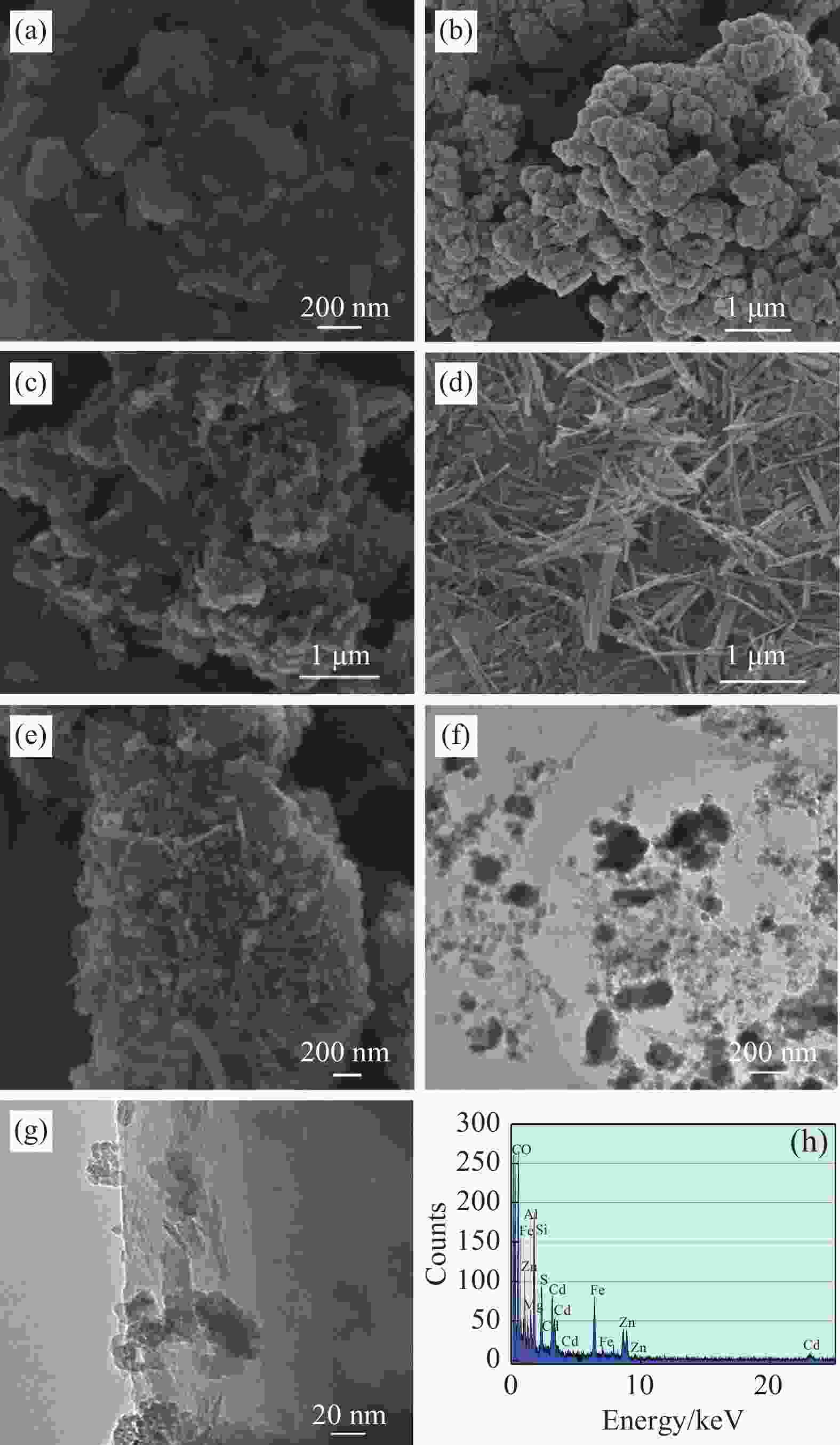
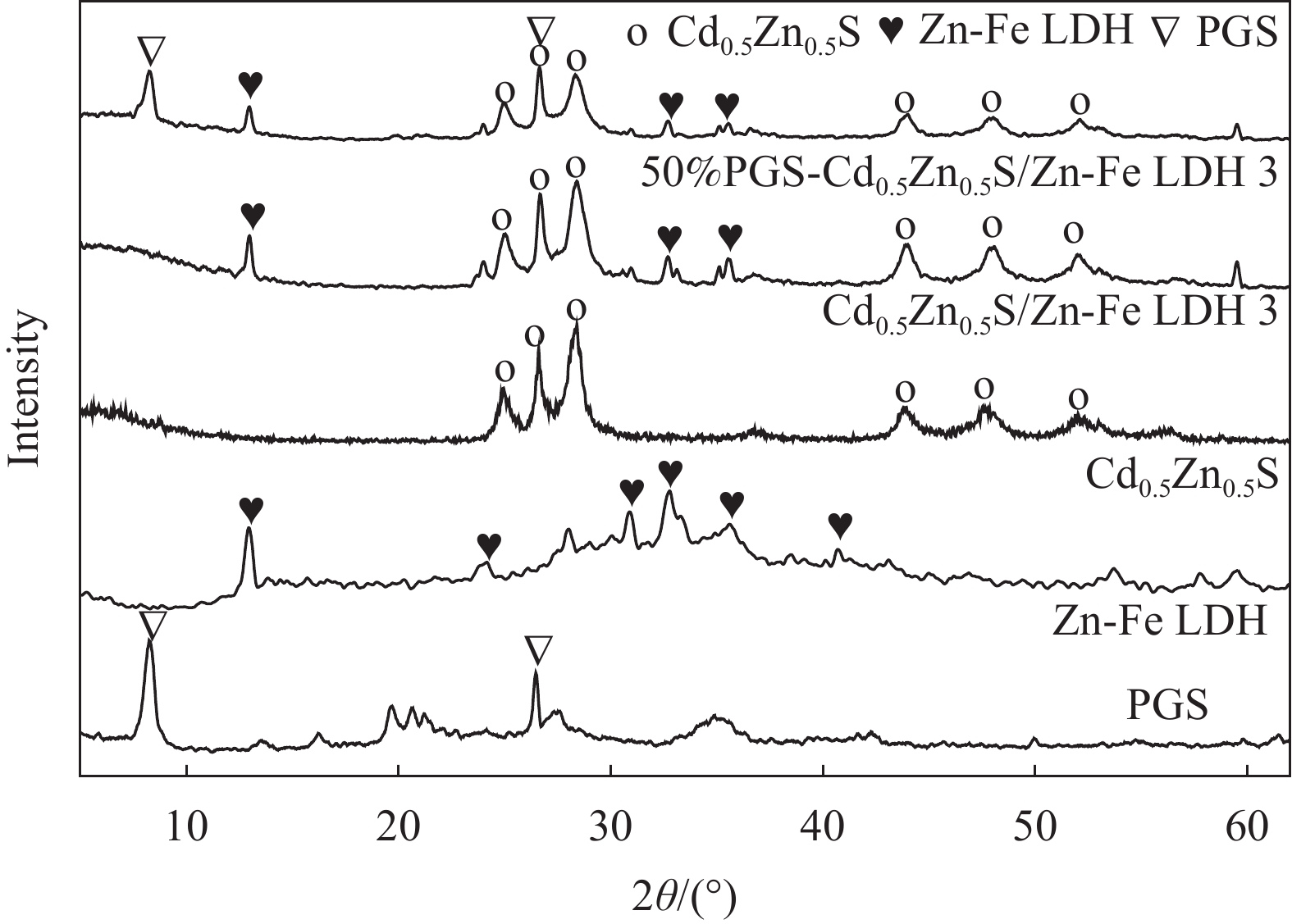
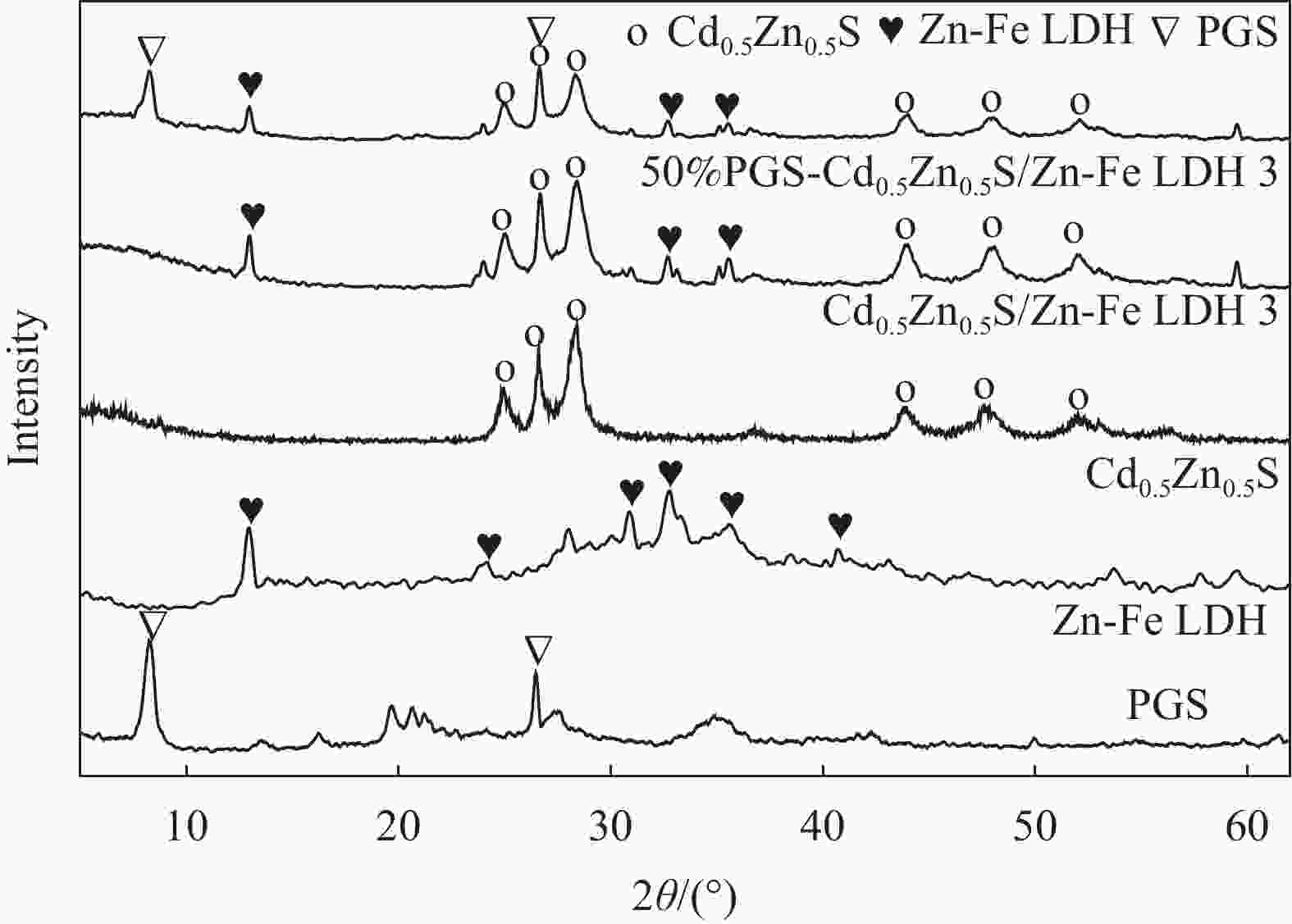
图 2 Zn-Fe LDH (a)、Cd0.5Zn0.5S (b)、Cd0.5Zn0.5S/Zn-Fe LDH 3 (c)、PGS (d)和50%PGS-Cd0.5Zn0.5S/Zn-Fe LDH 3 (e)的SEM图像;50%PGS-Cd0.5Zn0.5S/Zn-Fe LDH 3的TEM图像((f), (g))及EDS能谱图(h)
Figure 2. SEM images of Zn-Fe LDH (a), Cd0.5Zn0.5S (b), Cd0.5Zn0.5S/Zn-Fe LDH 3 (c), PGS (d) and 50%PGS-Cd0.5Zn0.5S/Zn-Fe LDH 3 (e); TEM images ((f), (g)) and EDS spectrum (h) of 50%PGS-Cd0.5Zn0.5S/Zn-Fe LDH 3
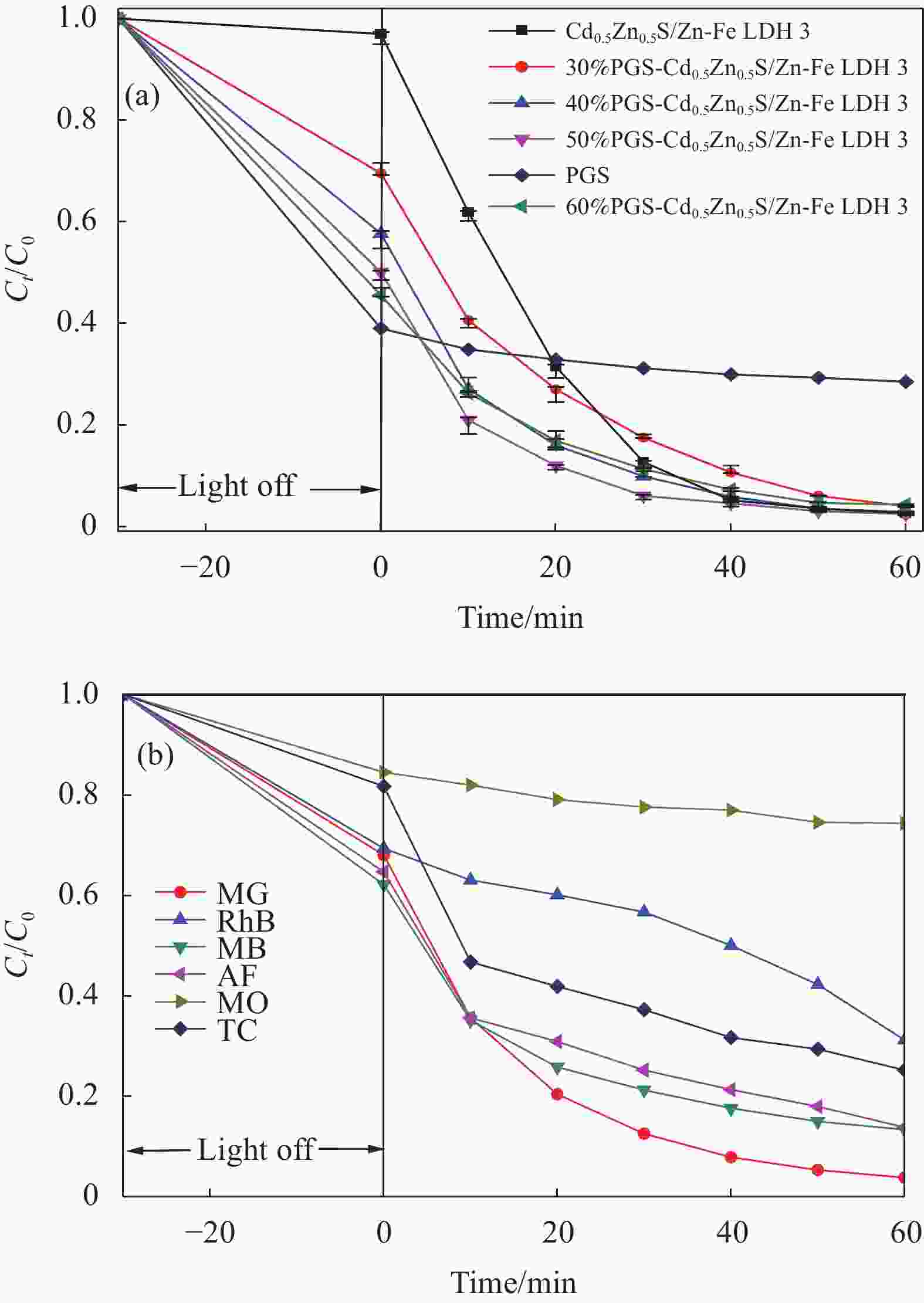
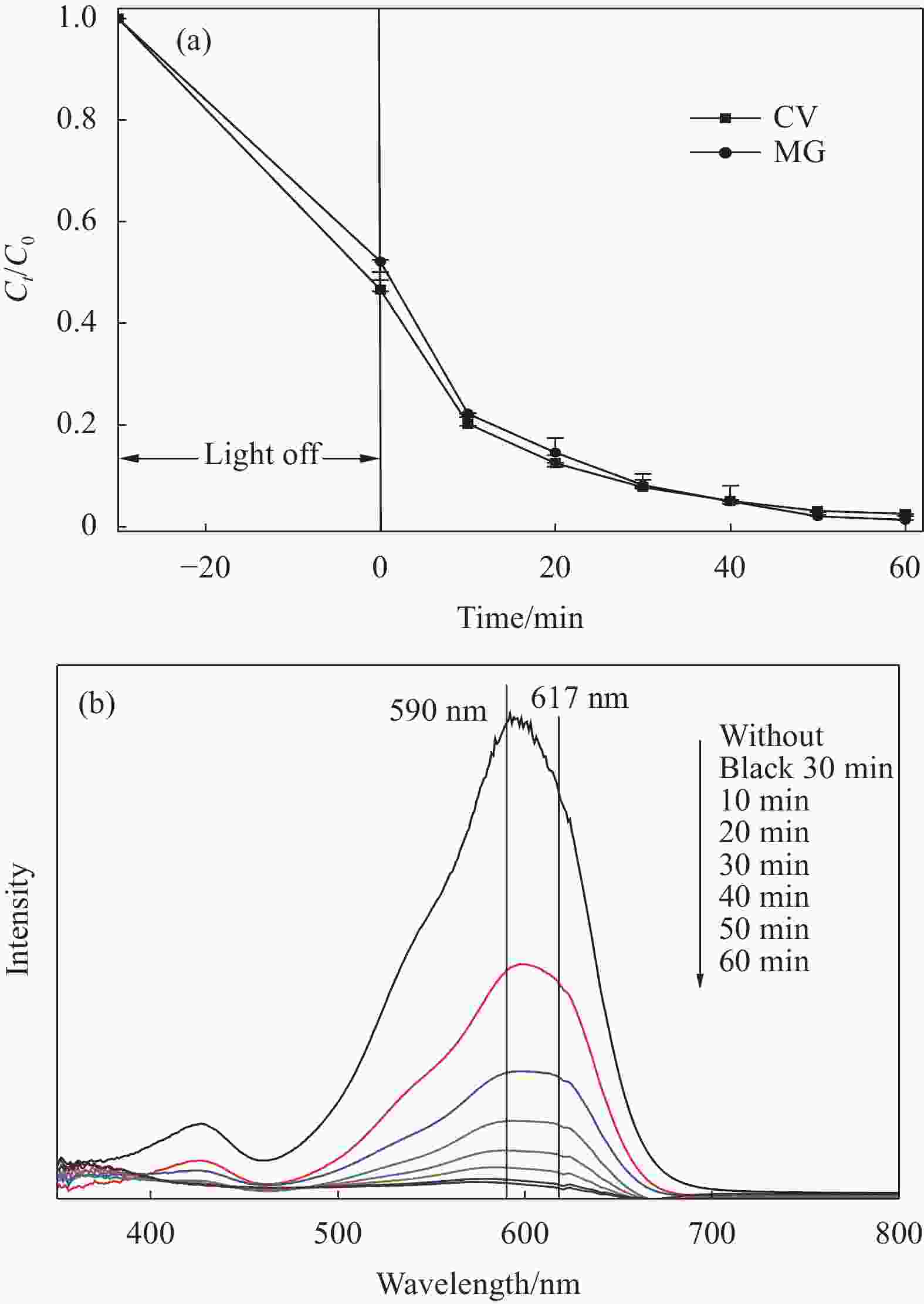
图 5 Cd0.5Zn0.5S、Zn-Fe LDH和Cd0.5Zn0.5S/Zn-Fe LDH复合材料对染料的光催化活性((a), (b))及光催化降解反应动力学(c)
Figure 5. Photocatalytic activity of Cd0.5Zn0.5S, Zn-Fe LDH and Cd0.5Zn0.5S/Zn-Fe LDH composites for dyes ((a), (b)) and photocatalytic degradation reaction kinetics (c)
Ct—Concentration after time t of degradation; C0—Initial concentration; MB—Methylene blue; CV—Crystal violet; RhB—Rhodamine B; AF—Acid fuchsin; MG—Malachite green; MO—Methyl orange
表 1 Cd0.5Zn0.5S/Zn-Fe层状双金属氢氧化物(LDH)复合材料的命名
Table 1. Nomenclature of Cd0.5Zn0.5S/Zn-Fe layered double hydroxides (LDH) composites
Sample Mass of Zn-Fe LDH/g Mass ratio of Cd0.5Zn0.5S to
Zn-Fe LDHCd0.5Zn0.5S/Zn-Fe LDH 1 0.052 7∶1 Cd0.5Zn0.5S/Zn-Fe LDH 2 0.104 7∶2 Cd0.5Zn0.5S/Zn-Fe LDH 3 0.156 7∶3 Cd0.5Zn0.5S/Zn-Fe LDH 4 0.208 7∶4 Cd0.5Zn0.5S/Zn-Fe LDH 5 0.260 7∶5 表 2 坡缕石(PGS)-Cd0.5Zn0.5S/Zn-Fe LDH复合材料的命名
Table 2. Naming of palygorskite (PGS)-Cd0.5Zn0.5S/Zn-Fe LDH composites
Sample Mass ratio of PGS/wt% Mass ratio of Cd0.5Zn0.5S/
Zn-Fe LDH 3/wt%30%PGS-Cd0.5Zn0.5S/
Zn-Fe LDH 330 100 40%PGS-Cd0.5Zn0.5S/
Zn-Fe LDH 340 100 50%PGS-Cd0.5Zn0.5S/
Zn-Fe LDH 350 100 60%PGS-Cd0.5Zn0.5S/
Zn-Fe LDH 360 100 -
[1] ZUBAIR M, DAUD M, MCKAY G, et al. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation[J]. Applied Clay Science,2017,143(1):279-292. [2] FENG J, HE Y, LIU Y, et al. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects[J]. Chemical Society Reviews,2015,44(15):5291-5319. doi: 10.1039/C5CS00268K [3] YAN K, WU G, JIN W. Recent advances in the synthesis of layered, double hydroxide-based materials and their application in hydrogen and oxygen evolution[J]. Energy Technology,2016,4(3):354-368. doi: 10.1002/ente.201500343 [4] DI G, ZHU Z, HUANG Q, et al. Targeted modulation of g-C3N4 photocatalytic performance for pharmaceutical pollutants in water using Zn Fe-LDH derived mixed metal oxides: Structure-activity and mechanism[J]. The Science of the Total Environment,2019,650(1):1112-1121. [5] SHAO M, HAN J, WEI M, et al. The synthesis of hierarchi-cal Zn-Ti layered double hydroxide for efficient visible-light photocatalysis[J]. Chemical Engineering Journal,2011,168(2):519-524. doi: 10.1016/j.cej.2011.01.016 [6] ABDERRAZEK K, NAJOUA F S, SRASRA E. Synthesis and characterization of [Zn-Al] LDH: Study of the effect of calcination on the photocatalytic activity[J]. Applied Clay Science,2016,119(2):229-235. [7] DAI D S, WANG L, XIAO N, et al. In-situ synthesis of Ni2P co-catalyst decorated Zn0.5Cd0.5S nanorods for high-quantum-yield photocatalytic hydrogen production under visible light irradiation[J]. Applied Catalysis B: Environmental,2018,233(1):194-201. [8] XUE W, CHANG W, HU X, et al. 2D mesoporous ultrathin Cd0.5Zn0.5S nanosheet: Fabrication mechanism and application potential for photocatalytic H2 evolution[J]. Chinese Journal of Catalysis,2021,42(1):152-163. doi: 10.1016/S1872-2067(20)63593-8 [9] MA A, TANG Z, SHEN S, et al. Controlled synthesis of ZnxCd1-xS nanorods and their composite with RGO for high-performance visible-light photocatalysis[J]. Royal Society of Chemistry Advances,2015,5(35):27829-27836. [10] HUANG H, FANG Z, YU K, et al. Visible-light-driven photocatalytic H2 evolution over CdZnS nanocrystal solid solutions: Interplay of twin structures, sulfur vacancies and sacrificial agents[J]. Journal of Materials Chemistry A,2020,8(7):3882-3891. doi: 10.1039/C9TA13836F [11] ISIMJAN T T, MAITY P, LLORCA J, et al. Comprehensive study of all-solid-state Z-scheme photocatalytic systems of ZnO/Pt/CdZnS[J]. Journal of Materials Chemistry A,2017,2(8):4828-4837. doi: 10.1021/acsomega.7b00767 [12] DIMITRIJEVIE N M, LI S B, GRATZEL M. Visible light induced oxygen evolution in aqueous CdS suspensions[J]. Journal of the American Chemical Society,1984,106(22):6565-6569. doi: 10.1021/ja00334a018 [13] MEI Z W, ZHANG B K, ZHENG J X, et al. Tuning Cu dopant of Zn0.5Cd0.5S nanocrystals enables high-performance photocatalytic H2 evolution from water splitting under visible-light irradiation[J]. Nano Energy,2016,26(1):405-416. [14] QI S, WANG D, ZHAO Y, et al. Core-shell g-C3N4@Zn0.5Cd0.5S heterojunction photocatalysts with high photocatalytic activity for the degradation of organic dyes[J]. Journal of Materials Science: Materials in Electronics,2019,30(5):5284-5296. doi: 10.1007/s10854-019-00828-w [15] WANG L, YAO Z, JIA F, et al. A facile synthesis of ZnxCd1-xS/CNTs nanocomposite photocatalyst for H2 production[J]. Dalton Transactions,2013,42(27):9976-9981. doi: 10.1039/c3dt50379h [16] SU J, WU X, CUI Y, et al. Preparation, characterization and photocatalytic degradation properties of Zn0.5Cd0.5S/SnO2 composites[J]. Journal of Materials Science: Materials in Electronics,2019,31(2):1585-1593. [17] WANG S, WANG Y, ZHUANG Y, et al. Synthesis of palygorskite supported spherical ZnS nanocomposites with enhanced photocatalytic activity[J]. CrystEngComm,2021,23(23):4229-4236. doi: 10.1039/D1CE00486G [18] 董秀芳, 洪均茂, 王超. 甘氨酸修饰Fe/Zn层状双金属氢氧化物的制备及其吸附性能[J]. 中北大学学报(自然科学版), 2021, 42(6):542-550.DONG Xiufang, HONG Junmao, WANG Chao. Preparation and adsorption performance of glycine-modified Fe/Zn layered double hydroxides[J]. Journal of North University of China (Natural Science Edition),2021,42(6):542-550(in Chinese). [19] THITE V, GIRIPUNJE S M. Giripunje structural characterization and photoluminescent behavior of CO32– intercalated Zn-Fe layered double hydroxide (LDH) and its colloids[J]. Journal of Electronic Materials,2020,50(4):1601-1607. [20] ZENG C, HU Y, ZHANG T, et al. A core-satellite structured Z-scheme catalyst Cd0.5Zn0.5S/BiVO4 for highly efficient and stable photocatalytic water splitting[J]. Journal of Materials Chemistry A,2018,6(35):16932-16942. doi: 10.1039/C8TA04258F [21] LUO Y T, WANG K, HU T, et al. Controlled synthesis of palygorskite/Bi5O7I hybrid microspheres with high efficient photodegradation of antibiotics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2021,616:126225. doi: 10.1016/j.colsurfa.2021.126225 [22] WU Y, WANG H, TU W, et al. Construction of hole-transported MoO3-x coupled with CdS nanospheres for boosting photocatalytic performance via oxygen-defects-mediated Z-scheme charge transfer[J]. Applied Organometallic Chemistry,2019,33(4):304-315. [23] HUANG L, XU H, LI Y, et al. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity[J]. Dalton Transactions,2013,42(24):8606-8616. doi: 10.1039/c3dt00115f [24] 刘成宝, 唐飞, 朱晨, 等. WO3-Ag/石墨相 C3N4 Z型复合光催化剂的合成及其光催化性能[J]. 复合材料学报, 2021, 38(1):209-220.LIU Chengbao, TANG Fei, ZHU Chen, et al. Preparation and photocatalytic properties of WO3-Ag/graphitic C3N4 Z-scheme composite photocatalyst[J]. Acta Materiae Compositae Sinica,2021,38(1):209-220(in Chinese). [25] 唐清海, 李梦佳, 曹珊珊, 等. CdS/Zn-Fe LDO复合材料光催化降解孔雀石绿[J]. 精细化工, 2020, 37(11):2342-2347.TANG Qinghai, LI Mengjia, CAO Shanshan, et al. Photocatalytic degradation of malachite green by CdS/Zn-Fe LDO composite materials[J]. Fine Chemicals,2020,37(11):2342-2347(in Chinese). [26] TAVAKOLI-AZAR T, MAHJOUB A R, SADJADI M S, et al. Synthesis and characterization of CdTiO3@S composite: Investigation of photocatalytic activity for the degradation of crystal violet under sunlight[J]. Journal of Inorganic and Organometallic Polymers and Materials,2020,30(2):667-683. [27] MANIKANDAN V, ELANCHERAN R, REVATHI P, et al. Efficient photocatalytic degradation of crystal violet by using graphene oxide/nickel sulphide nanocomposites[J]. Bulletin of Materials Science,2020,43(1):1-10. doi: 10.1007/s12034-019-1971-5 [28] PAWAR K K, CHAUDHARY L S, MALI S S, et al. In2O3 nanocapsules for rapid photodegradation of crystal violet dye under sunlight[J]. Journal of Colloid and Interface Science,2020,561(1):287-297. [29] WEN X, NIU C, HUANG D, et al. Study of the photocatalytic degradation pathway of norfloxacin and mineralization activity using a novel ternary Ag/AgCl-CeO2 photocatalyst[J]. Journal of Catalysis,2017,355(3):73-86. [30] LI X, YU J, FANG Y, et al. Engineering heterogeneous semiconductors for solar water splitting[J]. Journal of Materials Chemistry A,2015,3(6):2485-2534. doi: 10.1039/C4TA04461D [31] SAMADI-MAYBODI A, SHARIATI M. A study on the transfer of photo-excited charge carriers within direct and inverted type-I heterojunctions of CdS and ZnS QDs[J]. New Journal of Chemistry,2018,42(12):9808-9818. doi: 10.1039/C8NJ00584B [32] ZHENG Q, YAN T, LI W, et al. In situ anion exchange synthesis of In2S3/In(OH)3 heterostructures for efficient photocatalytic degradation of MO under solar light[J]. New Journal of Chemistry,2017,41(8):1-10. -







 下载:
下载: